Abstract
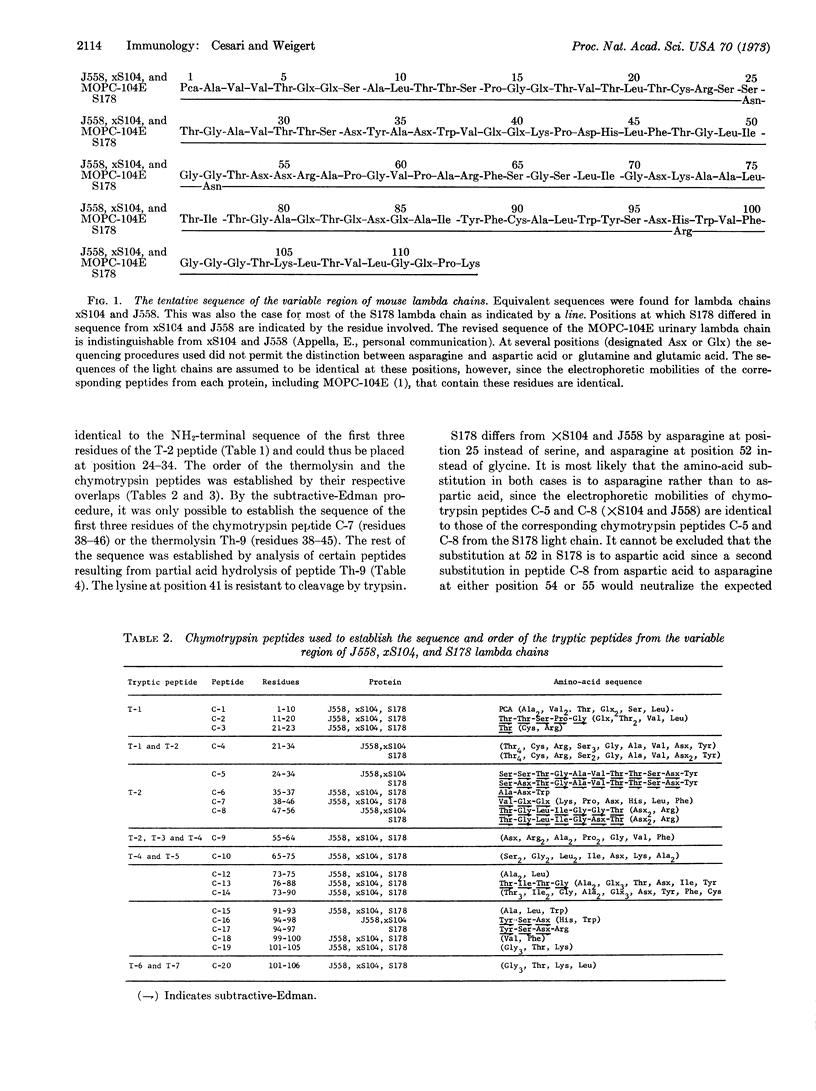
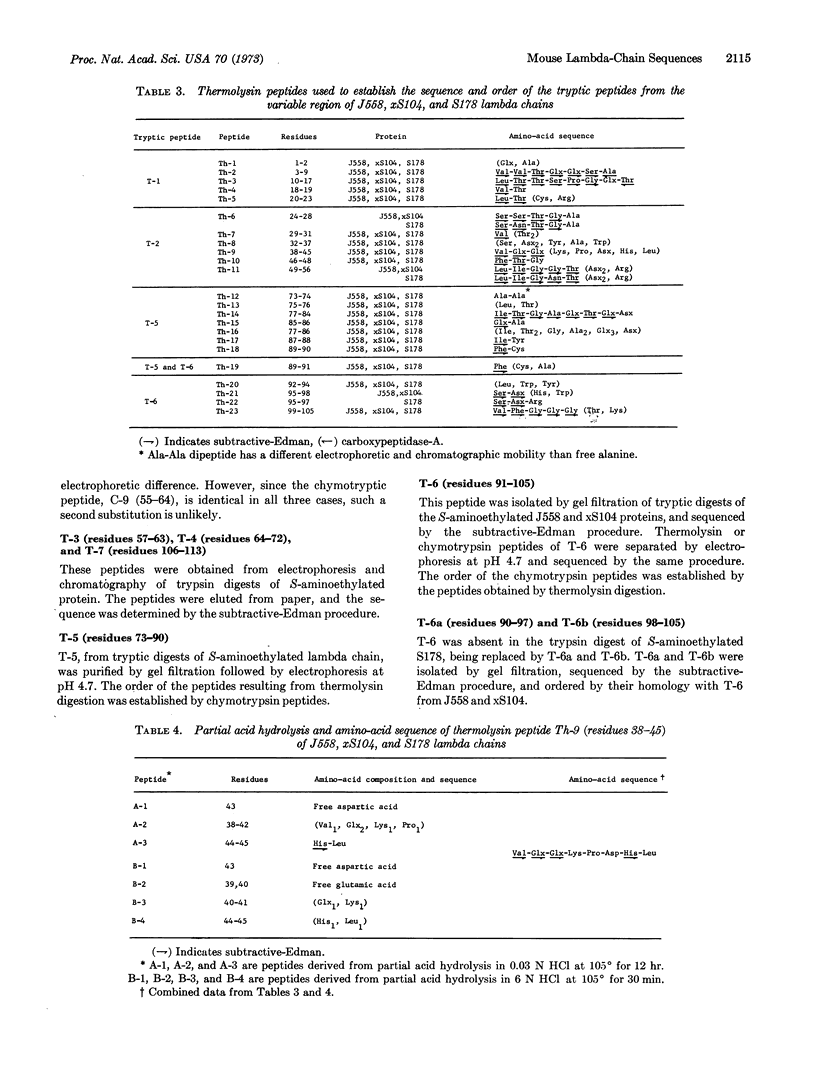
Amino-acid sequences of the variable regions of three lambda chains produced by plasmacytomas of BALB/c mice are compared. Two are almost certainly identical and one differs from these by three amino acids. These findings extend our earlier conclusion on the relative uniformity of sequences in this type of immunoglobulin light chain. With amino-acid sequence data on two additional lambda chains, eight mouse lambda chains studied to date are indistinguishable and four probably differ from these by one, two, or three amino acids.
Keywords: variable regions, plasmocytomas, BALB, c mice
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abel C. A., Grey H. M. Studies on the structure of mouse gamma-A myeloma proteins. Biochemistry. 1968 Jul;7(7):2682–2688. doi: 10.1021/bi00847a035. [DOI] [PubMed] [Google Scholar]
- Appella E. Amino acid sequences of two mouse immunoglobulin lambda chains. Proc Natl Acad Sci U S A. 1971 Mar;68(3):590–594. doi: 10.1073/pnas.68.3.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg B., Geckeler W. R., Weigert M. Genetics of the antibody response to dextran in mice. Science. 1972 Jul 14;177(4044):178–180. doi: 10.1126/science.177.4044.178. [DOI] [PubMed] [Google Scholar]
- FOLK J. E., SCHIRMER E. W. THE PORCINE PANCREATIC CARBOXYPEPTIDASE A SYSTEM. I. THREE FORMS OF THE ACTIVE ENZYME. J Biol Chem. 1963 Dec;238:3884–3894. [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- KONIGSBERG W., HILL R. J. The structure of human hemoglobin. III. The sequence of amino acids in the tryptic peptides of the alpha chain. J Biol Chem. 1962 Aug;237:2547–2561. [PubMed] [Google Scholar]
- Leon M. A., Young N. M., McIntire K. R. Immunochemical studies of the reaction between a mouse myeloma macroglobulin and dextrans. Biochemistry. 1970 Feb 17;9(4):1023–1030. doi: 10.1021/bi00806a043. [DOI] [PubMed] [Google Scholar]
- MAZUR R. H., ELLIS B. W., CAMMARATA P. S. A new reagent for detection of peptides, nucleotides, and other N-H-containing compounds on paper chromatograms. J Biol Chem. 1962 May;237:1619–1621. [PubMed] [Google Scholar]
- Matsubara H., Sasaki R. M. High recovery of tryptophan from acid hydrolysates of proteins. Biochem Biophys Res Commun. 1969 Apr 29;35(2):175–181. doi: 10.1016/0006-291x(69)90263-0. [DOI] [PubMed] [Google Scholar]
- RAFTERY M. A., COLE R. D. Tryptic cleavage at cysteinyl peptide bonds. Biochem Biophys Res Commun. 1963 Mar 25;10:467–472. doi: 10.1016/0006-291x(63)90381-4. [DOI] [PubMed] [Google Scholar]
- WEIGERT M. G., GAREN A. AMINO ACID SUBSTITUTIONS RESULTING FROM SUPPRESSION OF NONSENSE MUTATIONS. I. SERINE INSERTION BY THE SU-1 SUPPRESSOR GENE. J Mol Biol. 1965 Jun;12:448–455. doi: 10.1016/s0022-2836(65)80267-4. [DOI] [PubMed] [Google Scholar]
- Weigert M. G., Cesari I. M., Yonkovich S. J., Cohn M. Variability in the lambda light chain sequences of mouse antibody. Nature. 1970 Dec 12;228(5276):1045–1047. doi: 10.1038/2281045a0. [DOI] [PubMed] [Google Scholar]
- Wu T. T., Kabat E. A. An analysis of the sequences of the variable regions of Bence Jones proteins and myeloma light chains and their implications for antibody complementarity. J Exp Med. 1970 Aug 1;132(2):211–250. doi: 10.1084/jem.132.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S., Itano H. Phenanthrenequinone as an analytical reagent for arginine and other monosubstituted guanidines. Biochim Biophys Acta. 1966 Dec 28;130(2):538–540. doi: 10.1016/0304-4165(66)90256-x. [DOI] [PubMed] [Google Scholar]



