Abstract
The role of neuronal nicotinic acetylcholine receptors (nAChR) containing the β4 subunit in tolerance development and nicotinic binding site levels following chronic nicotine treatment was investigated. Mice differing in expression of the β4 nAChR subunit [wild-type (β4++), heterozygote (β4+−) and null mutant (β4−−)] were chronically treated for 10 days with nicotine (0, 0.5, 1.0, 2.0 or 4.0 mg/kg/hr) by constant intravenous infusion. Chronic nicotine treatment elicited dose-dependent tolerance development. β4−− mice developed significantly more tolerance than either β4++ or β4+− mice which was most evident following treatment with 4.0 mg/kg/hr nicotine. Subsets of [125I]-epibatidine binding were measured in several brain regions. Deletion of the β4 subunit had little effect on initial levels of cytisine-sensitive [125I]-epibatidine binding (primarily α4β2-nAChR sites) or their response (generally increased binding) to chronic nicotine treatment. In contrast, β4 gene-dose-dependent decreases in expression 5IA-85380 resistant [125I]-epibatidine binding sites (primarily β4*-nAChR) were observed. While these β4*nAChR sites were generally resistant to regulation by chronic nicotine treatment, significant increases in binding were noted for habenula and hindbrain. Comparison of previously published tolerance development in β2−− mice (less tolerance) to that of β4−− mice (more tolerance) supports a differential role for these receptor subtypes in regulating tolerance following chronic nicotine treatment.
Keywords: nicotinic acetylcholine receptor, beta4 null mutant mice, chronic nicotine treatment, epibatidine binding, nicotine tolerance, 5I-85380, cytisine
Several lines of evidence indicate that β4*-nAChR [nicotinic acetylcholine receptor, * indicates the presence of additional subunits (Lukas et al., 1999)] are important mediators of responses to both acute and chronic nicotine administration. For example, deletion of the β4 nAChR subunit virtually eliminated somatic signs of nicotine withdrawal in mice that had been chronically treated with nicotine (Salas et al., 2004b). In addition, β4 null mutant mice (β4−−) have greatly reduced seizures following acute administration of nicotine (Salas et al., 2004a). The β4 subunit is densely expressed in the medial habenula (Duvoisin et al., 1989), a brain region that has been demonstrated to be an important modulator of nicotinic responses ((Fowler and Kenny, 2012; Fowler et al., 2011)). The gene encoding the β4 subunit is part of a gene cluster that also includes the genes encoding the α3 and α5 subunits (Boulter et al., 1990), and both of these genes also are important regulators of nicotine-induced seizures (Salas et al., 2004a; Salas et al., 2003). Furthermore, the genome wide association studies have repeatedly demonstrated that variants in the α3α5β4 gene cluster affect many phenotypes related to nicotine use in humans (Stevens et al., 2008). Although most of the attention has focused on polymorphisms in the α5 gene, polymorphisms in the β4 gene also influence tobacco-related phenotypes (Kapoor et al., 2012).
We previously examined the effect of deletion of the β2 gene on tolerance development and nAChR binding sites following chronic nicotine treatment (McCallum et al., 2006). This study confirmed the deletion of the β2 subunit eliminated cytisine-sensitive epibatidine binding sites, which are the sites most responsive to chronic nicotine treatment. Furthermore, little tolerance, and even evidence for increased sensitivity, to the acute effects of nicotine were noted for β2−− mice following chronic nicotine treatment. This result suggests that tolerance may not develop to the responses mediated by residual, nonβ2*- nAChR following chronic nicotine treatment. In order to investigate the role of β4*nAChR in mediating response to chronic nicotine treatment, mice differing in expression of this subunit [β4++ (wild-type), β4+− (heterozygote) and β4−− (null mutant)] were chronically treated with one of several doses of nicotine. Response to the administration of acute nicotine on locomotion and body temperature was measured. In addition, several subsets of high-affinity [125I]-epibatidine binding were measured. The results indicate that β4−− mice develop tolerance more readily that do wild-type mice and that deletion of the β4 subunit has little effect on the regulation of the expression of cytisine-sensitive [125I]-epibatidine binding sites (primarily α4β2-nAChR sites).
2. Materials and Methods
2.1. Materials
[125I]-Epibatidine was obtained from Perkin-Elmer NEN, Boston, MA. NaCl, KCl, MgSO4, CaCl2, Na2HPO4, NaH2PO4, bovine serum albumin, polyethyleneglycol, polyethylenimine, nicotine, and cytisine were obtained from Sigma Chemical Co., St. Louis, MO. 5I-A-85380 was purchased from Tocris Bioscience, Bristol, UK. Ketamine, xylazine, acepromazine and buprenorphine were obtained from MWI Veterinary Supply, Nampa, ID. Sucrose was obtained from Roche Diagnostics, Indianapolis, IN. HEPES and NaHEPES (products of BDH) and silastic tubing (a product of Dow Chemical) were obtained through VWR International, Denver, CO. Glass filters Type B were products of MicroFiltration Systems, Dublin, CA and glass fiber filters Type A/E were products of Pall Life Sciences, Port Washington, NY. Nylon mesh and 22 gauge stainless steel tubing were obtained from AmazonSupply.com.
2.2. Mice
Animal production methods and experimental procedures using mice were reviewed and approved by the Animal Care and Utilization Committee at the University of Colorado, Boulder.
β4 null mutant mice (Xu et al., 1999) were originally obtained from Richard Paylor, Baylor College of Medicine, Houston, TX. Mice were bred in the Specific Pathogen Free Mouse Colony at the Institute for Behavioral Genetics, University of Colorado, Boulder. Heterozygous mice were mated to yield wild type (+/+), heterozygous (+/−) and homozygous (−/−) mutant mice. Mice were weaned at 25 days of age. Genotypes were determined from tail clippings as described previously (Salminen et al., 2004). Mice were housed in a vivarium maintained at 22°C and allowed free access to food and water. Lights were on from 7 AM to 7 PM.
2.3. Surgery and nicotine treatment
2.3.1. Surgery
Chronic nicotine treatment was performed by intravenous infusion through a cannula inserted in the right jugular vein of each mouse to assure careful control of dosing. A cannula constructed of silastic tubing was inserted in the right jugular vein of each mouse (Barr et al., 1979) and used successfully to treat mice with nicotine (Marks et al., 1983; McCallum et al., 2006). Briefly, mice were anesthetized with a cocktail composed of ketamine (80 mg/kg), xylazine (20 mg/kg) and acepromazine (1 mg/kg). An incision (approximately 1 cm) was made to expose the superficial right jugular vein. The silastic cannula (0.51 mm I.D. 0.94 mm O.D.) was inserted 8 mm into the right jugular vein through a small hole in the vein. The cannula, filled with isotonic saline containing 0.3% citric acid, was anchored to the underlying tissue with surgical thread. The cannula was attached to stainless steel tubing mounted on a nylon disk and from which a section of tubing passed through the back in the midscapular region. The disk with the attached tubing was then anchored to the skin with a wound clip. The mouse was injected with buprenorphine (0.1 mg/kg), placed in a clean cage and warmed until wakening.
2.3.2. Nicotine treatment
Following recovery from surgery each mouse was transferred to an individual infusion chamber (15 cm × 15 cm × 25 cm, length x width x height) and its cannula was attached to medical grade Tygon tubing connected to a 1 ml syringe mounted on an infusion pump (Harvard Apparatus, Holliston, MA). Sterile saline was continuously infused at a rate of 35μL/hr. After two days of saline infusion, nicotine treatment was begun. Mice were divided into five treatment groups that received the following nicotine doses: 0 mg/kg/hr (saline-infused control), 0.5 mg/kg/hr, 1.0 mg/kg/hr, 2.0 mg/kg/hr or 4.0 mg/kg/hr. All doses are free base. Nicotine solutions were prepared from liquid nicotine neutralized with HCl. Following ten days of treatment with the indicated nicotine dose, the cannula of each mouse was disconnected from the Tygon tubing and checked for free fluid flow. The total number of mice of each genotype and the number of mice treated with saline, 0.5 mg/kg/hr, 1.0 mg/kg/hr, 2.0 mg/kg/hr and 4.0 mg/kg/hr nicotine were: β4++: 84 mice total, 16, 15, 16, 19 and 18 in the five treatment groups, respectively; β4+−: 86 mice total, 16, 19, 18, 18 and 15 in the five treatment groups, respectively; and β4−− : 87 mice total, 15, 16, 17, 16 and 23 in the five treatment groups, respectively. Few mice died during the treatments and there was no significant difference in mortality among the mice differing in β4 expression.
2.3.3. Tolerance testing
Behavioral test measures were selected based on previous studies that have demonstrated dose-dependent responses to nicotine that can be altered by altering nAChR expression (Marks et al., 1985; O’Neill et al., 2013; Tritto et al., 2004). Chronic nicotine treatment had been discontinued for at least two hr before testing to allow clearance of nicotine (Petersen et al., 1984). Mice were habituated to the testing room for one hour prior to testing. Following the acclimation period, mice received an intraperitoneal injection of nicotine dissolved in sterile saline (0.01 ml/g). Testing was conducted as described previously (McCallum et al., 2006; Tritto et al., 2004). Mice were placed in a Y-maze apparatus 3 minutes after nicotine injection and both crosses and rears measured for 3 minutes. Immediately following Y-maze assessment, mice were placed in a bright light Med Associates circular open field (58 cm diameter) and locomotor activity was monitored for 5 minutes. Following completion of the open field test, mice were singly housed until temperature was measured 15 minutes post injection. Two hr after the initial test mice were injected with a second dose of nicotine and tested a second time. Preliminary experiments indicated little differences in test results provided the initial test was conducted with a lower nicotine dose. Results from both tests were used in the construction of dose-response curves. The following nicotine doses in mg/kg free base were used: 0, 0.25, 0.5, 0.75, 1.0, 1.5 and 2.0.
2.4. Assessment of nAChR binding sites with [125I]-epibatidine binding
2.4.1. Sample preparation
Two hr following the second tolerance test each mouse was euthanized by cervical dislocation. The brain was removed and placed on an ice cold surface and the following brain regions were dissected: olfactory bulb, cerebral cortex, thalamus, habenula, superior colliculus, inferior colliculus and hindbrain (pons and medulla not including the interpeduncular nucleus). Previous studies indicated that these regions differ in expression of nAChR subtypes. Each brain region was placed in a polypropylene test tube containing hypotonic salt solution (NaCl = 14.4 mM; KCl = 0.15 mM; CaCl2 = 0.2 mM, MgSO4 = 0.1 mM, HEPES = 2.5 mM, pH = 7.5) and homogenized using a Teflon pestle fitted to the test tube. The resulting homogenate was centrifuged at 20,000 × g for 15 min. The supernatant was discarded, the pellet suspended in hypotonic buffer and centrifuged again. This process was repeated twice more. Fresh hypotonic buffer was added to each tube and the samples were stored frozen until assay.
On the assay day, the samples were thawed, the pellets suspended in the overlying buffer and the samples were centrifuged at 20,000 × g for 15 min. The resulting pellet was suspended in water for use in the binding assay.
2.4.2. [125I]-Epibatidine binding
The binding was measured essentially as described previously (Whiteaker et al., 2000a). The binding assay was conducted in 96 well polyethylene microtiter plates in a final volume of 30 μL of isotonic buffer (NaCl = 144 mM, KCl = 1.5 mM, CaCl2 = 2 mM, MgSO4 = 1 mM, HEPES = 25 mM, pH = 7.5) containing 200 pM [125I]-epibatidine. In order to determine the cytisine-resistant sites, some samples included either 50 mM or 150 nM cytisine (Marks et al., 1998). In order to determine 5I-A85380-resistant sites other samples included either 10 nM or 50 nM 5I-A85380 (Whiteaker et al., 2000b). Blanks were established by including 10 μM nicotine in the incubation. Protein was adjusted to assure that less than 10% of the [125I]-epibatidine was bound. Following an incubation of 2 hr at 22°C, samples were filtered at 4°C using an Inotech Cell Harvester on holding two glass fiber filters that had been soaked in isotonic binding buffer containing 0.5% polyethylenimine (top filter MFS type B, bottom filter Pell type A/E). Filters were washed 4 times with ice-cold isotonic binding buffer. Filters were transferred to 96 well sample plates and 150 μL of Optiphase Supermix Scintillation Cocktail was added to each well. Samples were counted at 55% efficiency using a Perkin-Elmer Trilux Wallac 1500 Trilux scintillation counter.
2.4.3. Protein
Protein was measured using the Lowry method (Lowry et al., 1951) using bovine serum albumin as the standard.
2.5. Data analysis
2.5.1. Analysis of tolerance data
Results of the tolerance testing were analyzed several ways. Each test was initially analyzed using a three-way analysis of variance using IBM SPSS version 21 with the responses as the dependent variable and genotype, chronic treatment dose and acute challenge dose as the independent variables. In addition, the dose-response curves for each test for mice of each β4 genotype were fit to the following equations for activity measures:
Where RAD is the response measured after the acute nicotine challenge dose (NICAD), RSAL is the activity following saline administration (the initial response), ED50 is the acute nicotine dose eliciting half-maximal response and NH is the slope of the dose-response curve.
And for body temperature:
Where BTAD is the body temperature after the acute nicotine challenge dose, BTSAL is the body temperature following saline injection and BTFIN is the body temperature following administration of the maximally effective nicotine dose (approximately 31°C).
Initial curve fits for each genotype and each chronic treatment dose were constructed allowing all variables to float. No significant difference in NH was observed among the groups for each test, so subsequent calculations were made with a fixed value for NH: Y-maze crosses, NH = 2.66; Y-maze rears, NH = 2.14; Open-field distance, NH = 2.66; Body temperature, NH = 2.17.
2.5.2. Analysis of [125I]-epibatidine binding data
[125I]-Epibatidine binding to subsets of sites determined by differential inhibition by cytisine or 5I-A85380 was calculated using the following equation:
Where EPIBound is the measured binding of [125I]-epibatidine at each concentration of either cytisine or 5I-A85380 (INH), EPISen and EPIRes are the densities of [125I]-epibatidine binding sites that are more and less sensitive to inhibition with effective inhibition constants of IC50-1 and IC50-2, respectively. Inhibition constants were calculated in preliminary experiments using a wide range of inhibitor concentrations. Under the conditions of the experiments described in the current study IC50-1 and IC50-2 were 3 nM and 300 nM for cytisine and 0.3 nM and 300 nM for 5I-A85380. Two-way ANOVA with IBM SPSS version 21 was subsequently used to analyze the [125I]-epibatidine binding data with genotype and chronic treatment dose as the independent variables.
3. Results
3.1. Tolerance measurements
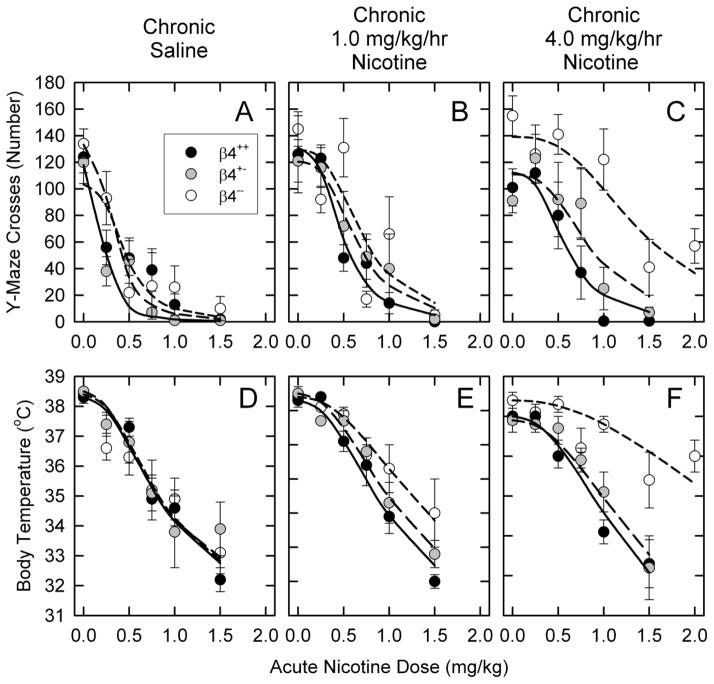
Mice differing in the expression of the β4 nAChR subunit were chronically treated for 10 days with 0, 0.5, 1.0, 2.0 or 4.0 mg/kg/hr nicotine by intravenous infusion. Mice were subsequently tested for tolerance to the acute effects of nicotine to evaluate the effects of nicotine treatment and the influence of differences in expression of the β4 subunit. Appendices A-D (results for Y-maze crosses, Y-maze rears, open field activity and body temperature, respectively) provide dose-response curves for responses of β4++, β4+−, and β4−mice to acute nicotine administration following chronic nicotine treatment following chronic treatment with each nicotine dose. Results in Figure 1 provide an abbreviated illustration of these data.
Figure 1. Dose-response curves for the effects of chronic nicotine treatment of mice differing in β4 genotype for select tests.
Mean ± SEM (N =4-9) for responses of β4++, β4+− and β−− mice treated with saline, 1.0 mg/kg/hr nicotine or 4.0 mg/kg/hr nicotine as indicated and acutely injected with the indicated nicotine dose. The lines are least squares curve fits of the results as described in the Methods.
3.1.1. Effect of β4 genotype on tolerance development following chronic nicotine treatment: Representative tolerance test results
The results presented in Figure 1 for Y-maze crosses and body temperature illustrate the responses of β4++ (wild-type), β4+− (heterozygote) and β4−− (null mutant) mice to acute injections of nicotine following chronic treatment with saline (control), 1.0 or 4.0 mg/kg/hr nicotine.
3.1.1. a. Y-Maze crosses
Acute nicotine injection elicited a dose-dependent decrease in locomotor activity in the Y-maze. The responses of β4++, β4+− and β4−− mice that had been chronically infused with saline (controls) exhibited very similar responses to acute nicotine injection (Figure 1A) as indicated by the results of a two-way ANOVA (main effect of genotype, F2,77 = 2.66, NS; main effect of acute nicotine dose, F5,77 = 22.41, P<0.001) and comparison of the ED50 values for nicotine also indicated that the response to acute nicotine for this test was very similar among the mice differing in β4 genotype (0.25±0.04, 0.40±0.09 and 0.39±0.08 mg/kg for the β4++, β4+− and β4−− mice, respectively).
Some tolerance development was noted following treatment with 1.0mg/kg/hr nicotine (Figure 1B, ED50 values: 0.47±0.07, 0.73±0.14 and 0.80±0.15 mg/kg for the β4++, β4+− and β4−− mice, respectively). As was the case with the saline-treated mice, the overall responses of the mice differing in β4 genotype following treatment with 1.0 mg/kg/hr nicotine were similar (two-way ANOVA, main effect of genotype F2,82 = 0.88).
Chronic treatment with a dose of 4.0 mg/kg/hr nicotine (Figure 1C) elicited greater tolerance that was particularly noteworthy for the β4−− mice (ED50 values: 0.58±0.10, 0.71±0.14 and 1.45±0.23 mg/kg for the β4++, β4+− and β4−− mice, respectively). The differences among the genotypes were also greater than those observed following treatment with the lower dose (two-way ANOVA, main effect of genotype F2,86 = 15.76, P<0.001). β4−− mice developed significantly more tolerance than either β4++ or β4+− mice.
3.1.1.b. Body temperature
Acute nicotine injection elicited a dose-dependent decrease in body temperature. The responses of β4++, β4+− and β4−− mice that had been chronically infused with saline (controls) exhibited virtually identical responses to acute nicotine injection (Figure 1D) as indicated both by the results of a two-way ANOVA (main effect of genotype, F2,76 = 0.28; main effect of acute nicotine dose, F5,76 = 21.94, P<0.001) and by comparison of the ED50 values (0.98±0.08, 1.01±0.12 and 0.96±0.12 mg/kg for the β4++, β4+− and β4−− mice, respectively).
Some tolerance was noted following treatment with 1.0mg/kg/hr nicotine (Figure 1E, ED50 values: 1.17±0.08, 1.38±0.10 and 1.96±0.24 mg/kg for the β4++, β4+− and β4−− mice, respectively). The extent of tolerance differed among the mice (two-way ANOVA, main effect of genotype F2,81 = 4.24, P = 0.018).
Chronic treatment with a dose of 4.0 mg/kg/hr nicotine (Figure 1F) elicited greater tolerance than that elicited by treatment with 1.0 mg/kg/hr nicotine. This difference was particularly noteworthy for the β4−− mice (ED50 values: 1.34±0.08, 1.64±0.15 and 4.12±0.57 mg/kg for the β4++, β4+− and β4−− mice, respectively). The differences among the genotypes was also greater than that observed following treatment with the 1.0 mg/kg/hr dose of nicotine (two-way ANOVA, main effect of genotype F2,86 = 15.41, P<0.001; and genotype by acute nicotine dose interaction F10,86 = 2.23, P=0.023). As was the case for Y-maze crosses, β4−− mice developed significantly more tolerance to the hypothermic effects of acute nicotine than did either β4++ or β4+− mice.
3.1.2. Effect of β4 genotype on tolerance development following chronic nicotine treatment: Four tests
In order to provide a more complete picture of the effects of deletion of the β4 subunit on tolerance development after chronic nicotine treatment, data for all four tests (shown in Appendices AD) were analyzed and the results of the three-way ANOVAs (independent variables were genotype, chronic treatment dose and acute challenge dose) are compiled in Appendix E. Significant main effects for response to acute nicotine administration were obtained for all four tests. In addition, significant main effects of chronic treatment dose indicated that tolerance had developed for each test. Furthermore, a significant main effect of β4 genotype was also obtained. While none of the three-way interactions were statistically significant, a variable pattern of significant two-way interactions was noted. For the measurement of body temperature, all three two-way interactions were statistically significant. In contrast, no two-way interactions achieved significance for open field activity.
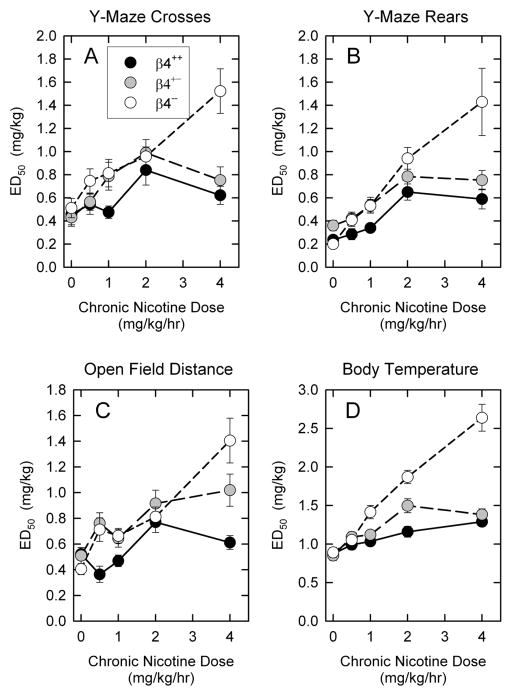
The results presented in Figure 2 summarize the effects of chronic nicotine treatment on ED50 values for each of the four tests and three genotypes. The overall pattern of the effects of chronic nicotine treatment is very similar for all four tests. The increases in ED50 values with increasing chronic nicotine dose provide further demonstration of tolerance development. However, significantly more tolerance development was observed for the β4−− mice, particularly following treatment with the highest nicotine dose (4 mg/kg/hr). The response of β4++ and β4+− mice to chronic nicotine treatment was quite similar, although the β4+− mice tended to develop somewhat more tolerance than did the β4++ mice.
Figure 2. ED50 values following chronic nicotine treatment for mice differing in β4 genotype.
Mean ± SEM of ED50 values calculated by non-linear least squares curve fits for dose-response curves from each of the four tests for β4++, β4+− and β4−− that had been chronically treated with the indicated doses of nicotine.
3.2. Nicotinic binding sites
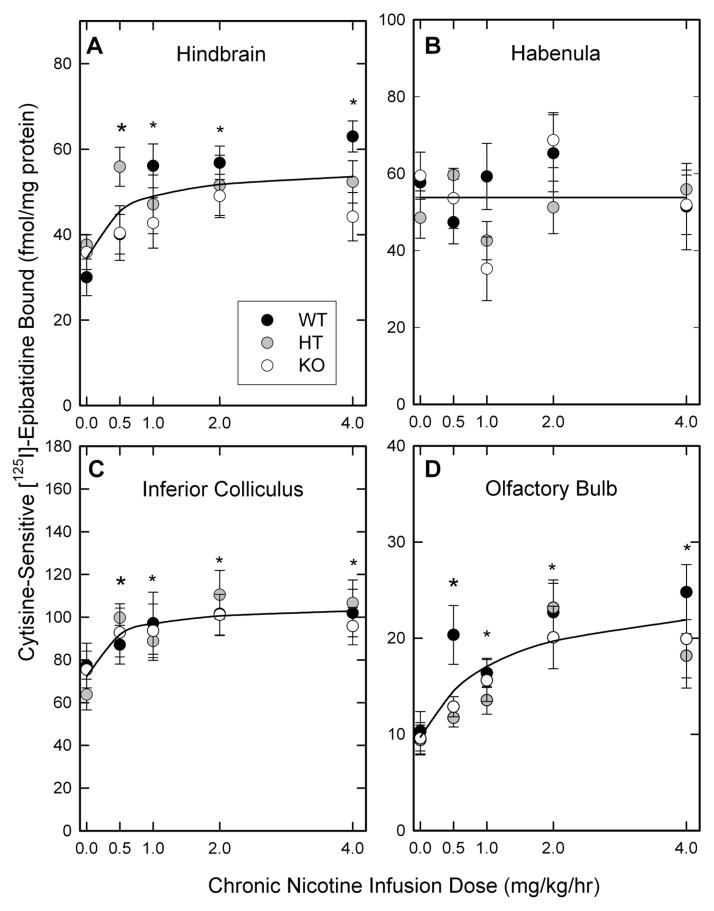
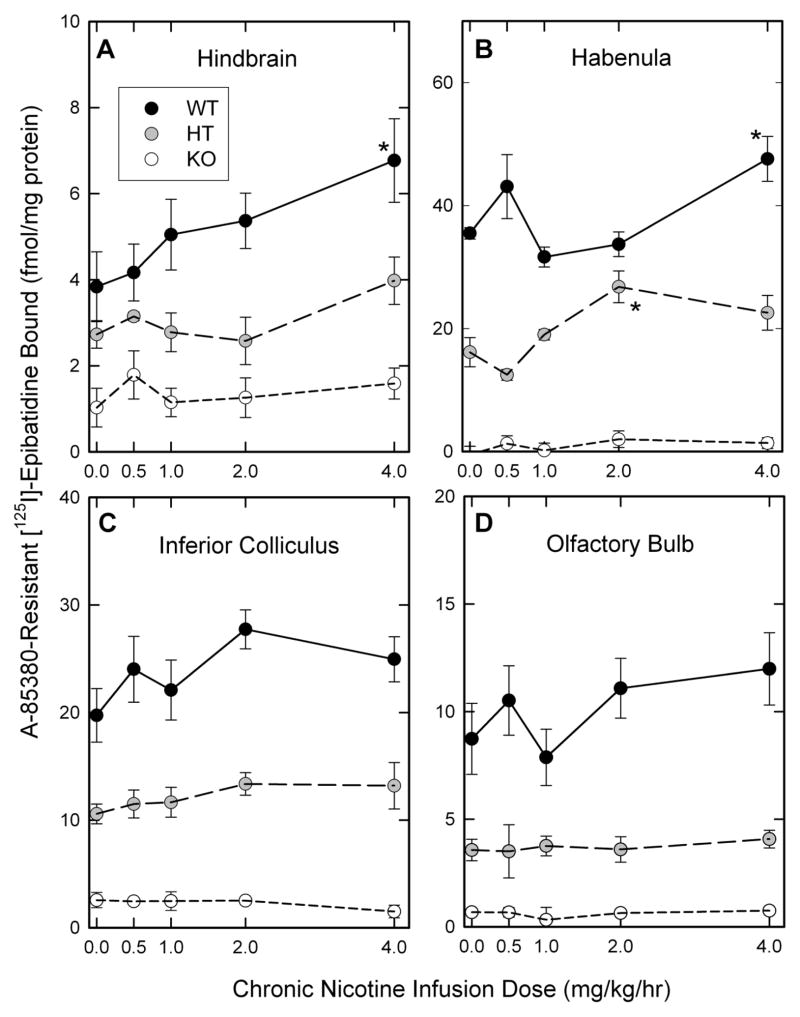
In order to examine the effects of chronic nicotine treatment on nAChR expression, the binding of [125I]-epibatidine was measured. [125I]-Epibatidine binds with relatively equal affinity to heteromeric nAChR subtypes in mouse brain (Marks et al., 1998; Whiteaker et al., 2000b). However, nAChR subtypes display different affinities for ligands other than epibatidine: Cytisine displays higher affinity for α4β2*-nAChR (Baddick and Marks, 2011; Marks et al., 1998) and A85380 (or 5I-A85380) displays higher affinity for β2*-nAChR than these ligands do for other subtypes (Mukhin et al., 2000; Whiteaker et al., 2000b). Consequently, assay conditions were selected to measure several [125I]-epibatidine subtypes using differential inhibition by cytisine or A85380. Levels of nAChR binding sites in seven brain regions of mice differing in β4 subunit expression as well as the results of the two-way ANOVAs are presented in Appendix F. Graphical presentation of results for cytisine-sensitive [125I]-epibatidine binding and 5I-A85380-resistant [125I]-epibatidine are shown in Figures 3 and 4 for visual presentation of the results.
Figure 3. Effects of chronic nicotine treatment on expression of cytisine-sensitive [125I]-epibatidine binding sites.
The expression of cytisine-sensitive[125I]-epibatidine binding sites in membranes prepared from four brain regions of β4++, β4+− and β4−− mice treated with the indicated chronic doses of nicotine was calculated as described in the methods. Points represent the mean ± SEM of 5-11 independent samples. The lines in each panel are nonlinear least squares curve fits of the data. Cytisine-sensitive[125I]-epibatidine binding in each brain region except habenula was significantly higher than that of saline treated mice after nicotine treatment as indicated by the asterisks (*).
Figure 4. Effects of chronic nicotine treatment on expression of 5I-A-85380 resistant [125I]-epibatidine binding sites.
The expression of 5I-A-85380-resistant [125I]-epibatidine binding sites in membranes prepared from four brain regions of β4++, β4+− and β4−− mice treated with the indicated chronic doses of nicotine as described in the methods. Points represent the mean ± SEM of 5-11 independent samples. Lines in all panels merely connect the data points. Values significantly different from saline-treated mice of the same genotype are indicated by asterisks (*).
3.2.1. Cytisine-sensitive [125I]-epibatidine binding (primarily α4β2*-nAChR sites)
The effects of chronic nicotine treatment on the levels of cytisine-sensitive [125I]-epibatidine binding are shown in Figure 3. Chronic nicotine treatment elicited significant, saturable, dose-dependent increases in cytisine-sensitive [125I]-epibatidine binding sites (primarily α4β2*-nAChR sites) in hindbrain (Figure 3A, F4,174 = 5.14, P<0.001), inferior colliculus (Figure 3C, F4, 104 = 4.51, P<0.01) and olfactory bulb (Figure 3D, F4,148 = 11.46, P<0.001). The pattern noted for the response to chronic nicotine treatment of each β4 genotype was very similar (there were no significant genotype by nicotine treatment interactions). However, modest overall differences among the genotypes were noted for hindbrain (F2,174 =3.33, P=0.033) and olfactory bulb (F2,148 =3.23, P=0.042). In contrast to the increased binding noted for hindbrain, inferior colliculus and olfactory bulb, cytisine-sensitive [125I]-epibatidine binding in habenula was unaffected by chronic nicotine treatment (Figure 3B, F4,64 = 1.76, P>0.05).
Cytisine-sensitive [125I]-epibatidine binding was also measured in cortex, thalamus and superior colliculus and values for these regions are included in Appendix F. Significant nicotine dose-dependent increases in these sites were for each region. However, the average maximal increases measure in thalamus (29%) and superior colliculus (27%) were relatively modest compared to the increase in cortex (47%).
3.2.2. Cytisine-resistant [125I]-epibatidine binding (mostly non α4β2*-nAChR sites)
The effects of chronic nicotine treatment on cytisine-resistant [125I]-epibatidine binding sites are summarized in Appendix F. Effects of β4 gene deletion and chronic nicotine treatment provide general information about the response of this diverse set of nAChR binding sites to chronic nicotine treatment.
Deletion of the β4 subunit did not significantly affect cytisine-resistant [125I]-epibatidine binding in cortex, thalamus or superior colliculus. While these sites are relatively minor components of total [125I]-epibatidine binding in cortex and thalamus, they do comprise a significant fraction of binding in superior colliculus. No statistically significant effects of chronic nicotine treatment were observed in these three regions as well.
Deletion of the β4 subunit significantly reduced cytisine-resistant [125I]-epibatidine binding in habenula (F2,64 = 184.71, P <0.001), hindbrain (F2,174 = 30.23, P <0.001), inferior colliculus (F2,104 = 77.30, P < 0.001) and olfactory bulb (F2,148 = 188.39, P < 0.001) demonstrating that β4*-nAChR comprise a significant fraction of these sites in these four brain regions. Furthermore, chronic nicotine treatment elicited significant changes in habenula (F4,64 = 2.91, P < 0.05), hindbrain (F4,174 = 4.51, P <0.01) and olfactory bulb (F4,148 = 4.03, P <0.01). The significant genotype by chronic treatment dose interactions observed in habenula (F8,64 = 2.23 P < 0.05) and olfactory bulb (F8,148, P < 0.05) suggests that response to chronic nicotine treatment varied among the mice differing in expression of the β4 subunit. Consequently, subsets of cytisine-resistant [125I]-epibatidine binding were measured as described below.
3.2.3. 5I-A85380-resistant [125I]-epibatidine binding (primarily β4*-nAChR sites)
The effects of chronic nicotine treatment on the levels of 5I-A85380-resistant [125I]-epibatidine binding are shown in Figure 4. In contrast to the modest effects on cytisine-sensitive [125I]-epibatidine binding, deletion of the β4 nAChR subunit significantly reduced 5I-A-85380-resistant [125I]-epibatidine binding in each of the brain regions shown in Figure 4 (main effects of genotype: 4A, hindbrain, F2,174 = 49.32, P<0.001; 4B, habenula, F2,64 = 308.59, P<0.001; 4C, inferior colliculus, F2,104 = 212.49, P<0.001; 4D, olfactory bulb, F2, 148 = 122.71, P<0.001). 5I-A85380-resistant [125I]-epibatidine binding was reduced by more than 90% in habenula, inferior colliculus and olfactory bulb and by more than 70% in hindbrain following deletion of the β4 nAChR subunit. Chronic nicotine treatment had less effect on 5I-A85380-resistant [125I]-epibatidine binding sites (β4*-nAChR sites) than on the α4β2-nAChR sites. However, significant effects of nicotine treatment were observed for the β4*-nAChR sites in hindbrain (Figure 4A, F4, 174 = 2.98, P <0.05) and habenula (Figure 4B, F4, 64 = 4.60, P < 0.01). The increases were most evident for the β4++ mice following treatment with 4.0 mg/kg/hr nicotine. Deletion of the β4subunit eliminated these nicotine-induced increases.
5I-A85380-resistant [125I]-epibatidine binding was also measured in three other regions (Appendix F). However, these sites are sparsely expressed in these regions as indicated by the percentage of total epibatidine binding sites: cortex (2.5%), superior colliculus (3.3%) and thalamus (2.0%).
3.2.4. Cytisine-resistant, 5I-A85380-sensitive [125I]-epibatidine binding sites (mostly non-α4β2-nAChR, β2*-nAChR sites)
The effects of chronic nicotine treatment on cytisine-resistant, A-85380-sensitive [125I]-epibatidine binding sites are also compiled in Appendix F. These binding sites comprise a relatively large fraction of total [125I]-epibatidine binding sites in superior colliculus, where neither β4 gene deletion nor chronic nicotine treatment elicited significant changes in binding site density. However, in olfactory bulb where these sites also comprise a significant fraction of total [125I]-epibatidine binding sites deletion of the β4subunit significantly reduced binding. The diversity of responses for these sites that represent a mixed population of nAChR subtypes is further indicated in hindbrain where deletion of β4 reduced the density of these sites and also eliminated the increased binding elicited by nicotine treatment in the wild-type mice. This subset of sites represents less than 5% of total epibatidine binding in the other five brain regions that were assayed.
4. Discussion
Cytisine-sensitive [125I]-epibatidine binding sites (primarily α4β2-nAChR sites) are relatively unaffected by deletion of the β4 nAChR subunit. In addition, deletion of β4 has virtually no effect on the nicotine-induced changes in cytisine-sensitive [125I]-epibatidine binding sites in most brain regions. However, somewhat less up-regulation of these sites was observed in the hindbrain and olfactory bulb of β4−− mice than in β4++ mice suggesting that a subset of these binding sites in hindbrain may express a complex nAChR containing both β2 and β4 subunits. Since mRNA encoding both of these subunits is expressed in these brain regions, assembly of complex receptor subtypes is possible. Selective immunoprecipitation studies in other brain areas have identified nAChR subtypes that co-express β2 and β4 subunits (Gotti et al., 2007; Mao et al., 2006; Mineur et al., 2009; Turner and Kellar, 2005).
Heteromeric A85380-resistant [125I]-epibatidine binding sites have been considered to be primarily β4*-nAChR since A85380 is highly selective for β2*-nAChR (Mukhin et al., 2000). The expression of this subset of binding sites is relatively high in olfactory bulb, habenula and inferior colliculus. These sites are reduced by at least 90% following deletion of the β4 subunit. Most of the β4*-nAChR binding sites are likely α3β4 *-nAChR, but although the presence of receptors assembled from more than two types of subunits (including α4, α5, β2 and β3) has been demonstrated in several neuronal preparations (Grady et al., 2009; Mao et al., 2006; Turner and Kellar, 2005). While deletion of the β4 subunit significantly reduced the levels of 5I-A-85380 resistant [125I]-epibatidine binding sites in hindbrain, superior colliculus and thalamus, substantial residual binding remained in each of these regions suggesting that a subset of these binding sites do not fit the normal classification as strictly β4*-nAChR sites. Chronic nicotine treatment elicited significant increases in 5I-A85380-resistant [125I]-epibatidine binding sites only in habenula and hindbrain. These increases were most apparent in the samples from mice treated with the 4 mg/kg/hr nicotine dose. Treatment with this dose of nicotine produces plasma concentrations over 1 μM (Marks et al., 2004), suggesting that some β4*-nAChR binding sites respond to exposure to high nicotine concentrations. The fact that no up-regulation was observed following deletion of the β4 subunit indicates brain region selective β4*-nAChR up-regulation occurred. This selective increase may reflect the region specific expression of β4*-nAChR with complex pentameric composition. Only a few studies have examined the effect of chronic nicotine treatment in vivo on the expression of [125I]-epibatidine binding to putative α3β4-nAChR sites and these studies indicated that the α3β4*-nAChR are resistant to nicotine-induced upregulation (Marks et al., 2004; McCallum et al., 2006; Nguyen et al., 2003). However, upregulation of α3β4*-nAChR has been observed in cultured cells after exposure to relatively high concentrations of nicotine (Peng et al., 1997). nAChR in habenula have been identified as being important regulators of nicotine self-administration and withdrawal (Fowler and Kenny, 2012; McCallum et al., 2012; Salas et al., 2009), so alteration of their expression by chronic nicotine treatment, if confirmed, could have important implications for the regulation of responsiveness to the drug as suggested previously (Olale et al., 1997). Several compounds that appear to act relatively selectively on α3β4*-nAChR are being actively investigated as useful pharmacological agents (Glick et al., 2000; Glick et al., 2011; Toll et al., 2012).
The [125I]-epibatidine binding sites that are resistant to inhibition by cytisine, but sensitive to inhibition by 5I-A85380 comprise a diverse set of receptors that may include nAChR assembled with α2, α3 and/or α6 subunits the expression of which varies among brain regions (Baddick and Marks, 2011). The fact that these sites are sensitive to inhibition by 5I-A85380 implies the presence of the β2 subunit, as well (Mukhin et al., 2000). Significant expression of these sites was found in inferior colliculus, olfactory bulb and, particularly, superior colliculus. However, response following deletion of the β4 subunit indicates that the receptor composition in these regions differs. Deletion of the β4 subunit had little effect on these sites in either superior or inferior colliculus, but reduced them in olfactory bulb, indicating the presence of a relatively complex set of receptors in this region. Such complexity could be expected owing to the diversity of nAChR subunit mRNA expression in olfactory bulb a brain region that includes relatively high levels of mRNA encoding α2, α3, β2 and β4 nAChR subunits (Dineley-Miller and Patrick, 1992; Wada et al., 1989) and the expression of α2*-nAChR in olfactory bulb has been demonstrated (Whiteaker et al., 2009). Characterization of nAChR expression in superior colliculus has revealed the existence of significant receptor populations that include α3 and α6 subunits (McClure-Begley et al., 2014). Chronic nicotine treatment had no significant effect on the expression of this subset of receptors in any brain region, except hindbrain, where a dose-dependent up-regulation was observed for wild-type mice that was lost in both the β4+− and β4−− mice. The observation that chronic nicotine treatment elicits up-regulation of all three major subsets of high affinity [125I]-epibatidine binding sites in hindbrain suggests that the receptors in this brain region may be uniquely responsive to chronic nicotine treatment.
Deletion of the β4 nAChR subunit has relatively little effect on the nicotine-elicited responses of saline-infused (control) mice for any of the four tests used in the current study. This observation is consistent with previous reports that β2*-nAChR appear to modulate responses to relatively low doses of nicotine for these tests (Tritto et al., 2004). However, deletion of the β4 subunit significantly affects the extent of tolerance development following chronic nicotine treatment that was particularly evident in mice treated with 4 mg/kg/hr nicotine, the highest dose used in the current study. This substantial tolerance contrasts with the relatively feeble tolerance observed for β2−− mice treated with nicotine using the same paradigm (McCallum et al., 2006). These results suggest that functional changes mediated by β2*-nAChR responses observed following chronic nicotine treatment are primarily responsible for the observed tolerance, and that these effects are more apparent in the absence of expression of β4*-nAChR. In contrast, functional changes mediated by β4*-nAChR do not seem to be significant contributors to tolerance development, at least for the four tests used in the current study. These changes may actually render mice slightly more sensitive to nicotine following chronic treatment with this drug. This observation is consistent with the demonstration that β4*-nAChR are important in mediating somatic signs of nicotine withdrawal (Salas et al., 2004b).
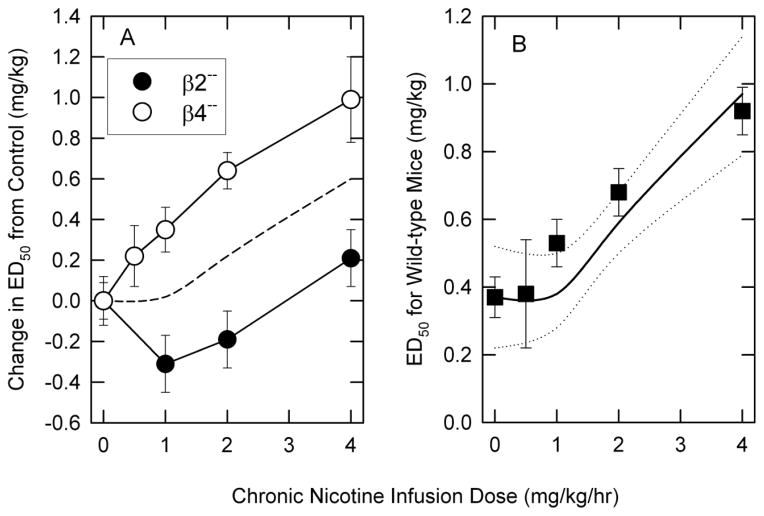
The relative contribution of responses mediated by β4- and β2-nAChR was evaluated by comparing the effects of deletion of either of these genes on tolerance development. The changes in ED50 values for the combined responses following chronic nicotine treatment of either β2− (McCallum et al., 2006) or β4− (current study) are illustrated in Figure 5A. As noted previously for the individual tests, significant, dose-dependent tolerance (likely mediated by β2*-nAChR elicited responses) develops in β4−− mice, while relatively little tolerance and perhaps even some super-sensitivity (likely mediated by β4*-nAChR elicited responses) develops in β2−− mice. In order to test whether the alteration of the acute responses to nicotine by chronic nicotine treatment of wild-type mice (tolerance to the effects of nicotine) can be approximated by averaging the responses of the two knockout mouse lines, changes in ED50 values of wild-type mice were compared to the average of extent of tolerance development mediated by β2*- and β4*-nAChR and are illustrated in Figure 5B. In general, this analysis is consistent with the hypothesis that development of tolerance to chronic nicotine treatment in wild-type mice is relatively well approximated by the contributions of β2*-nAChR (dose-dependent tolerance observed in β4−− mice) and β4*-nAChR (modest super-sensitivity at lower nicotine doses observed in β2−− mice). Given that many of the effects of these nAChR subtypes are mediated by their effects on neurotransmitter and/or hormone release, the exact mechanisms for the interactions between these two populations of nAChRs remains to be determined.
Figure 5. Comparison of the effects of deletion of the β2 or β4 nAChR on the development of tolerance to nicotine to that of wild-type mice.
The results in Panel A compare the change in ED50 values (mean ± SEM) for nicotine of β2−− and β4−− mice following chronic treatment with the indicated doses of nicotine. The dotted line is the average of the ED50 values for the two genotypes at each dose. The results in panel B compare ED50 values for wild-type C57BL/6 mice to the average change in ED50 values for β2−− and β4−mice added to the ED50 for saline-infused mice (solid line). The dotted lines are one SEM above and below the average line.
Conclusions
Chronic nicotine treatment elicits more tolerance in β4−− mice than that in either β4++ or β4+− mice suggesting that responses mediated by nAChR remaining in the β4−− mice, probably β2*-nAChR, contribute significantly to tolerance development following chronic nicotine treatment. In contrast, responses mediated by β4*-nAChR don’t substantially contribute to the tolerance of the behaviors measured here. Deletion of the β4 subunit has relatively little effect on the subset of [125I]-epibatidine binding sites (cytisine-sensitive sites) thought to be primarily α4β2-nAChR sites. However, the subset of [125I]-epibatidine binding sites thought to have a significant contribution of β4*-nAChR (A-85380-resistant sites) were indeed reduced in the brains of β4−mice. The significant and brain-region selective increase in 5I-A85380 resistant [125I]-epibatidine binding sites in several brain regions of wild-type mice indicates that some β4*-nAChR, perhaps those also containing the β2 subunit, indicates that the β4*-nAChR are not universally resistant to increases elicited by chronic nicotine treatment.
Supplementary Material
Highlights.
Mice differing in beta4 nicotinic subunit expression received nicotine chronically
Beta4 null mutants develop more nicotine tolerance than wild-types or heterozygotes
Deletion of beta4 has selective effects on subsets of epibatidine binding sites
Responses to chronic nicotine of receptors with beta4 differ among brain regions
Both beta2 and beta4 receptors appear to influence nicotine tolerance development
Acknowledgments
This work was supported by the following grants to Michael J. Marks from the National Institutes of Health, National Institute on Drug Abuse: R01 DA003194 and P30 DA015663. Jill Miyamoto-Ditman provided some technical assistance. We also wish to thank Sharon R. Grady and Allan C. Collins for critical reading of the manuscript.
Abbreviations
- nAChR
nicotinic acetylcholine receptor
- ED50
effective dose giving 50% maximal response
- 5I-A85380
3-[(2S)-2-azetidylmethoxy]-5-iodopyridine
- HEPES
4-(2-hydroxyethyl)piperazine-1-ethanesulonic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Erin E. Meyers, Email: erin.meyers@gmail.com.
Esteban C. Loetz, Email: esteban.loetz@colorado.edu.
Michael J. Marks, Email: marksm@colorado.edu.
References
- Baddick CG, Marks MJ. An autoradiographic survey of mouse brain nicotinic acetylcholine receptors defined by null mutants. Biochem Pharmacol. 2011;82:828–841. doi: 10.1016/j.bcp.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr JE, Holmes DB, Ryan LJ, Sharpless SK. Techniques for the chronic cannulation of the jugular vein in mice. Pharmacol Biochem Behav. 1979;11:115–118. doi: 10.1016/0091-3057(79)90307-1. [DOI] [PubMed] [Google Scholar]
- Boulter J, O’Shea-Greenfield A, Duvoisin RM, Connolly JG, Wada E, Jensen A, Gardner PD, Ballivet M, Deneris ES, McKinnon D, et al. Alpha 3, alpha 5, and beta 4: three members of the rat neuronal nicotinic acetylcholine receptor-related gene family form a gene cluster. J Biol Chem. 1990;265:4472–4482. [PubMed] [Google Scholar]
- Dineley-Miller K, Patrick J. Gene transcripts for the nicotinic acetylcholine receptor subunit, beta4, are distributed in multiple areas of the rat central nervous system. Brain Res Mol Brain Res. 1992;16:339–344. doi: 10.1016/0169-328x(92)90244-6. [DOI] [PubMed] [Google Scholar]
- Duvoisin RM, Deneris ES, Patrick J, Heinemann S. The functional diversity of the neuronal nicotinic acetylcholine receptors is increased by a novel subunit: beta 4. Neuron. 1989;3:487–496. doi: 10.1016/0896-6273(89)90207-9. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Kenny PJ. Habenular signaling in nicotine reinforcement. Neuropsychopharmacology. 2012;37:306–307. doi: 10.1038/npp.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471:597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Dickinson HA. 18-MC reduces methamphetamine and nicotine self-administration in rats. Neuroreport. 2000;11:2013–2015. doi: 10.1097/00001756-200006260-00041. [DOI] [PubMed] [Google Scholar]
- Glick SD, Sell EM, McCallum SE, Maisonneuve IM. Brain regions mediating alpha3beta4 nicotinic antagonist effects of 18-MC on nicotine self-administration. Eur J Pharmacol. 2011;669:71–75. doi: 10.1016/j.ejphar.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Gaimarri A, Zanardi A, Clementi F, Zoli M. Heterogeneity and complexity of native brain nicotinic receptors. Biochem Pharmacol. 2007;74:1102–1111. doi: 10.1016/j.bcp.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Grady SR, Moretti M, Zoli M, Marks MJ, Zanardi A, Pucci L, Clementi F, Gotti C. Rodent habenulo-interpeduncular pathway expresses a large variety of uncommon nAChR subtypes, but only the alpha3beta4* and alpha3beta3beta4* subtypes mediate acetylcholine release. J Neurosci. 2009;29:2272–2282. doi: 10.1523/JNEUROSCI.5121-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor M, Wang JC, Bertelsen S, Bucholz K, Budde JP, Hinrichs A, Agrawal A, Brooks A, Chorlian D, Dick D, Hesselbrock V, Foroud T, Kramer J, Kuperman S, Manz N, Nurnberger J, Jr, Porjesz B, Rice J, Tischfield J, Xuei X, Schuckit M, Edenberg HJ, Bierut LJ, Goate AM. Variants located upstream of CHRNB4 on chromosome 15q25.1 are associated with age at onset of daily smoking and habitual smoking. PLoS One. 2012;7:e33513. doi: 10.1371/journal.pone.0033513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Lukas RJ, Changeux JP, Le Novere N, Albuquerque EX, Balfour DJ, Berg DK, Bertrand D, Chiappinelli VA, Clarke PB, Collins AC, Dani JA, Grady SR, Kellar KJ, Lindstrom JM, Marks MJ, Quik M, Taylor PW, Wonnacott S. International Union of Pharmacology. XX. Current status of the nomenclature for nicotinic acetylcholine receptors and their subunits. Pharmacol Rev. 1999;51:397–401. [PubMed] [Google Scholar]
- Mao D, Yasuda RP, Fan H, Wolfe BB, Kellar KJ. Heterogeneity of nicotinic cholinergic receptors in rat superior cervical and nodose Ganglia. Mol Pharmacol. 2006;70:1693–1699. doi: 10.1124/mol.106.027458. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Burch JB, Collins AC. Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. J Pharmacol Exp Ther. 1983;226:817–825. [PubMed] [Google Scholar]
- Marks MJ, Romm E, Bealer SM, Collins AC. A test battery for measuring nicotine effects in mice. Pharmacol Biochem Behav. 1985;23:325–330. doi: 10.1016/0091-3057(85)90577-5. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Rowell PP, Cao JZ, Grady SR, McCallum SE, Collins AC. Subsets of acetylcholine-stimulated 86Rb+ efflux and [125I]-epibatidine binding sites in C57BL/6 mouse brain are differentially affected by chronic nicotine treatment. Neuropharmacology. 2004;46:1141–1157. doi: 10.1016/j.neuropharm.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Smith KW, Collins AC. Differential agonist inhibition identifies multiple epibatidine binding sites in mouse brain. J Pharmacol Exp Ther. 1998;285:377–386. [PubMed] [Google Scholar]
- McCallum SE, Collins AC, Paylor R, Marks MJ. Deletion of the beta 2 nicotinic acetylcholine receptor subunit alters development of tolerance to nicotine and eliminates receptor upregulation. Psychopharmacology (Berl) 2006;184:314–327. doi: 10.1007/s00213-005-0076-6. [DOI] [PubMed] [Google Scholar]
- McCallum SE, Cowe MA, Lewis SW, Glick SD. alpha3beta4 nicotinic acetylcholine receptors in the medial habenula modulate the mesolimbic dopaminergic response to acute nicotine in vivo. Neuropharmacology. 2012;63:434–440. doi: 10.1016/j.neuropharm.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure-Begley TD, Grady SR, Marks MJ, Collins AC, Stitzel JA. Presynaptic GABA autoreceptor regulation of nicotinic acetylcholine receptor mediated [H]-GABA release from mouse synaptosomes. Biochem Pharmacol. 2014 doi: 10.1016/j.bcp.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Brunzell DH, Grady SR, Lindstrom JM, McIntosh JM, Marks MJ, King SL, Picciotto MR. Localized low-level re-expression of high-affinity mesolimbic nicotinic acetylcholine receptors restores nicotine-induced locomotion but not place conditioning. Genes Brain Behav. 2009;8:257–266. doi: 10.1111/j.1601-183X.2008.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhin AG, Gundisch D, Horti AG, Koren AO, Tamagnan G, Kimes AS, Chambers J, Vaupel DB, King SL, Picciotto MR, Innis RB, London ED. 5-Iodo-A-85380, an alpha4beta2 subtype-selective ligand for nicotinic acetylcholine receptors. Mol Pharmacol. 2000;57:642–649. doi: 10.1124/mol.57.3.642. [DOI] [PubMed] [Google Scholar]
- Nguyen HN, Rasmussen BA, Perry DC. Subtype-selective up-regulation by chronic nicotine of high-affinity nicotinic receptors in rat brain demonstrated by receptor autoradiography. J Pharmacol Exp Ther. 2003;307:1090–1097. doi: 10.1124/jpet.103.056408. [DOI] [PubMed] [Google Scholar]
- O’Neill HC, Laverty DC, Patzlaff NE, Cohen BN, Fonck C, McKinney S, McIntosh JM, Lindstrom JM, Lester HA, Grady SR, Marks MJ. Mice expressing the ADNFLE valine 287 leucine mutation of the Beta2 nicotinic acetylcholine receptor subunit display increased sensitivity to acute nicotine administration and altered presynaptic nicotinic receptor function. Pharmacol Biochem Behav. 2013;103:603–621. doi: 10.1016/j.pbb.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olale F, Gerzanich V, Kuryatov A, Wang F, Lindstrom J. Chronic nicotine exposure differentially affects the function of human alpha3, alpha4, and alpha7 neuronal nicotinic receptor subtypes. J Pharmacol Exp Ther. 1997;283:675–683. [PubMed] [Google Scholar]
- Peng X, Gerzanich V, Anand R, Wang F, Lindstrom J. Chronic nicotine treatment up-regulates alpha3 and alpha7 acetylcholine receptor subtypes expressed by the human neuroblastoma cell line SH-SY5Y. Mol Pharmacol. 1997;51:776–784. doi: 10.1124/mol.51.5.776. [DOI] [PubMed] [Google Scholar]
- Petersen DR, Norris KJ, Thompson JA. A comparative study of the disposition of nicotine and its metabolites in three inbred strains of mice. Drug Metab Dispos. 1984;12:725–731. [PubMed] [Google Scholar]
- Salas R, Cook KD, Bassetto L, De Biasi M. The alpha3 and beta4 nicotinic acetylcholine receptor subunits are necessary for nicotine-induced seizures and hypolocomotion in mice. Neuropharmacology. 2004a;47:401–407. doi: 10.1016/j.neuropharm.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Salas R, Orr-Urtreger A, Broide RS, Beaudet A, Paylor R, De Biasi M. The nicotinic acetylcholine receptor subunit alpha 5 mediates short-term effects of nicotine in vivo. Mol Pharmacol. 2003;63:1059–1066. doi: 10.1124/mol.63.5.1059. [DOI] [PubMed] [Google Scholar]
- Salas R, Pieri F, De Biasi M. Decreased signs of nicotine withdrawal in mice null for the beta4 nicotinic acetylcholine receptor subunit. J Neurosci. 2004b;24:10035–10039. doi: 10.1523/JNEUROSCI.1939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Sturm R, Boulter J, De Biasi M. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J Neurosci. 2009;29:3014–3018. doi: 10.1523/JNEUROSCI.4934-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, Grady SR. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol. 2004;65:1526–1535. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- Stevens VL, Bierut LJ, Talbot JT, Wang JC, Sun J, Hinrichs AL, Thun MJ, Goate A, Calle EE. Nicotinic receptor gene variants influence susceptibility to heavy smoking. Cancer Epidemiol Biomarkers Prev. 2008;17:3517–3525. doi: 10.1158/1055-9965.EPI-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll L, Zaveri NT, Polgar WE, Jiang F, Khroyan TV, Zhou W, Xie XS, Stauber GB, Costello MR, Leslie FM. AT-1001: a high affinity and selective alpha3beta4 nicotinic acetylcholine receptor antagonist blocks nicotine self-administration in rats. Neuropsychopharmacology. 2012;37:1367–1376. doi: 10.1038/npp.2011.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritto T, McCallum SE, Waddle SA, Hutton SR, Paylor R, Collins AC, Marks MJ. Null mutant analysis of responses to nicotine: deletion of beta2 nicotinic acetylcholine receptor subunit but not alpha7 subunit reduces sensitivity to nicotine-induced locomotor depression and hypothermia. Nicotine Tob Res. 2004;6:145–158. doi: 10.1080/14622200310001656966. [DOI] [PubMed] [Google Scholar]
- Turner JR, Kellar KJ. Nicotinic cholinergic receptors in the rat cerebellum: multiple heteromeric subtypes. J Neurosci. 2005;25:9258–9265. doi: 10.1523/JNEUROSCI.2112-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Whiteaker P, Jimenez M, McIntosh JM, Collins AC, Marks MJ. Identification of a novel nicotinic binding site in mouse brain using [(125)I]-epibatidine. Br J Pharmacol. 2000a;131:729–739. doi: 10.1038/sj.bjp.0703616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteaker P, McIntosh JM, Luo S, Collins AC, Marks MJ. 125I-alpha-conotoxin MII identifies a novel nicotinic acetylcholine receptor population in mouse brain. Mol Pharmacol. 2000b;57:913–925. [PubMed] [Google Scholar]
- Whiteaker P, Wilking JA, Brown RW, Brennan RJ, Collins AC, Lindstrom JM, Boulter J. Pharmacological and immunochemical characterization of alpha2* nicotinic acetylcholine receptors (nAChRs) in mouse brain. Acta Pharmacol Sin. 2009;30:795–804. doi: 10.1038/aps.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Orr-Urtreger A, Nigro F, Gelber S, Sutcliffe CB, Armstrong D, Patrick JW, Role LW, Beaudet AL, De Biasi M. Multiorgan autonomic dysfunction in mice lacking the beta2 and the beta4 subunits of neuronal nicotinic acetylcholine receptors. J Neurosci. 1999;19:9298–9305. doi: 10.1523/JNEUROSCI.19-21-09298.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.