Abstract
Background
Step asymmetries during gait in persons after stroke can occur in temporal or spatial domains. Prior studies have shown that split-belt locomotor adaptation can temporarily mitigate these asymmetries.
Objective
We investigated whether baseline gait asymmetries affected how patients adapt and store new walking patterns.
Methods
Subjects with stroke and age-matched controls were studied walking at a 2:1 speed ratio on the split-belt during adaptation and assessed for retention of the learned pattern (the after-effect) with both belts at the same speed.
Results
Those with stroke adapted more slowly (P < .0001), though just as much as healthy older adults. During split-belt walking, the participants with stroke adapted toward their baseline asymmetry (eg, F = 14.02, P < .01 for step symmetry), regardless of whether the subsequent after-effects improved or worsened their baseline step asymmetries. No correlation was found between baseline spatial and temporal measures of asymmetry (P = .38). Last, the initial spatial and temporal asymmetries predicted after-effects independently of one another. The after-effects in the spatial domain (ie, center of oscillation difference) are only predicted by center of oscillation difference baseline (F = 15.3, P = .001), while all other parameters were nonsignificant (all Ps > .17). Temporal coordination (ie, phasing) after-effects showed a significant effect only from phasing baseline (F = 26.92, P < .001, all others P > .33).
Conclusion
This work demonstrates that stroke patients adapt toward their baseline temporal and spatial asymmetries of walking independently of one another. We define how a given split-belt training session would affect asymmetries in these domains, which must be considered when developing rehabilitation interventions for stroke patients.
Keywords: locomotor rehabilitation, split-belt treadmill, motor adaptation, poststroke, kinematics, walking
Introduction
One limitation for stroke survivors, even years after rehabilitation, is a slow, unstable gait that can interfere with activities of daily living,1,2 decreased independence in the home and community,3 and increased susceptibility to falls.4,5 Gait asymmetry is a particularly important problem for stroke survivors. Temporal asymmetries are perhaps most common and are related to slow walking speeds, especially when the asymmetry is large.6 Spatial asymmetries tend to occur more commonly in people that have large temporal asymmetries,6 are related to deficits in propulsive force production7 and may affect the efficiency of walking.8 Indeed symmetric gait has been shown to be more energetically efficient in healthy individuals and in computational models.8–10 Walking asymmetry may be problematic for stroke survivors for many reasons, including reductions in speed and efficiency of walking, reductions in stability of dynamic balance, musculoskeletal imbalances that can lead to pain, reductions in general activity levels, and reductions in loading which can affect bone density.11,12 As such, rehabilitation targeting gait symmetry is an important consideration.
Rehabilitation relies on an individual’s ability to learn through training.13–15 One learning mechanism studied extensively is adaptation—an error-driven process that adjusts existing sensorimotor mappings of well-learned movements to account for new, predictable demands.16 We have previously shown that gait symmetry can be temporarily improved in stroke patients through split-belt training,17 an adaptation paradigm where one leg is driven to move faster than the other. In these experiments, subjects typically learn a new spatial and temporal coordination pattern (ie, sensorimotor mapping) between the limbs when the belts are driven at different speeds.18
Although split-belt training can improve gait symmetry, there are important considerations for rehabilitation that are not well understood. First, do the adaptation rates of stroke patients differ from healthy older adults? It has been shown that adaptation of spatial and temporal parameters relies on the cerebellum,17,19 although there is evidence that cerebral damage may affect spatial components.18 We hypothesized that these patients would be slower at adapting spatial components of walking. Second, how do baseline asymmetries affect a subject’s adaptation and storage of a new walking pattern? Hemiparesis can result in spatial and temporal asymmetries that differ in size and direction across individuals. We have previously shown a dissociation between spatial and temporal control of locomotion18,20; therefore, we hypothesized that specific spatial and temporal baseline asymmetries would predict the adaptation and storage of the novel gait pattern. Furthermore, based on the directionality of after-effects in healthy adults induced by the split-belt, we hypothesized that patients with a larger hemiparetic step would show better gait symmetry when the hemiparetic limb was adapted on the slow belt.
Materials and Methods
Subjects
Twenty-two individuals who had sustained a stroke more than 6 months prior to the study (14 men, 8 women; mean age, 51.4 years) participated in this study. Table 1 shows clinical information for the participants. Subjects were excluded if they had other neurological, orthopedic or cardiovascular conditions, and if they had evidence of damage to the cerebellum. Subjects who typically wore an ankle-foot orthotic were able to wear it during the experiment. Seven healthy volunteers (2 men, 5 women; mean age, 53.1 years) were studied for comparison. All subjects gave informed written consent before participating. Protocols were approved by the Johns Hopkins Institutional Review Board.
Table 1.
Subject Characteristics.
| Subject ID | Age (Years) |
Hemiparetic Limb |
LE Fugl-Meyer Score |
Fast Walking Speed (m/s) |
Left Monofilament Threshold (g) |
Right Monofilament Threshold (g) |
Lesion Information |
|---|---|---|---|---|---|---|---|
| LS0259 | 58 | L | 18/34 | nt | 2 | 300 | R putamen hemorrhagic |
| LS0261 | 38 | L | 33/34 | 1.42 | 0.4 | 0.4 | R parietal hemorrhagic |
| LS0263 | 61 | R | 23/34 | 0.9 | 300 | 300 | L MCA |
| LS0265 | 56 | R | 28/34 | 1.01 | 4 | 4 | R hemisphere |
| LS0267 | 43 | R | 18/34 | 0.97 | nt | >300 | L parietal |
| LS0272 | 62 | R | 26/34 | 1.22 | 4 | 2 | L hemisphere |
| LS0359 | 27 | L | 31/34 | 1.15 | 2 | 0.4 | R MCA |
| LS0387 | 87 | L | 27/34 | 0.97 | 2 | 300 | Small vessel disease |
| LS0406 | 45 | L | 16/34 | 0.73 | 0.4 | 0.4 | R MCA |
| LS0409 | 20 | L | 18/34 | 0.44 | 4 | 0.4 | R hemisphere |
| LS0410 | 65 | L | 27/34 | 1.28 | 300 | >300 | R hemisphere |
| LS0413 | 49 | L | 30/34 | 1.27 | 2 | 0.4 | Ventral medial thalamic lacune |
| LS0418 | 44 | R | 20/34 | 1.03 | 2 | 2 | L hemisphere |
| LS0420 | 50 | L | 22/34 | 1.26 | 0.4 | 2 | R corona radiata of frontal lobe and putamen |
| LS0422 | 50 | R | 29/34 | 1.64 | 300 | 300 | L MCA |
| LS0434 | 69 | R | 32/34 | 1.79 | 2 | 300 | L frontal/SMA |
| LS0439 | 45 | L | 24/34 | 0.85 | 4 | 4 | R premotor/postsensory cortex |
| LS0440 | 54 | L | 30/34 | 1.22 | 0.4 | 0.07 | R basal ganglia and internal capsule |
| LS0441 | 68 | L | 19/34 | 0.78 | 4 | 300 | Small vessel disease |
| LS0444 | 24 | R | 28/34 | 1.55 | 0.4 | 0.4 | L frontal lobe |
| LS0447 | 61 | R | 19/34 | 1.35 | 0.4 | 2 | L MCA |
| LS0452 | 54 | R | 31/34 | 1.36 | 2 | 2 | L thalamic and internal capsule |
Abbreviations: L, left; R, right; LE, lower extremity; nt, not tested; MCA, middle cerebral artery; SMA, supplementary motor area.
Clinical Examination
Subjects who had previously sustained a stroke underwent a clinical examination on the day of the experiment. Proprioceptive ability was measured at the great toe and ankle joints, by asking subjects to determine the direction (flexion or extension) of ~10° movements. Touch sensation was tested at the great toe with graded monofilaments; threshold was determined as the last graded monofilament that the subject could accurately detect 4 out of 6 trials. Fast walking speed was measured as the average speed of 3 trials along a 6-meter walkway. The lower extremity Fugl-Meyer score was used as a measure of sensorimotor control of the involved limb.21
Data Collection
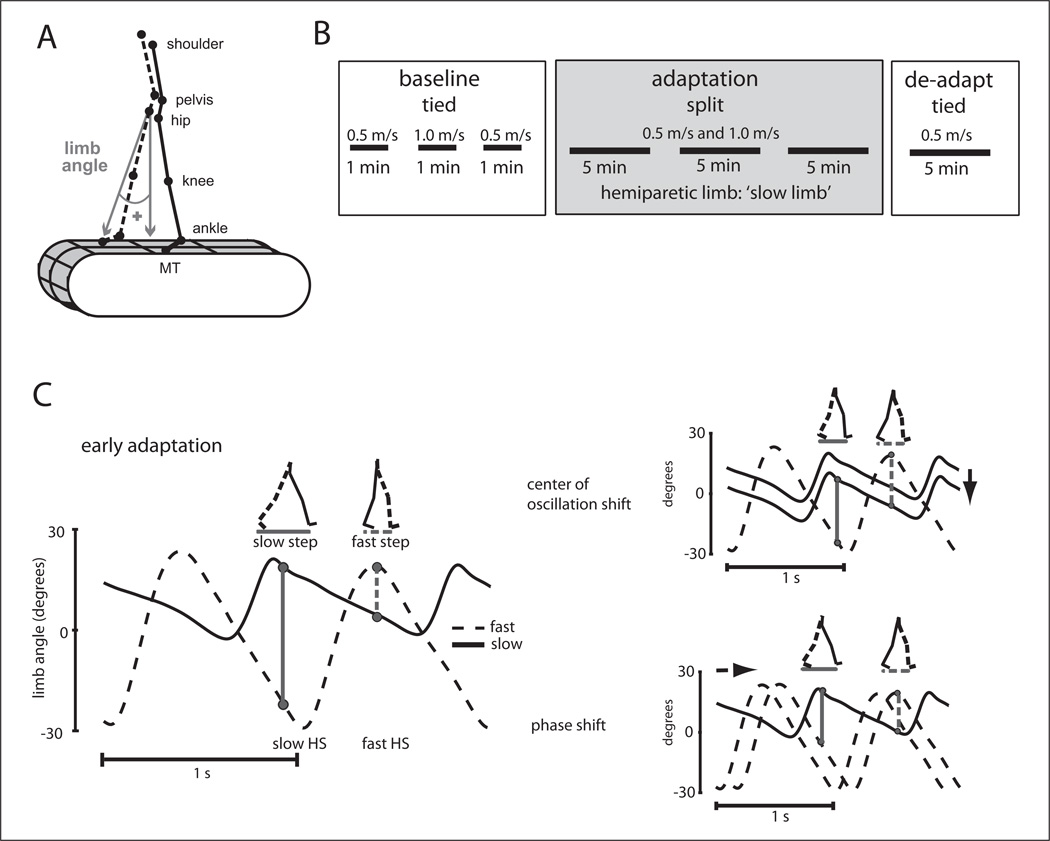
Kinematic data were collected at 100 Hz using Optotrak (Northern Digital, Waterloo, Ontario, Canada). Infrared-emitting markers were placed bilaterally over the toe (fifth metatarsal head), ankle (lateral malleolus), knee (lateral femoral epicondyle), hip (greater trochanter), pelvis (iliac crest), and shoulder (acromion process; Figure 1A). Voltages reflecting treadmill belt speeds were recorded directly from the treadmill motor output at 1000 Hz. Marker position and analog data (treadmill belt speeds) were synchronized and sampled simultaneously using Optotrak software. Heel strike times were approximated using the maximum (positive) angle of the limb (Figure 1A); toe-off time was approximated to be the minimum (negative) limb angle.
Figure 1.
(A) Diagram of marker location and limb angle diagram. (B) Experimental paradigm showing the phases of the experiment: baseline (tied belts), adaptation (split-belts), and de-adaptation (tied belts). All stroke patients had their hemiparetic limb on the “slow” belt during adaptation. (C) Schematic showing how step symmetry can be changed during adaptation. On the left are the limb angle trajectories during early adaptation where step lengths are asymmetric. Two possible ways to make stepping more symmetric are shown on the right. The center of oscillation shift (top) is a spatial change where the limbs gradually shift to oscillate about a midpoint closer to a vertical line intersecting the hip (ie, center of oscillation = 0). Alternatively, the timing of limb motion (ie, phase shift) can be altered to equalize stepping (shown at bottom).
Figure adapted from Malone et al.18
Testing Paradigm
Split-belt walking adaptation was studied using a custombuilt treadmill (Woodway, Waukesha, WI) that had 2 separate belts driven by independent motors—these belts could be driven at the same speed (“tied-belts”) or at different speeds (“split-belts”). Speed commands for each belt were sent to the treadmill through a custom MATLAB (MathWorks, Natick, MA) computer interface. Subjects wore a safety harness suspended from the ceiling that did not support their body weight and were positioned in the middle of the treadmill with one leg on each belt. The belts were initially stationary and subjects were not informed of the speeds of the belts. Subjects were asked to refrain from looking down at the belts and to hold onto a ground-referenced rail in front of them during the entire walking period. Although holding onto the rail could potentially affect the walking pattern, we had all subjects hold on for safety reasons.
The experimental paradigm is shown in Figure 1B. All subjects began with 3 baseline periods during which the belts were tied at 0.5, 1.0 and 0.5 m/s. Subjects were then exposed to three 5-minute periods of split-belts (belts split at 0.5 and 1.0 m/s; slow belt was always on the dominant (healthy) or hemiparetic (stroke patients) limb. For the patients, these rest breaks were variable (~1–11 minutes), based on the individual subject’s fatigue level. For the healthy older adults, the breaks were 5 minutes. All subjects completed a de-adaptation period for 5 minutes, where the belts were again tied at 0.5 m/s. This speed was chosen because the largest after-effects are seen when the belts are tied at the split “slow” belt speed.22 In this study, we chose to keep the belt speeds and “slow” belt designation the same across subjects so that variability in after-effect magnitudes could be reduced and the study could assess how the same training would affect patients with different baseline asymmetries.
Data Analysis
For this study, our primary measurement was step length symmetry which has previously been shown to adapt robustly.17,18,22–26 Step symmetry (SS) was defined as the normalized difference between the step lengths (SL) of the “fast” and “slow” leg, or
| (1) |
Normalization was done so subjects of different heights who take different sized steps could be compared. A value of 0 indicates symmetry. Positive step symmetry means that the fast step was larger than the slow step, and vice versa for negative values. Since the “slow” limb was defined as the hemiparetic limb, when step symmetry was positive, it meant that subjects took a smaller step with their hemiparetic limb.
Step symmetry is the primary measure since our previous work suggested that it is a “global” measure of walking coordination, which can be altered with modifications to either spatial, temporal, or a combination of both parameters (Figure 1C).18 Subjects can alter their step lengths by shifting the “center of oscillation” (spatial coordination: where the limbs are placed during walking) for each leg or by changing the “phasing” (temporal coordination: when the limbs are placed) between the legs.18
The measure of spatial coordination, “center of oscillation,” quantified whether the leg was oscillating about a flexed, extended or neutral (vertical) axis. This was calculated on a stride-by-stride basis as the midpoint of the limb angle between heel strike and toe off for each leg. Limb angle was defined as the angle between a vertical line and the vector from the hip to the foot on an x–y plane (Figure 1A); it was positive when the foot was in front of the hip (flexion) and negative behind (extension). When the limb was oscillating symmetrically around a vertical axis drawn through the hip, the center of oscillation value was defined as zero (eg, solid black line, Figure 1D). The center of oscillation of the “fast leg” was subtracted from that of the “slow leg” to give the center of oscillation difference (COD) between the 2 legs. When the COD was zero, stepping in the spatial realm was symmetric. For stroke patients, when the COD was positive, subjects were walking with their hemiparetic limb more flexed than their nonparetic limb.
The measure of temporal coordination, “phasing,” was determined using the time series of limb angles for each leg.24 It was calculated as the lag time at peak cross-correlation (Signal Processing Toolbox, MATLAB) of the limb angle trajectories over one stride cycle.24 The slow leg was the reference leg in this analysis (solid black line, Figure 1C). In other words, the limb angle trajectory for the fast leg was sequentially shifted in time until it matched the slow leg’s trajectory most closely (ie, peak cross-correlation). The lag time is the percentage of the stride time that the fast leg has to be shifted to reach the maximum correlation. Possible phasing values ranged from 0 to1 stride cycles, with symmetric walking having a value of 0.5. Therefore, when patients had phasing values smaller than 0.5 at baseline, it meant that their hemiparetic limb was lagging behind.
To compare adaptation and de-adaptation behavior between stroke patients (n = 22) and healthy older adults (n = 7), we subtracted out individual baseline asymmetries (average of last 30 seconds of baseline walking at the slow speed, 0.5 m/s) from the adaptation and de-adaptation data (ie, “0” indicates baseline walking). The first 5 strides of adaptation and de-adaptation were used to measure the initial perturbation and after-effects, respectively. Plateau values were calculated by averaging the last 30 strides of each experimental period. Rates of adaptation and de-adaptation were quantified with repeated-measures analyses of variance (ANOVAs) using epochs of 5 strides for the first 50 strides in adaptation and 25 strides in de-adaptation.
All remaining analyses were done within only the group of stroke patients (n = 22) and baseline asymmetries were not subtracted out (ie, “0” indicates zero asymmetry). This allowed us to assess the effects of split-belt training on individual subject asymmetries in the spatial and temporal domains. Baseline asymmetry was quantified as the average of the last 30 seconds of tied walking at the slow speed (0.5 m/s). The adaptation plateau was defined as the average of the last 30 strides of split-belts, while the after-effect was the average of the first 5 strides in de-adaptation (tied belts).
Statistical Analysis
Repeated-measures ANOVAs were used to compare adaptation and de-adaptation rates between the healthy older adults and stroke patients. Post hoc analysis was performed using Fisher’s least significant difference test. T tests were used to assess differences in initial perturbations, after-effects, and final plateaus from baseline between the stroke patients and healthy older adults. General linear models were used to test for correlations in the group of stroke patients. Baseline asymmetries and lower extremity Fugl-Meyer score were tested as regressors for adaptation plateau and after-effect. Statistica (StatSoft, Tulsa, OK) was used for all statistical analysis and the α level was set at P = .05.
Results
Healthy Older Adults Versus Stroke Patients
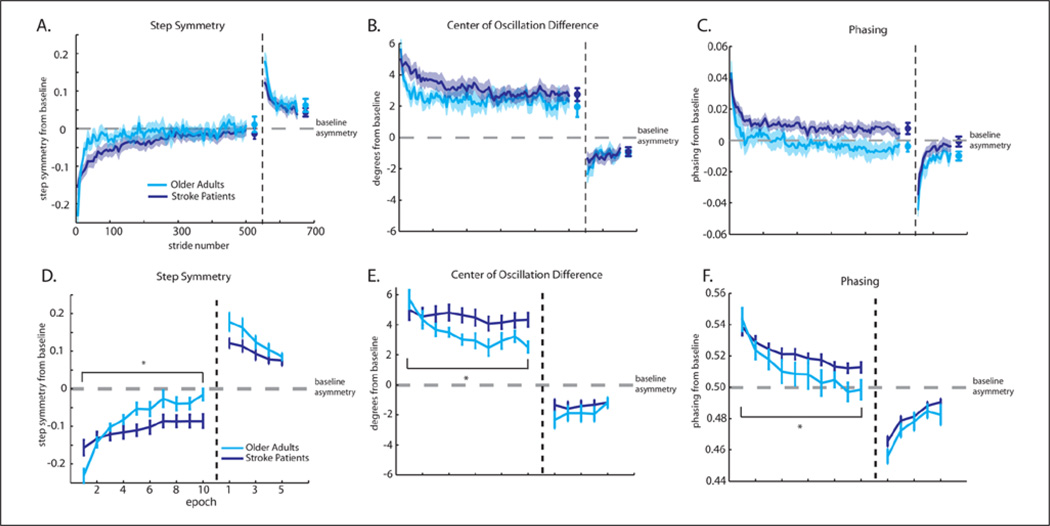
All subjects were able to complete the walking task without difficulty. First, we assessed whether patients could adapt and de-adapted spatial and temporal coordination at similar rates to healthy older adults. Figure 2 shows group means for adaptation and de-adaptation behavior. Note that in these figures, baseline asymmetry is subtracted, so that zero does not represent absolute symmetry (taking equal steps, etc). The global measure, step symmetry, is presented in Figure 2A, while COD (spatial parameter) and phasing (temporal parameter) are Figure 2B and C, respectively.
Figure 2.
(A–C) Group average curves and shaded standard errors for adaptation and de-adaptation. Plateau values are shown by the symbols at the end of the curves. Adaptation and de-adaptation curves are subtracted from baseline, so a value of zero represents baseline asymmetry in all subjects. Stroke patients show a slower adaptation, but eventually reach the same plateau in all parameters. (A) Step symmetry is presented. Stroke patients have slightly smaller initial perturbations and after-effects than healthy older adults. (B) Center of oscillation difference is our spatial parameter. Groups are not significantly different in initial perturbation or aftereffects. (C) Phasing (temporal parameter) is presented. Groups are not significantly different in initial perturbation or after-effects. (D–F) Adaptation and de-adaptation rates. Mean and standard errors are shown for epochs of 5 strides (10 epochs for adaptation and 5 epochs for de-adaptation). Repeated-measures analyses of variance found significant differences in adaptation rate for step symmetry (D), center of oscillation difference (E), and phasing (F).
The initial perturbation and after-effects tended to be larger for healthy older adults in step symmetry (P = .06 and P = .05). No comparable differences were found for the COD (P = .58 and P = .22) or phasing (P = .63 and P = .15). Additionally, there was no difference in either adaptation or de-adaptation plateau for step symmetry (P = .37 and P = .40, respectively), COD (P = .34 and P = .57, respectively), or phasing (P = .11 and P = .13, respectively). These results show that stroke patients have a slightly smaller initial perturbation, and therefore have smaller after-effects, compared to healthy older adults. However, both groups had similar plateau values with respect to their baseline.
Figure 2D–F illustrates the rates of adaptation over the first 50 strides and de-adaptation over 25 strides. Repeated-measures ANOVA showed significant epoch×group interactions in all 3 parameters for adaptation: step symmetry, F(9, 243) = 7.15, P < .001; COD, F(9, 243) = 2.36, P = .01; and phasing, F(9, 243 = 2.85, P < .01. Thus, stroke patients adapted more slowly than healthy older adults. However, we did not find significant interactions of epoch × group for any parameter in de-adaptation: step symmetry, F(4, 108) = 1.19, P = .32; COD, F(4, 108) = 0.83, P = .51; or phasing, F(4, 108) = 0.72; P = .58. These results indicate a slower rate of adaptation in stroke patients compared with healthy older adults. However, after 15 minutes of training all subjects were able to reach the same plateau relative to baseline (Figure 2A–C).
Stroke Patients’ Baseline Asymmetries
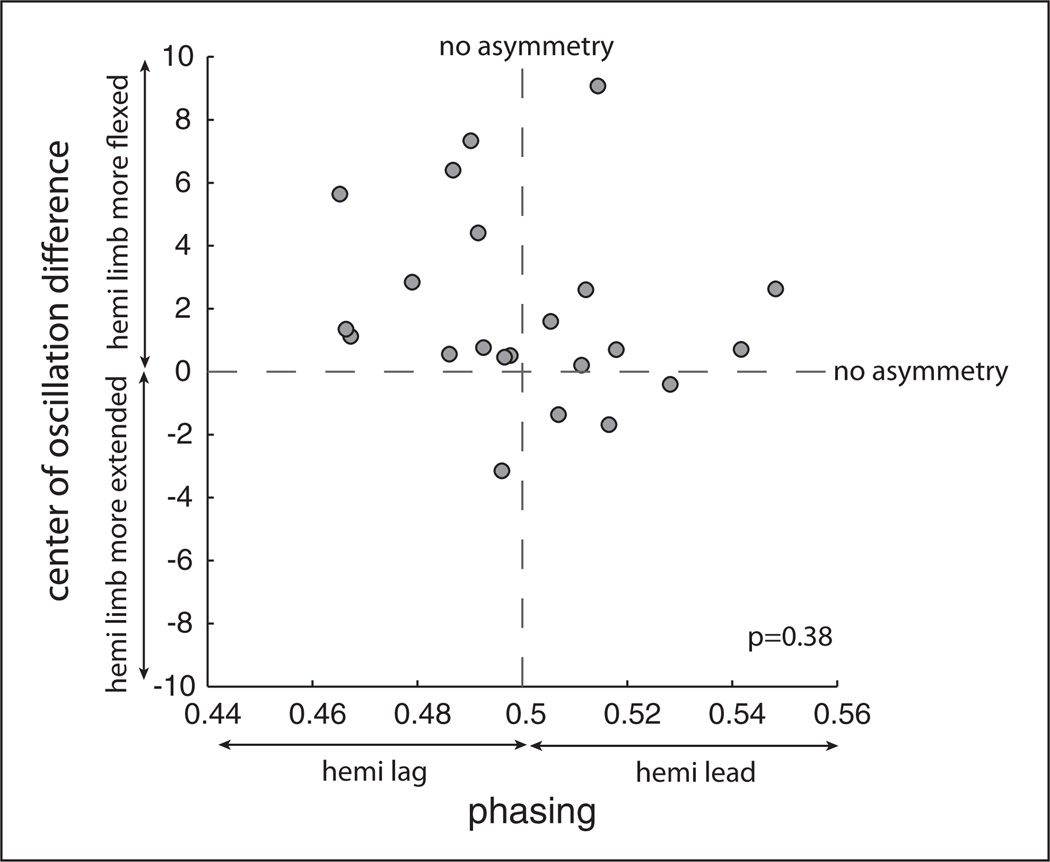
This analysis included only stroke patients (n = 22). We assessed whether spatial and temporal baseline asymmetries were correlated. Figure 3 shows each individual patient’s baseline asymmetry for COD versus phasing. The hemiparetic limb tended to be in a more flexed position (via COD measure) than the nonparetic limb for most subjects. The phasing of the hemiparetic limb was more equally distributed between leading and lagging legs across individuals. No significant correlation was found using phasing baseline asymmetry to predict COD baseline asymmetry (P = .38), which suggests independence of spatial and temporal locomotor control.
Figure 3.
Spatial (center of oscillation difference) and temporal (phasing) baseline asymmetries for stroke patients. Absolute symmetry for each parameter is shown by the dotted lines: a value of 0 for center of oscillation difference and a value of 0.5 for phasing.
Stroke Patients’ Adaptation Plateau Correlates With Baseline Asymmetry
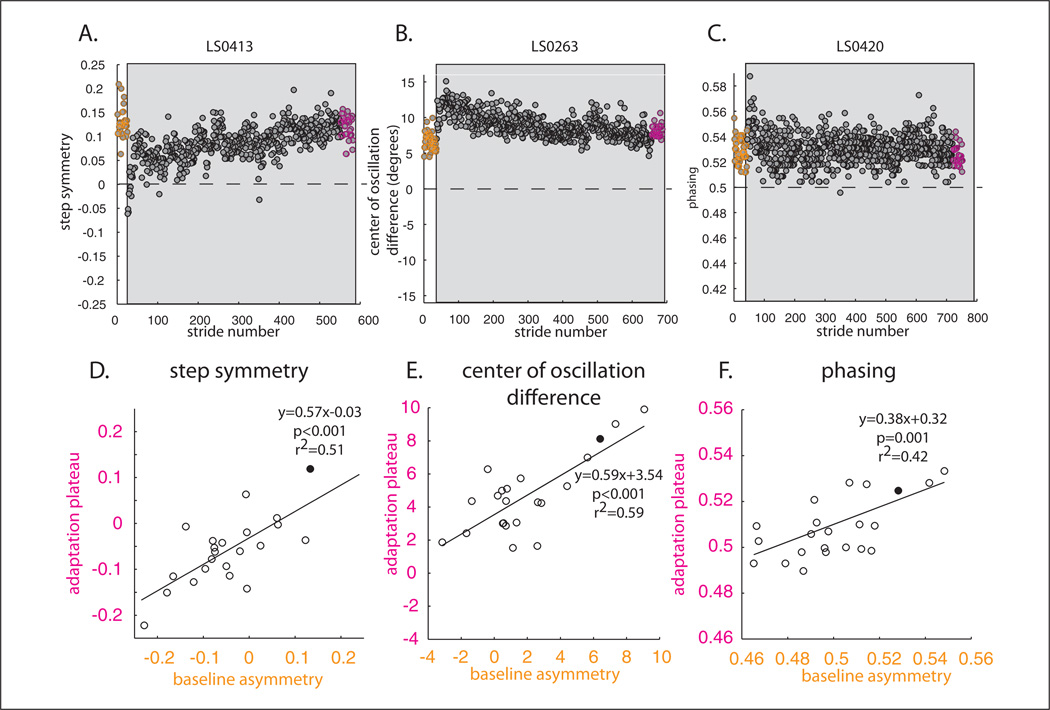
Figure 4 demonstrates how baseline asymmetries (orange circles) are related to adaptation plateau (the last 30 strides in adaptation, pink circles). Here baseline asymmetry was not subtracted out and only the baseline and adaptation periods are shown. We found that subjects adapted toward their baseline asymmetry in all 3 parameters. This occurred regardless of whether we use “corrective training” during adaptation (ie, correcting a patient’s walking asymmetry during early adaptation) or “error-augmenting training” (ie, exacerbating a patient’s walking asymmetry). However, corrective and error-augmenting training showed different results in de-adaptation, which is discussed in the next section. Figure 4A is a good example of corrective training, where the split-belt treadmill was used to correct a patient’s asymmetry (early in adaptation step symmetry is zero). This subject did not maintain a symmetric pattern but rather returned to his baseline asymmetry by the end of adaptation (compare pink and orange circles). Figure 4B and C show examples of error-augmenting training for COD and phasing. By the end of adaptation, subjects once again do not achieve absolute symmetry (zero), but rather tended to plateau around their baseline asymmetries. The adaptive process occurs in stroke patients to return walking behavior back toward baseline gait asymmetries, whether the split-belt perturbation is corrective or error-augmenting.
Figure 4.
Comparison of baseline asymmetries and adaptation plateaus. Here, individual baseline asymmetries are not subtracted out from adaptation and de-adaptation data; therefore, zero represents absolute symmetry. Adaptation is highlighted in the gray area. (A) Example subject’s baseline and adaptation shown stride-by-stride. The orange circles represent baseline walking. Adaptation plateau (last 30 strides) is highlighted in pink. Note in this example, that the split-belts caused the subject to walk symmetrically early in adaptation (ie, step symmetry is zero in the first strides of adaptation). Rather than maintaining this symmetric gait, the subject returns toward his baseline asymmetry (ie, pink and orange circles are equal). (B) Example subject of center of oscillation difference. Once again subject returns close to baseline symmetry by the end of adaptation; however, this subject was perturbed in the opposite direction. Split-belts caused the subject to initially be more asymmetric early in adaptation. (C) Phasing example similar to panel B. Remaining panels display how individual subjects’ baseline asymmetries correlated with adaptation plateaus for step symmetry (D), center of oscillation difference (E), and phasing (F). Example subjects are shown as the filled circles. Note that only the specific parameter’s baseline correlates with the adaptation plateau.
A general linear model was used to determine what parameters predict adaptation plateau. Results indicate that the step symmetry plateau at the end of the split-belt adaptation was predicted by its baseline, F = 14.02, P < .01, and neither by the Fugl-Meyer score, F = 0.12, P = .73, nor the other walking parameters (baseline COD, F = 1.79, P = .20 and phasing F = 0.01, P = .94). As such, we show only the statistically significant correlation for step symmetry, baseline asymmetry versus plateau, in Figure 4D.
Similar results were found for the COD and phasing plateaus. For COD, the baseline predicted the adaptation plateau (F = 16.70, P < .001), while other parameters were not significant: Fugl-Meyer (F = 0.94, P = .34), step symmetry baseline (F = 1.2, P = .29), and phasing baseline (F = 2.16, P = .16). Figure 4E shows the correlation between COD at baseline and plateau. Finally, phasing adaptation plateau is only predicted by phasing baseline asymmetry (F = 13.02, P < .01), and not Fugl-Meyer (F = 1.92, P = .18), step symmetry baseline (F < 0.01, P = .98), or COD baseline (F = 0.01, P = .93). Figure 4F shows the correlation between phasing baseline and adaptation plateau. Taken together, these results suggest that adaptation plateau is related to baseline asymmetry of a given parameter—for example, step symmetry at baseline does not predict how phasing will adapt. Subjects tend to adapt toward their own baselines for spatial and temporal walking parameters.
After-Effects Correlate With Specific Baseline Asymmetries
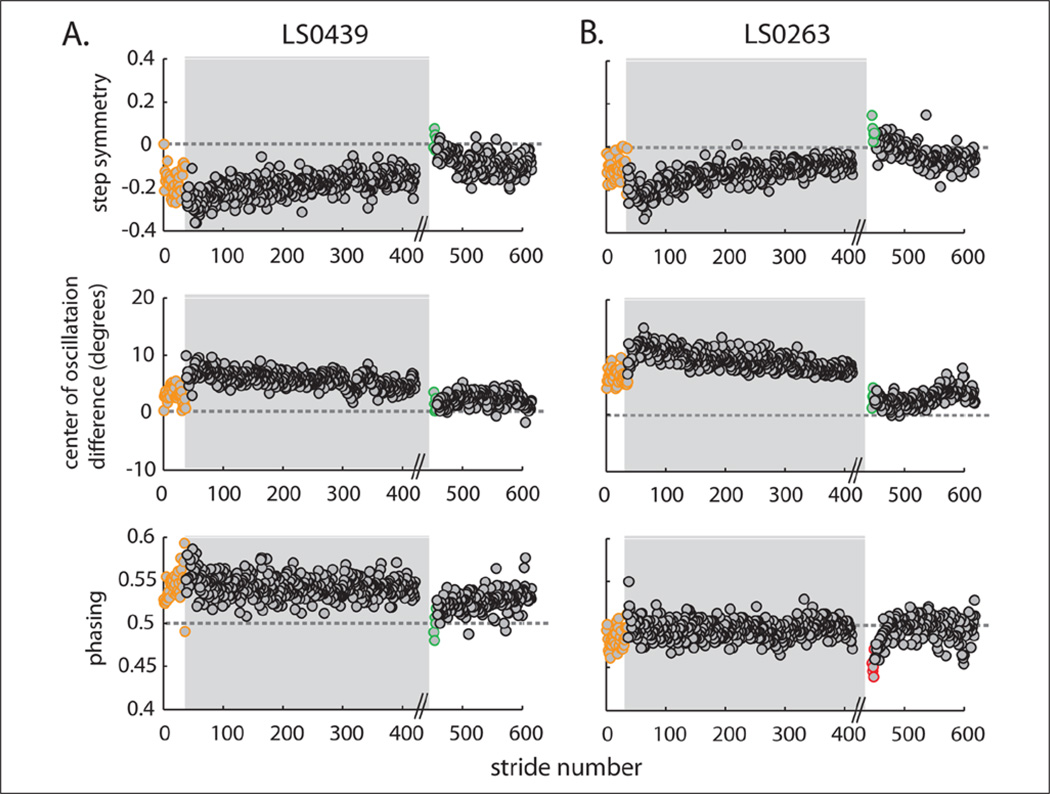
Are baseline asymmetries related to after-effects in de-adaptation? Once again, baseline asymmetries were not subtracted out from this analysis. Figure 5A shows a stride-by-stride plot in which split-belt training allowed the patient to exhibit more symmetric after-effects compared with baseline (compare green circles (after-effects) with orange circles (baseline)). This example subject is closer to absolute zero in early de-adaptation, meaning that we have “improved” the walking pattern across all 3 parameters. This subject was adapted with the impaired leg on the slow belt—we hypothesize that if this individual was adapted with the impaired leg on the fast belt, the opposite would occur and split-belt after-effects would exaggerate their baseline asymmetries.17
Figure 5.
Stride by stride plots of example subjects highlighting after-effects compared to baseline. Adaptation period is highlighted in gray. Absolute symmetry is shown by gray dotted line. One subject showed an improvement in gait symmetry since his after-effects (green circles) are closer to absolute symmetry than at baseline (orange circles) for all parameters (A). Another example subject showed that the same split-belt perturbation can make baseline asymmetries worse in one domain and better in the other. His after-effects (red circles) are farther from absolute symmetry than baseline (orange circles) in phasing, but better in center of oscillation difference (B).
Figure 5B shows that it is possible that an asymmetry can be improved in one domain and made worse in the other. Note how the green circles (after-effects) are closer to absolute symmetry than the orange circles (baseline) for step symmetry and the COD, but the opposite is true for phasing (compare red and orange circles). Thus, baseline asymmetry directionality can differ across parameters—2 individuals may have the same step asymmetry but opposite hemiparetic leg phase deficits (ie, one lags, one leads). Therefore, in some patients, split-belt adaptation can improve both parameters whereas in others it can improve one.
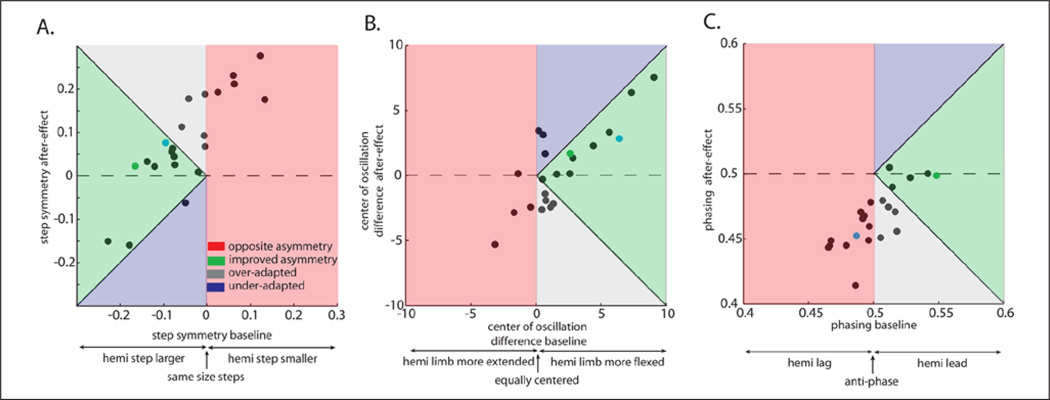
The correlation between baseline asymmetries and aftereffects is shown for all subjects in Figure 6. Subjects whose asymmetries were improved in de-adaptation are found in the green section; those that were worsened are found in the red portion of the graphs. Additionally, some subjects were overadapted (after-effects went past absolute symmetry, gray section), while other subjects were underadapted (blue section). We suggest that the green range is the desired, target range and show here that many subjects fell into that target range. Note that all subjects received the same adaptation speeds during split-belt walking (hemiparetic leg 0.5 m/s, nonparetic leg 1 m/s). This argues that speeds must be tailored for the individual in order to move the subjects in the red zone to the green zone.
Figure 6.
Specific baseline asymmetries (last 30 strides of tied belts) are related to spatial and temporal after-effects (first 5 strides in de-adaptation). The same split-belt (2:1 speed ratio with hemiparetic limb on slow belt) perturbation can have varying effects on a subject’s walking pattern. Patients whose baseline asymmetries were improved are shown in the green section. Other subjects had their baseline asymmetries exaggerated by the training, which are shown in red. Those patients who were overadapted (ie, training caused subjects to change their walking pattern too much) are shown in gray, while those who did not store enough of the walking pattern are shown in blue. Results are shown for step symmetry (A), center of oscillation difference (spatial) (B), and phasing (temporal) (C). Example subjects are shown with green (5A) and blue (5B) filled circles.
Here again a general linear model was used to determine what predicts the after-effects for specific parameters. Step symmetry after-effects are significantly predicted by step symmetry baseline (F = 13.97, P = .001), but not Fugl- Meyer (F = 0.02, P = .88), COD (F = .09, P = .78), or phasing (F = 0.46, P = .51). The after-effects in COD are only predicted by COD baseline (F = 15.3, P = .001), while all other parameters were nonsignificant (all Ps > .17). Finally, phasing after-effects showed a significant effect only from phasing baseline (F = 26.92, P < .001, all others P > .33). In sum, the same perturbation can have different after-effects on each individual, which is based on his or her baseline asymmetry. Since after-effects are correlated solely with baseline asymmetries in the specific parameters, spatial and temporal asymmetries can act independently and possibly asymmetries in one domain can be improved while the other is exaggerated.
Discussion
Here we show that stroke patients adapted more slowly, but as much as a sample of healthy older adults in both spatial and temporal domains. There was a clear relationship between each patient’s baseline asymmetry and adaptation plateau—they tended to adapt toward their own asymmetric baseline suggesting that they view this as their “default” state. Finally, the results demonstrate how the same split-belt adaptation leads to different after-effects depending on the initial asymmetry of the patient. Walking asymmetry can be improved or worsened in post-adaptation depending on a patient’s asymmetry and which limb is on the “slow” belt of the split-belt treadmill. Importantly, in some individuals, it is even possible to improve their spatial pattern but worsen their temporal pattern (or vice versa). As such, we define how the same split-belt perturbation would affect different asymmetries in the spatial and temporal domain of walking.
Neural Substrates of Walking Adaptation
The walking adaptation studied here undoubtedly involves the interplay between many regions of the brain and spinal cord.19,27,28 Previous work has shown that the cerebellum might be a key structure involved in adaptation in both spatial and temporal domains, since it is required for adaptation of both spatial and temporal parameters.19 Yet, more recently, results found for a dissociation between spatial and temporal locomotor adaptation in healthy adults and children.18,29,30 These studies showed that spatial coordination is influenced by conscious efforts whereas temporal coordination was not,18 temporal adaptation could be adapted while preventing spatial adaptation,29 and that temporal walking adaptation is developed at a younger age than spatial walking adaptation.30 It was speculated that spatial coordination might require cerebellar interactions with cerebral structures, while temporal coordination might use cerebellar brainstem and spinal control in healthy individuals.18,30 We therefore expected to see slower spatial parameter adaptation in stroke patients. Instead, results indicated slower adaptation for both spatial and temporal parameters of gait. Thus, cerebral mechanisms may be involved in adaptation of both spatial and temporal coordination, or may indirectly affect temporal coordination by altering brainstem or cerebellar networks. It is difficult to know exactly why spatial and temporal coordination was affected in stroke patients, but because neural networks rewire after permanent injury there might be differences between healthy people and stroke patients. Either way, the effect of cerebral damage was only to slow adaptation slightly. This result agrees with a recent walking study where unilateral perturbing weights were applied in stroke patients to resist forward leg movement, which showed slower adaptation to the leg loading than healthy adults.31
Baseline Asymmetry in Stroke Can Predict Adaptation Extent and After-Effects
The finding that stroke patients adapt back to their individual baselines, and not to absolute symmetry, has important implications. First, it suggests that individuals with stroke have developed a “default state” that is their asymmetric pattern—they return to it despite having the ability to walk with symmetry (eg, during the after-effect). We see at least 2 reasons for why this might be—patients might have developed a “habit,” which is an asymmetric motor pattern that is suboptimal, but has been useful and reinforced during recovery. This may represent their default walking pattern, and will not be reset within a single session. Or, it is possible that asymmetry represents the optimal pattern for patients with a unilateral cerebral lesion. We cannot distinguish between these alternatives in this study. However, we speculate that for many patients it might be the former, that is, habit, since weeks of error-augmenting split-belt training can drive some individuals to a more symmetric default or baseline pattern.32
There are different training methods that can be used for gait rehabilitation. One strategy is to use the split-belt treadmill to assist the patients, immediately correcting baseline asymmetries. In other words, if patients have a baseline step symmetry value greater than zero (ie, hemiparetic step is smaller than nonparetic step), we would split the belts so that early in adaptation subjects had symmetric stepping (example shown in Figure 5A). The idea behind this training is that subjects would be able to experience a symmetric gait that the nervous system could practice. Based on the results of this study, we believe that patients will be unlikely to practice the symmetric gait induced by the split-belts, because as is shown in Figure 4A, subjects adapt and tend to approach values proportional to their baseline asymmetries (Figure 4D–F). Thus, during the adaptive process, their damaged nervous systems are not moving toward absolute symmetry. Rather, patients are adapting toward their baseline asymmetry.
We think that training patients with an error-augmenting split-belt technique (ie, increase initial asymmetry early in adaptation), is the best way to use the adaptive learning process for rehabilitation. It results in stroke patients exhibiting more symmetric after-effects, when belts are tied following adaptation. This would allow them to practice a symmetric walking pattern that they produce themselves—not one driven by the treadmill. Recent studies have demonstrated that stroke patients can maintain this more symmetric pattern for up to 3 months posttraining.32,33
Therefore, it is essential to consider each individual’s baseline asymmetry prior to starting any rehabilitation therapy for chronic stroke patients, because the same split-belt perturbation can result in different after-effects. Table 2 shows the different types of asymmetries and how a split-belt treadmill could effectively be used in error-augmenting training for each type of asymmetry. For example, if patients take a smaller step with their hemiparetic limb, we would suggest training them with their nonparetic limb on the slow belt. While we cannot predict the magnitude of after-effects that patients will demonstrate, we can predict the directionality, which is perhaps the most important component. We know which way to split the belts in order to improve their gait, but there still exists some variability in the amount that subjects adapt. One important caveat is that because after-effects were correlated with baseline asymmetries specific to spatial and temporal coordination, in some patients, it is possible that training may improve asymmetries in one domain while exaggerating the other, as shown in Figure 5B. In these patients, it may not always be possible to “fix” all asymmetries, but therapists can target the one that is most severe.
Table 2.
Predicted Split-Belt Protocols for “Error-Augmenting” Training Given Different Baseline Asymmetries.
| Baseline Asymmetry | Limb on Slow Belt |
|---|---|
| Step symmetry | |
| Larger hemiparetic step | Hemiparetic |
| Larger nonparetic step | Nonparetic |
| Center of oscillation difference | |
| Hemiparetic more extended than nonparetic | Nonparetic |
| Hemiparetic more flexed than nonparetic | Hemiparetic |
| Phasing | |
| Hemiparetic lags | Nonparetic |
| Hemiparetic leads | Hemiparetic |
Conclusion and Rehabilitation Consequences
In sum, these results indicate that both spatial and temporal coordination of locomotion can be adapted to improve gait asymmetries in patients with hemiparesis. Prior studies in our lab have shown that split-belt treadmill training could be an effective rehabilitation tool in stroke patients because the learned pattern generalizes to over-ground walking.26 However, studies of long-term training are now needed to understand if targeting these spatial and temporal asymmetries with error-augmenting split-belt training can lead to more symmetric walking patterns. Preliminary results indicate that this might be the case. We have shown that 4 weeks of error-augmenting training can mitigate asymmetries up to 3 months following training.32,33 In the current study, we emphasize that the intervention be specific to each patient’s baseline asymmetries, since all patients do not respond the same way to training.
Acknowledgments
The authors would like to thank Katie Amenabar and Russell Rasquinha for assistance with data collection.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Institutes of Health grant R01 HD048741.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Jonsdottir J, Cattaneo D. Reliability and validity of the dynamic gait index in persons with chronic stroke. Arch Phys Med Rehabil. 2007;88:1410–1415. doi: 10.1016/j.apmr.2007.08.109. [DOI] [PubMed] [Google Scholar]
- 2.Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke. 1995;26:982–989. doi: 10.1161/01.str.26.6.982. [DOI] [PubMed] [Google Scholar]
- 3.Krishnamoorthy V, Hsu WL, Kesar TM, et al. Gait training after stroke: a pilot study combining a gravity-balanced orthosis, functional electrical stimulation, and visual feedback. J Neurol Phys Ther. 2008;32:192–202. doi: 10.1097/NPT.0b013e31818e8fc2. [DOI] [PubMed] [Google Scholar]
- 4.Diller L, Weinberg J. Evidence for accident-prone behavior in hemiplegic patients. Arch Phys Med Rehabil. 1970;51:358–363. [PubMed] [Google Scholar]
- 5.Nyberg L, Gustafson Y. Patient falls in stroke rehabilitation. A challenge to rehabilitation strategies. Stroke. 1995;26:838–842. doi: 10.1161/01.str.26.5.838. [DOI] [PubMed] [Google Scholar]
- 6.Patterson KK, Parafianowicz I, Danells CJ, et al. Gait asymmetry in community-ambulating stroke survivors. Arch Phys Med Rehabil. 2008;89:304–310. doi: 10.1016/j.apmr.2007.08.142. [DOI] [PubMed] [Google Scholar]
- 7.Balasubramanian CK, Bowden MG, Neptune RR, Kautz SA. Relationship between steplength asymmetry and walking performance in subjects with chronic hemiparesis. Arch Phys Med Rehabil. 2007;88:43–49. doi: 10.1016/j.apmr.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Finley JM, Bastian AJ, Gottschall JS. Learning to be economical: the energy cost of walking tracks motor adaptation. J Physiol. 2013;591:1081–1095. doi: 10.1113/jphysiol.2012.245506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Platts MM, Rafferty D, Paul L. Metabolic cost of over ground gait in younger stroke patients and healthy controls. Med Sci Sports Exerc. 2006;38:1041–1046. doi: 10.1249/01.mss.0000222829.34111.9c. [DOI] [PubMed] [Google Scholar]
- 10.Srinivasan M. Fifteen observations on the structure of energy-minimizing gaits in many simple biped models. J R Soc Interface. 2011;8:74–98. doi: 10.1098/rsif.2009.0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patterson KK, Nadkarni NK, Black SE, McIlroy WE. Gait symmetry and velocity differ in their relationship to age. Gait Posture. 2012;35:590–594. doi: 10.1016/j.gaitpost.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jorgensen L, Crabtree NJ, Reeve J, Jacobsen BK. Ambulatory level and asymmetrical weight bearing after stroke affects bone loss in the upper and lower part of the femoral neck differently: bone adaptation after decreased mechanical loading. Bone. 2000;27:701–707. doi: 10.1016/s8756-3282(00)00374-4. [DOI] [PubMed] [Google Scholar]
- 13.Barreca S, Wolf SL, Fasoli S, Bohannon R. Treatment interventions for the paretic upper limb of stroke survivors: a critical review. Neurorehabil Neural Repair. 2003;17:220–226. doi: 10.1177/0888439003259415. [DOI] [PubMed] [Google Scholar]
- 14.Krakauer JW. Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol. 2006;19:84–90. doi: 10.1097/01.wco.0000200544.29915.cc. [DOI] [PubMed] [Google Scholar]
- 15.Webster BR, Celnik PA, Cohen LG. Noninvasive brain stimulation in stroke rehabilitation. NeuroRx. 2006;3:474–481. doi: 10.1016/j.nurx.2006.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. II. Specificity and storage of multiple gaze-throw calibrations. Brain. 1996;119(pt 4):1199–1211. doi: 10.1093/brain/119.4.1199. [DOI] [PubMed] [Google Scholar]
- 17.Reisman DS, Wityk R, Silver K, Bastian AJ. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain. 2007;130:1861–1872. doi: 10.1093/brain/awm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malone LA, Bastian AJ. Thinking about walking: effects of conscious correction versus distraction on locomotor adaptation. J Neurophysiol. 2010;103:1954–1962. doi: 10.1152/jn.00832.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptations during split-belt treadmill walking. J Neurosci. 2006;26:9107–9116. doi: 10.1523/JNEUROSCI.2622-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torres-Oviedo G, Bastian AJ. Seeing is believing: effects of visual contextual cues on learning and transfer of locomotor adaptation. J Neurosci. 2010;30:17015–17022. doi: 10.1523/JNEUROSCI.4205-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient.a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 22.Vasudevan EV, Bastian AJ. Split-belt treadmill adaptation shows different functional networks for fast and slow human walking. J Neurophysiol. 2010;103:183–191. doi: 10.1152/jn.00501.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi JT, Vining EP, Reisman DS, Bastian AJ. Walking flexibility after hemispherectomy: split-belt treadmill adaptation and feedback control. Brain. 2009;132:722–733. doi: 10.1093/brain/awn333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi JT, Bastian AJ. Adaptation reveals independent control networks for human walking. Nat Neurosci. 2007;10:1055–1062. doi: 10.1038/nn1930. [DOI] [PubMed] [Google Scholar]
- 25.Reisman DS, Block HJ, Bastian AJ. Interlimb coordination during locomotion: what can be adapted and stored? J Neurophysiol. 2005;94:2403–2415. doi: 10.1152/jn.00089.2005. [DOI] [PubMed] [Google Scholar]
- 26.Reisman DS, Wityk R, Silver K, Bastian AJ. Split-belt treadmill adaptation transfers to overground walking in persons poststroke. Neurorehabil Neural Repair. 2009;23:735–744. doi: 10.1177/1545968309332880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drew T, Prentice S, Schepens B. Cortical and brainstem control of locomotion. Prog Brain Res. 2004;143:251–261. doi: 10.1016/S0079-6123(03)43025-2. [DOI] [PubMed] [Google Scholar]
- 28.Grillner S. Biological pattern generation: the cellular and computational logic of networks in motion. Neuron. 2006;52:751–766. doi: 10.1016/j.neuron.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Malone LA, Bastian AJ, Torres-Oviedo G. How does the motor system correct for errors in time and space during locomotor adaptation? J. Neurophysiol. 2012;108:672–683. doi: 10.1152/jn.00391.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vasudevan EVL, Torres-Oviedo G, Morton SM, Yang JF, Bastian AJ. Younger is not always better: development of locomotor adaptation from childhood to adulthood. J Neurosci. 2011;31:3055–3065. doi: 10.1523/JNEUROSCI.5781-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savin DN, Tseng S, Whitall J, Morton SM. Poststroke hemiparesis impairs the rate but not magnitude of adaptation of spatial and temporal locomotor features. Neurorehabil Neural Repair. 2013;27:24–34. doi: 10.1177/1545968311434552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reisman DS, McLean H, Keller J, Danks KA, Bastian AJ. Repeated split-belt treadmill training improves poststroke step length asymmetry. Neurorehabil Neural Repair. 2013;27:460–468. doi: 10.1177/1545968312474118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reisman DS, McLean H, Bastian AJ. Split-belt treadmill training poststroke: a case study. J Neurol Phys Ther. 2010;34:202–207. doi: 10.1097/NPT.0b013e3181fd5eab. [DOI] [PMC free article] [PubMed] [Google Scholar]