Abstract
Alternative donor transplantation is increasingly used for high risk lymphoma patients. We analyzed 1593 transplant recipients (2000 to 2010) and compared transplant outcomes in recipients of 8/8 allele human leukocyte antigen (HLA)-A, -B, -C, and DRB1 matched unrelated donors (MUD; n=1176), 7/8 allele HLA-matched unrelated donors (MMUD; n=275) and umbilical cord blood donors (1 or 2 units UCB; n=142). Adjusted 3-year non-relapse mortality of MMUD (44%) was higher as compared to MUD (35%; p=0.004), but similar to UCB recipients (37%; p=0.19), although UCB had lower rates of neutrophil and platelet recovery compared to unrelated donor groups. With a median follow-up of 55 months, 3-year adjusted cumulative incidence of relapse was lower after MMUD compared with MUD (25% vs 33%, p=0.003) but similar between UCB and MUD (30% vs 33%; p=0.48). In multivariate analysis UCB recipients had lower risks of acute and chronic graft versus host disease compared with adult donor groups (UCB vs MUD: HR=0.68, p=0.05; HR=0.35; p<0.001). Adjusted 3-year overall survival was comparable (43% MUD, 37% MMUD and 41% UCB). Data highlight that patients with lymphoma have acceptable survival after alternative donor transplantation. MMUD and UCB can expand the curative potential of allotransplant to patients who lack suitable HLA-matched sibling or MUD.
Keywords: Umbilical Cord Blood, Lymphoma, Alternative Donor Transplantation
INTRODUCTION
Allogeneic hematopoietic cell transplants (HCT) has been shown to be a valuable and potentially curative strategy to treat patients with high-risk lymphoma.1-6 Reduced-intensity conditioning (RIC) regimens have further expanded the use of allogeneic HCT to those who relapse after autologous HCT, older patients and persons with significant pre-transplant co-morbidities.6-10
Donor availability is a potential barrier for patients who are candidates for allogeneic HCT, but lack an adequately human leukocyte antigen (HLA)-matched and clinically suitable sibling donor. While Caucasian patients have a 60-70% probability of identifying an 8/8 allele level HLA-matched unrelated donor (MUD), for ethnic minority groups fewer than 30% find a well-matched donor.11 In the past 10 years, a growing number of reports supported an expanding utilization of HLA-mismatched unrelated donors (MMUD), umbilical cord blood (UCB) and partially HLA-matched family donors (haploidentical) as valuable alternatives to fill the gap in donor availability.12-14
However, data on the relative efficacy of alternative donor HCT for adults with high-risk lymphoma are limited and there are no data on comparison of 7/8 versus 8/8 HLA-matched unrelated donors and UCB.7,9,15-20 Thus, we performed a retrospective registry based analysis studying the outcomes of patients with advanced lymphoma who received an allograft from MUD, MMUD or UCB using data from the Center for International Blood and Marrow Transplant Research (CIBMTR).
PATIENTS AND METHODS
Data source
The CIBMTR, a voluntary working group of more than 450 transplantation centers worldwide, collects data on consecutive allogeneic HCTs at a statistical center housed at both the Medical College of Wisconsin (Milwaukee, WI) and the National Marrow Donor Program (Minneapolis, MN). Patients are observed longitudinally with yearly follow-up. Computerized checks for errors and onsite audits of participating centers ensure data quality. The present study was conducted with a waiver of informed consent and in compliance with Health Insurance Portability and Accountability Act regulations as determined by the Institutional Board and the Privacy Officer of the Medical College of Wisconsin.
Study Population
In this comparative study, we included patients ≥ 18 years-old with non-Hodgkin lymphoma (NHL) and Hodgkin lymphoma (HL) who underwent transplant with an 8/8 allele HLA-matched donor (MUD), 1 antigen or allele MMUD and UCB transplanted in the United States between 2000-2010. We verified HLA matching for all cases included in this study. Forty-nine percent were retrospectively typed using stored samples for NMDP/CIBMTR research repository;21 43% were NMDP facilitated transplants and 9% had HLA typing reported by the transplant center. A contemporary haploidentical related donor cohort had only 39 patients with a short median follow-up of 14 months and was excluded from this analysis. Patients with planned second transplants, ex-vivo manipulated grafts and those with rare aggressive histologies (ie, aggressive NK cell neoplasms, lymphoblastic lymphoma, Burkitt lymphoma, primary central nervous system lymphoma) were excluded. Preparative regimens were classified either as RIC or myeloablative conditioning (MAC) according to published consensus definitions.22 RIC regimens included melphalan ≤ 140 mg/m2, busulfan ≤ 9 mg/kg orally, total body irradiation <5 Gy, fludarabine-total body irradiation combinations, or fludarabine-based conditioning. The MAC preparative regimens included mostly total body irradiation or busulfan-based combinations.
Definitions, Study Endpoints and Statistical Analysis
The primary objective was to compare overall survival (OS) after HCT between patients undergoing MUD, MMUD and UCB transplants, while adjusting for patient, disease, and transplant-related characteristics. Patient, disease and transplant-related factors were compared between groups using the Chi-square test for categorical variables and the Wilcoxon sample test for continuous variables. Surviving patients were censored at the time of last contact. Secondary endpoints were progression-free survival (PFS), relapse, non-relapse mortality (NRM), grade II-IV acute graft versus host disease (GvHD), and chronic GvHD.23,24 Adjusted survival probabilities of OS and PFS for the 3 donor groups were estimated based on Cox proportional hazards models.25 Adjusted cumulative incidence rates were calculated for relapse and non-relapse mortality (NRM) to accommodate competing risks.26 Acute and chronic GVHD were defined calculated using cumulative incidence function. Multivariate analysis used Cox's proportional hazard model.27 All clinical variables were tested for proportional hazards assumptions. Factors violating the proportional hazards assumption were adjusted through stratification. We stratified models for OS, PFS, relapse and NRM based on same set of variables (i.e., Karnofsky performance score, lymphoma subset, GvHD prophylaxis, disease status). Stepwise model building procedures used a significance threshold of 0.05 for both entry and retention in the models. The main effect variable of donor type (MUD vs. MMUD vs. UCB) was forced into the models, and a random effect in the model was used to adjust for the center effect. Interactions between the main effect variable and adjusted covariates were tested at a significance level of 0.01. No significant interactions between the donor type variable and adjusted covariates were detected in any of the models. The results are reported at 3 years post-transplant.
RESULTS
Patients, Disease, and Transplant Characteristics
We studied 1593 patients with NHL and HL treated at 119 centers. Baseline patient, disease, and transplant-related characteristics of UCB (n=142), MMUD (1 allele mismatched n=106; 1 antigen mismatched n=169) and MUD (n=1176) recipients are summarized in Table 1. The median age at transplant was 50 (MUD), 45 (MMUD) and 45 (UCB) years. The MUD cohort included more males, more often had mantle cell NHL and less often had HL. Both MUD and MMUD graft types were mostly peripheral blood male-male donor-recipient sex matched (Table 1). About half of recipients in three donor groups were cytomegalovirus sero-positive. More UCB recipients were non-Caucasian, had higher Karnofsky performance score, more had chemotherapy-sensitive disease and received prior radiation-therapy. Sixty-three percent (n=90) of UCB transplants used two UCB unit grafts. The median TNC dose of combined UCB units was 2.8 × 10e7/kg (range, 0.2-9.5) and were mostly HLA locus 5/6 (28%) or 4/6 (55%) matched. Notably, 45% (n=23) of single and 29% (n=26) of double UCB grafts were small providing <2.5× 10e7 TNC/kg. UCB HCT had the shortest interval from diagnosis to transplant (median 27 months). In each donor group, about 70% received a RIC transplant. The proportion of patients with prior autograft, chemosensitive disease and type of conditioning in different lymphoma subsets were similar in each donor group. Recipients of MUD and MMUD were more likely to receive a tacrolimus based GvHD prophylaxis regimen and anti-thymocyte globulin (ATG) or alemtuzumab than UCB recipients. GvHD prophylaxis for UCB transplants more often included cyclosporine plus mycophenolate mofetil. Donor/recipient sex, donor/recipient cytomegalovirus status, and graft type (marrow vs blood) were similar in adult unrelated donors. The median follow-up of survivors in the MUD, MMUD and UCB groups was 57 months (range 6-129), 65 months (range 12-125) and 25 months (range 6-73; p<0.001), respectively.
Table 1.
Characteristics of patients that underwent allogeneic hematopoietic cell transplantation for NHL and HL reported to the CIBMTR between 2000 and 2010, by graft type.
| Characteristics of patients | MUD | MMUD | UCB | P-value |
|---|---|---|---|---|
| Number of patients | 1176 | 275 | 142 | |
| Age, median (range), years* | 50 (18-75) | 45 (18-71) | 45 (19-73) | <0.001 |
| Male sex* | 749 (64) | 164 (60) | 79 (56) | 0.106 |
| Karnofsky performance score* | 0.097 | |||
| <90% | 349 (30) | 98 (36) | 37 (26) | |
| ≥90% | 709 (60) | 152 (55) | 96 (68) | |
| Missing | 118 (10) | 25 ( 9) | 9 ( 6) | |
| Race* | <0.001 | |||
| Caucasian | 1122 (95) | 246 (89) | 110 (77) | |
| Black | 21 ( 2) | 16 ( 6) | 18 (13) | |
| Others** | 33 ( 3) | 13 ( 5) | 14 (10) | |
| Interval from diagnosis to transplant, months* | 34 (3-312) | 32 (3-247) | 27 (2-203) | 0.168 |
| Previous autologous transplant* | 485 (41) | 134 (49) | 64 (45) | 0.067 |
| Interval from autoHCT to alloHCT, months* | 20 (6-175) | 19 (6-154) | 18 (6-139) | 0.894 |
| Histology* | 0.074 | |||
| Hodgkin lymphoma | 233 (20) | 74 (27) | 39 (27) | |
| Follicular/ other indolent lymphoma | 294 (25) | 59 (21) | 30 (21) | |
| DLBCL/other aggressive B cell lymphoma | 282 (24) | 70 (25) | 39 (27) | |
| Mantle cell lymphoma | 212 (18) | 38 (14) | 13 ( 9) | |
| Mature T cell and NK cell neoplasm | 155 (13) | 34 (12) | 21 (15) | |
| Chemosensitive status prior to transplant* | 818 (69) | 183 (67) | 107 (76) | 0.201 |
| Disease status prior to transplant | 0.363 | |||
| First partial remission | 143 (12) | 27 (10) | 23 (16) | |
| PIF resistant | 128 (11) | 34 (12) | 13 ( 9) | |
| CR1 | 72 ( 6) | 13 ( 5) | 15 (11) | |
| Second partial remission | 315 (27) | 77 (28) | 34 (24) | |
| REL resistant | 230 (20) | 58 (21) | 22 (16) | |
| CR2+ | 220 (18) | 54 (20) | 26 (18) | |
| REL untreated/unknown | 26 ( 2) | 7 ( 2) | 2 ( 1) | |
| Missing | 42 ( 4) | 5 ( 2) | 7 ( 4) | |
| Prior radiation therapy* | 751 (64) | 194 (71) | 116(82) | <0.001 |
| Graft type* | NA | NA | ||
| Bone marrow | 259 (22) | 74 (27) | ||
| Peripheral blood | 913 (78) | 201 (73) | ||
| Recipient Cytomegalovirus serology* | 0.089 | |||
| Positive | 622 (53) | 136 (49) | 79 (56) | |
| Negative | 552 (47) | 138 (50) | 61 (43) | |
| Missing | 2 (<1) | 1 (<1) | 2 ( 1) | |
| One antigen/allele mismatch by locus | NA | NA | NA | |
| HLA-A | 78 (28) | |||
| HLA-B | 38 (14) | |||
| HLA-C | 130 (47) | |||
| HLA-DRB1 | 29 (11) | |||
| Donor-Recipient sex match | NA | NA | ||
| Male-Male | 531 (45) | 112 (41) | ||
| Male-Female | 277 (24) | 58 (21) | ||
| Female-Male | 189 (16) | 51 (19) | ||
| Female-Female | 132 (11) | 53 (19) | ||
| Year of transplant* | <0.001 | |||
| 2000-2003 | 338 (29) | 97 (35) | 21 (15) | |
| 2004-2006 | 463 (39) | 134 (49) | 34 (24) | |
| 2007-2010 | 375 (32) | 44 (16) | 87 (61) | |
| Conditioning regimen* | <0.001 | |||
| Myeloablative | 302 (26) | 81 (29) | 41 (29) | |
| Reduced intensity | 874 (74) | 194 (71) | 101 (71) | |
| Total number chemotherapy lines, median | 4 (1-5) | 4 (1-5) | 3 (1-5) | <0.001 |
| ATG/alemtuzumab* | <0.001 | |||
| ATG and alemtuzumab | 1 (<1) | 1 (<1) | 0 | |
| ATG alone | 296 (25) | 88 (32) | 51 (36) | |
| alemtuzumab alone | 138 (12) | 38 (14) | 1 ( 1) | |
| No ATG or alemtuzumab | 740 (63) | 148 (54) | 89 (63) | |
| Graft versus host disease prophylaxis* | <0.001 | |||
| Tacrolimus + others | 809 (69) | 179 (65) | 56 (39) | |
| Cyclosporine + others | 177 (15) | 43 (16) | 65 (46) | |
| Other*** | 28 ( 4) | 4 ( 3) | 7 ( 5) | |
| Median follow-up of survivors (range), months | 57 (6-129) | 65 (12-125) | 25 (6-73) |
Abbreviations: UCB umbilical cord blood; MUD matched unrelated donor; MMUD mismatched unrelated donor; DLBCL diffuse large B cell lymphoma, TBI total body irradiation, ATG antithymocyte globulin
Variables tested in Cox proportional hazards regression models.
Other race includes: Asian/Pacific Islander n=18 (UCB=7, MUD=8, MMUD=3), Middle East or Northcoast of Africa n=2 (MUD=1, MMUD=1), Hispanic n=5 (MUD=3, MMUD=2) and others (UCB=7, MUD=21, MMUD=7).
Other graft versus host disease prophylaxis includes: ATG only=1, ATG/Methotrexate=1, Methotrexate only=1, Missing=26.
Neutrophil and platelet engraftment
Neutrophil engraftment at day 28 and day 100 was significantly more frequent in MUD and MMUD recipients as compared to UCB (Table 2). Platelet recovery to ≥20 × 109/L at day 100 was also significantly better in MUD and MMUD than UCB (Table 2). In MUD, MMUD and UCB groups, median time to neutrophil recovery was 13 (0-106), 16 (1-75) and 21 (0-66) days and median time to platelet recovery was 16 (0-394), 25 (1-49) and 45 (0-334) days, respectively.
Table 2.
Outcomes after hematopoietic cell transplantation by donor type
| Outcomes | MUD N=1173 | MMUD N=274 | UCB N=140 | P-value |
|---|---|---|---|---|
| % (95% confidence interval) | ||||
| Neutrophil recovery | ||||
| at 28 days | 94 (92-95) | 94 (90-96) | 66 (57-73) | <0.001 |
| at 100 days | 95 (94-96) | 95 (92-97) | 87 (80-92) | 0.023 |
| Platelet recovery 3 20 × 109 | ||||
| at 100 days | 86 (84-88) | 85 (80-89) | 68 (59-76) | <0.001 |
| Acute GvHD (II-IV) | ||||
| at 100 days | 37 (35-40) | 49 (43-55) | 26 (19-34) | <0.001 |
| Acute GvHD (III-IV) | ||||
| at 100 days | 20 (18-22) | 24 (19-29) | 17 (11-23) | 0.17 |
| Chronic GvHD | ||||
| at 3 years | 51 (48-54) | 48 (42-54) | 22 (15-30) | <0.001 |
Abbreviations: UCB umbilical cord blood, MMUD 1 Ag or 1 allele mismatched unrelated donor, MUD matched unrelated donor, GvHD graft versus host disease
Non relapse mortality
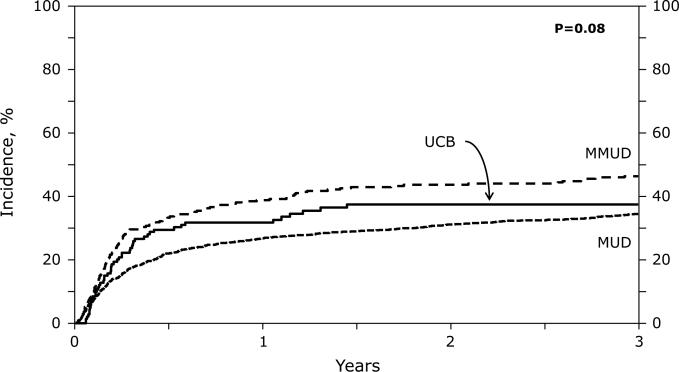
The adjusted cumulative incidences of NRM at 3 years were 35% (MUD 95%CI 32-38%), 44% (MMUD 95%CI 39-50%) and 37% (UCB 95%CI 28-46%) (Table 3; Figure 1A). In multivariate analysis, the NRM risk was significantly higher in MMUD compared to MUD recipients, while there was no difference between MMUD vs. UCB and MUD vs. UCB groups (Figure 1A; Table 4). UCB graft cell dose did not significantly impact the NRM risk (UCB NC < 2.5×10e7 versus ≥2.5×10e7 HR 1.37; p=0.13). The most common non-relapse cause of death among MUD and MMUD patients was infections (n=16 and 16), followed by GvHD (n=14 and 13). Organ failure (n=15 and 13) and non-engraftment were infrequent (n=3 and 1). In the UCB group the most frequent causes of NRM were infection (n=15), organ failure (n=11), non-engraftment (n=11), GvHD (n=4), and lymphoproliferative disorder (n=4). Graft failure was managed by 2nd (n=10) or 3rd transplant (n=1); only 2 patients with graft failure survived, both UCB recipients following 2nd HCT.
Table 3.
Three-year adjusted probabilities.
| Outcomes | MUD | MMUD | UCB | UCB vs MUD | UCB vs MMUD | MMUD vs MUD |
|---|---|---|---|---|---|---|
| N=1173 | N=274 | N=140 | p-value | p-value | p-value | |
| % (95% confidence interval) | ||||||
| Non relapse mortality | 35 (32-38) | 44 (39-50) | 37 (28-46) | 0.63 | 0.19 | 0.004 |
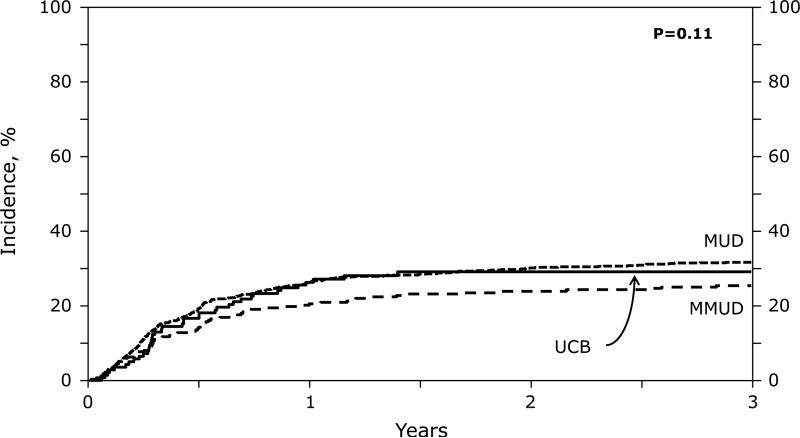
| Relapse | 33 (30-36) | 25 (20-30) | 30 (22-38) | 0.48 | 0.27 | 0.003 |
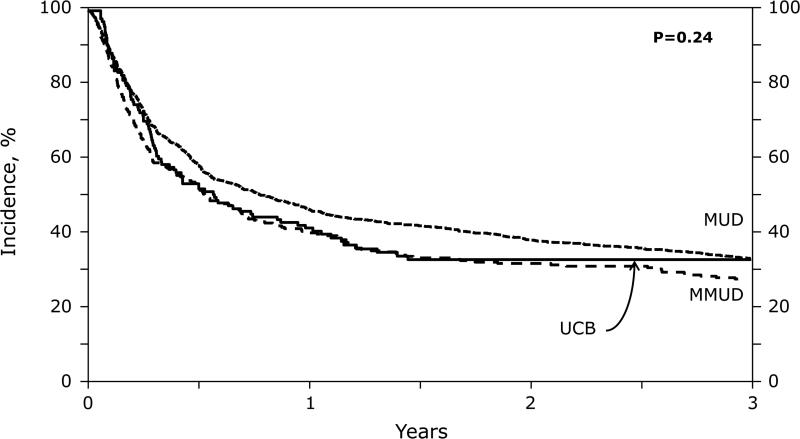
| Progression-free survival | 33 (30-36) | 30 (25-35) | 31 (23-39) | 0.72 | 0.81 | 0.35 |
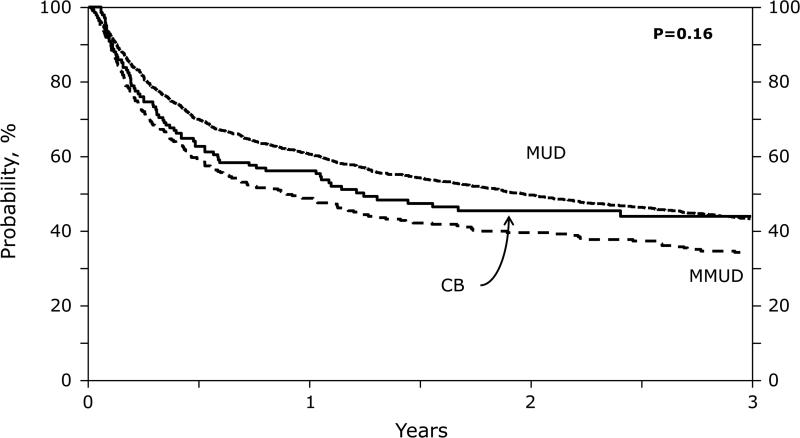
| Overall survival | 43 (40-46) | 37 (32-43) | 41 (33-50) | 0.77 | 0.45 | 0.073 |
Abbreviations: UCB umbilical cord blood, MMUD mismatched unrelated donor, MUD matched unrelated donor, Adjusted probabilities of progression-free survival, overall survival, relapse and non-relapse mortality for the 3 donor groups were based on a stratified Cox regression model. Karnofsky performance score, lymphoma subset, Graft versus host disease prophylaxis and disease status violated the proportionality assumption, and therefore, all the models were stratified on these variables.
Figure 1.
(A) Non-Relapsed Mortality: Adjusted 3-year non-relapse mortality by donor groups
(B) Relapse: Adjusted 3 year relapse rate by donor groups.
(C) Progression-Free Survival: Adjusted 3 year progression-free survival by donor groups.
(D) Overall Survival: Adjusted 3 years overall survival by donor groups.
Table 4.
Multivariate analysis of factors associated with risk of NRM, acute GvHD, chronic GvHD, relapse, PFS and OS.
| Variable | HR (95% Confidence interval) | P-value |
|---|---|---|
| Non relapse mortalitya | ||
| MUD | Ref | Poverall=0.08 |
| UCB | 1.22 (0.87-1.72) | 0.24 |
| MMUD | 1.32 (1.03-1.69) | 0.02 |
| MMUD vs. UCB | 1.07 (0.76-1.52) | 0.68 |
| Grade II-IV acute GvHDb | ||
| MUD | Ref | Poverall<0.001 |
| UCB | 0.68 (0.46-1.00) | 0.050 |
| MMUD | 1.44 (1.18-1.75) | <0.001 |
| MMUD vs UCB | 2.12 (1.52-2.95) | <0.001 |
| Chronic GvHDc | ||
| MUD | Ref | Poverall<0.001 |
| UCB | 0.35 (0.21-0.56) | <0.001 |
| MMUD | 1.15 (0.90-1.48) | 0.240 |
| MMUD vs UCB | 3.32 (1.99-5.54) | <0.001 |
| Relapsed | ||
| MUD | Ref | Poverall=0.11 |
| UCB | 1.08 (0.72-1.63) | 0.70 |
| MMUD | 0.75 (0.58-0.98) | 0.03 |
| MMUD vs UCB | 0.69 (0.42-1.14) | 0.15 |
| Progression-free survivale | ||
| MUD | Ref | Poverall=0.24 |
| UCB | 1.22 (0.96-1.54) | 0.09 |
| MMUD | 1.07 (0.88-1.29) | 0.49 |
| MMUD vs UCB | 0.88 (0.67-1.13) | 0.31 |
| Overall Survivalf | ||
| MUD | Ref | Poverall=0.16 |
| UCB | 1.14 (0.89-1.47) | 0.29 |
| MMUD | 1.19 (0.98-1.45) | 0.08 |
| MMUD vs UCB | 1.04 (0.77-1.40) | 0.77 |
Abbreviations: HCT hematopoietic cell transplantation, UCB umbilical cord blood; MUD matched unrelated donor, MMUD 1 Ag or allele mismatched unrelated donor, GvHD graft versus host disease, ATG antithymocyte globulin, CsA cyclosporine, MAC myeloablative conditioning, RIC reduced intensity conditioning.
Other prognostic factors in the models
Age, time from diagnosis to HCT, race, conditioning regimen, prior auto HCT, & year of HCT
ATG/alemtuzumab use, GvHD prophylaxis, time from diagnosis to HCT, & disease status.
ATG/alemtuzumab use
ATG/alemtuzumab use
Year of HCT
Age, time from diagnosis to HCT, conditioning regimen, & year.
Graft versus host disease
Grade II-IV aGvHD was more frequent in MMUD and MUD as compared to UCB recipients (Table 2). Grade III-IV occurred at similar rate (Table 2). The cumulative incidence of chronic GvHD at 3 year was 2-fold higher in MMUD and MUD cohorts as compared to UCB (Table 2). In multivariate analysis the risk of aGvHD was significantly lower in UCB recipients as compared to MUD and MMUD (Table 4). The risk of chronic GvHD was highly significantly decreased in UCB recipients (Table 4).
Relapse/Progression
The 3-year risk of relapse/progression was lower in MMUD transplants but was not different in recipients of MUD and UCB grafts (Tables 3 and 4; Figure 1B). Relapse was not influenced by single or double unit UCB grafts or by total UCB TNC dose infused (data not shown). Relapse was the most frequent cause of death in all 3 donor groups affecting 285 (39%) in MUD, 64 (32%) in MMUD and 22 (29%) in UCB recipients. Twenty-five patients received donor lymphocyte infusion (DLI) for relapse; 23 (MUD) and 2 (MMUD). Only eight MUD recipients survive between 16 and 96 months after DLI.
Survival
Adjusted PFS at 3 years was 33% (MUD 95%CI 30-36%), 30% (MMUD 95%CI 25-35%) and 31% (UCB 95%CI 23-39%) (Table 3, Figure 1C) with the risk of treatment failure not significantly associated with graft source (Table 4). Due to higher NRM and lower relapse risks in the MMUD group, the OS in 3 groups were similar (Table 4). Adjusted OS at 3 years in the 3 groups was 43% (95%CI 40-46%) in MUD, 37% (95%CI 32-43%) in MMUD and 41% (95%CI 33-50%) in UCB recipients (Figure 1D). In UCB group, overall mortality was not influenced by TNC dose (low vs high HR 1.24; p=0.42).
DISCUSSION
In this large registry-based study, we analyzed the differences in transplant risks and clinical benefits in adults with HL and NHL receiving transplants from alternative donors. Comparative data are increasingly needed by the patients and their physicians to guide the decision-making regarding hematopoietic transplant donor options. The main findings of our study were that 1) survival was similar for three donor types; 2) the risk of acute and, in particular chronic GvHD was significantly lower in recipients of UCB; 3) there was quicker hematopoietic recovery in recipients of MUD and MMUD as compared to UCB, yet without significant influence on NRM and 4) MMUD recipients had lower risk of relapse as compared to MUD; however, this benefit was offset by increased NRM. Overall, between 37-43% patients with relapsed or refractory lymphoma using alternative donors survived beyond 3 years and the graft source did not significantly influence PFS or OS. These promising results compare favorably even to HLA-matched sibling donor transplants, yet the heterogeneity in subjects and lymphoma histology likely contribute to modest differences.1,4,28,29 It is important to recognize that our cohort of lymphoma patients undergoing allograft is heterogeneous and skewed with high proportion of patients who were chemorefractory (27%), had failed autologous HCT (50%) and radiation therapy (70%). Thus some patients were heavily pre-treated and these unrelated donor HCTs were delayed and used after other modalities failed to control their disease. Furthermore, the UCB HCT were more recent and follow-up was shorter. Because some critical prognostic variables such as disease status and lymphoma subtype violated the proportional hazard assumption in 3 donor groups, we controlled for them by stratified analysis to answer the donor source risk association; thus the analysis was not designed to address influence of disease and patient-related factors on outcomes. Some potentially important variables such as comorbidity index were not available in this cohort. Despite several adverse features and heterogeneity of this cohort, these encouraging results clearly suggest that allotransplantation offers potentially curative therapy which can be extended to almost all patients with high-risk lymphoma, even those without an available HLA matched sibling. Future studies investigating different lymphoma subsets are needed to refine our conclusions.
Importantly, our results highlight the acceptable transplant outcomes of MMUD and UCB HCT.9,15,28 In MMUD, the HLA-mismatch seems to have driven greater alloreactivity as evidenced by higher incidences of aGvHD and cGvHD and a lower risk of relapse. The benefit of lower relapse was offset by higher risk of NRM resulting to similar survival as compared to UCB and MUD. Future efforts to improve MMUD HCT need to focus on better patient selection and innovative strategies to reduce GvHD. Recent much larger registry studies demonstrated impairment of survival after single allele mismatch and adverse effect of HLA-C antigen mismatching, therefore we acknowledge that our results maybe impacted by smaller cohort size.30-32 Validation in larger study and cautious interpretation is therefore warranted.
We observed a lower risk of acute and chronic GvHD in UCB recipients as compared to MUD and MMUD, although in vivo T-cell depletion that can reduce the risk for acute GvHD was used frequently in MUD and MMUD. Lower risk of GvHD and greater HLA–mismatch in UCB HCT did not compromise the alloreactivity against lymphomas. As GvHD contributes to morbidity and mortality and can compromise the quality of life of long-term survivors, a lower risk of both acute and chronic GvHD after UCB HCT may be an additional favorable feature influencing donor choice. UCB transplant were used more frequently for ethnic minorities since suitable UCB units mismatched in 1 or 2 HLA loci can provide a graft for 90-95% of patients with minority backgrounds, who less often identify a MUD.33
These data demonstrate that successful allogeneic donor HCT can be available for all adult lymphoma patients including those of minority ethnic groups with rare HLA haplotypes. Our study supports prospective testing of UCB and MMUD in lymphoma such as randomized CTN trial comparing UCB to haploidentical donor. Our results mandate that patients with lymphoma in whom allograft is indicated have wider access to alternative donor options.
ACKNOWLEDGMENTS
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-12-1-0142 and N00014-13-1-0039 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals; Allos Therapeutics, Inc.; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; *Blue Cross and Blue Shield Association; *Celgene Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; *Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.;*Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Jeff Gordon Children's Foundation; Kiadis Pharma; The Leukemia & Lymphoma Society; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; *Milliman USA, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; *Remedy Informatics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; St. Baldrick's Foundation; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; *Tarix Pharmaceuticals; *TerumoBCT; *Teva Neuroscience, Inc.; *THERAKOS, Inc.; University of Minnesota; University of Utah; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
Footnotes
Financial Disclosure Statement: The authors do not have any conflicts to disclose.
*Corporate Members
REFERENCES
- 1.Peniket AJ, Ruiz de Elvira MC, Taghipour G, Cordonnier C, Gluckman E, de Witte T, et al. An EBMT registry matched study of allogeneic stem cell transplants for lymphoma: Allogeneic transplantation is associated with a lower relapse rate but a higher procedure-related mortality rate than autologous transplantation. Bone Marrow Transplant. 2003;31:667–678. doi: 10.1038/sj.bmt.1703891. [DOI] [PubMed] [Google Scholar]
- 2.Ratanatharathorn V, Uberti J, Karanes C, Abella E, Lum LG, Momin F, et al. Prospective comparative trial of autologous versus allogeneic bone marrow transplantation in patients with non-hodgkin's lymphoma. Blood. 1994;84:1050–1055. [PubMed] [Google Scholar]
- 3.van Besien K, Carreras J, Bierman PJ, Logan BR, Molina A, King R, et al. Unrelated donor hematopoietic cell transplantation for non-hodgkin lymphoma: Long-term outcomes. Biol Blood Marrow Transplant. 2009;15:554–563. doi: 10.1016/j.bbmt.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lazarus HM, Zhang MJ, Carreras J, Hayes-Lattin BM, Ataergin AS, Bitran JD, et al. A comparison of HLA-identical sibling allogeneic versus autologous transplantation for diffuse large B cell lymphoma: A report from the CIBMTR. Biol Blood Marrow Transplant. 2010;16:35–45. doi: 10.1016/j.bbmt.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khouri IF, McLaughlin P, Saliba RM, Hosing C, Korbling M, Lee MS, et al. Eight-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab. Blood. 2008;111:5530–5536. doi: 10.1182/blood-2008-01-136242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khouri IF, Saliba RM, Giralt SA, Lee MS, Okoroji GJ, Hagemeister FB, et al. Nonablative allogeneic hematopoietic transplantation as adoptive immunotherapy for indolent lymphoma: Low incidence of toxicity, acute graft-versus-host disease, and treatment-related mortality. Blood. 2001;98:3595–3599. doi: 10.1182/blood.v98.13.3595. [DOI] [PubMed] [Google Scholar]
- 7.Tomblyn M, Brunstein C, Burns LJ, Miller JS, MacMillian M, DeFor TE, et al. Similar and promising outcomes in lymphoma patients treated with myeloablative or nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14:538–545. doi: 10.1016/j.bbmt.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hale GA, Shrestha S, Le-Rademacher J, Burns LJ, Gibson J, Inwards DJ, et al. Alternate donor hematopoietic cell transplantation (HCT) in non-hodgkin lymphoma using lower intensity conditioning: A report from the CIBMTR. Biol Blood Marrow Transplant. 2012;18:1036–1043. doi: 10.1016/j.bbmt.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodrigues CA, Rocha V, Dreger P, Brunstein C, Sengeloev H, Finke J, et al. Alternative donor hematopoietic stem cell transplantation for mature lymphoid malignancies after reduced-intensity conditioning regimen: Similar outcomes with umbilical cord blood and unrelated donor peripheral blood. Haematologica. 2014;99:370–377. doi: 10.3324/haematol.2013.088997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sureda A, Robinson S, Canals C, Carella AM, Boogaerts MA, Caballero D, et al. Reduced-intensity conditioning compared with conventional allogeneic stem-cell transplantation in relapsed or refractory hodgkin's lymphoma: An analysis from the lymphoma working party of the european group for blood and marrow transplantation. J Clin Oncol. 2008;26:455–462. doi: 10.1200/JCO.2007.13.2415. [DOI] [PubMed] [Google Scholar]
- 11.Anasetti C, Aversa F, Brunstein CG. Back to the future: Mismatched unrelated donor, haploidentical related donor, or unrelated umbilical cord blood transplantation? Biol Blood Marrow Transplant. 2012;18:S161–5. doi: 10.1016/j.bbmt.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Brunstein CG, Laughlin MJ. Extending cord blood transplant to adults: Dealing with problems and results overall. Semin Hematol. 2010;47:86–96. doi: 10.1053/j.seminhematol.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Lee SJ, Klein J, Haagenson M, Baxter-Lowe LA, Confer DL, Eapen M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 14.Ballen KK, Klein JP, Pedersen TL, Bhatla D, Duerst R, Kurtzberg J, et al. Relationship of race/ethnicity and survival after single umbilical cord blood transplantation for adults and children with leukemia and myelodysplastic syndromes. Biol Blood Marrow Transplant. 2012;18:903–912. doi: 10.1016/j.bbmt.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunstein CG, Cantero S, Cao Q, Majhail N, McClune B, Burns LJ, et al. Promising progression-free survival for patients low and intermediate grade lymphoid malignancies after nonmyeloablative umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2009;15:214–222. doi: 10.1016/j.bbmt.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majhail NS, Weisdorf DJ, Wagner JE, Defor TE, Brunstein CG, Burns LJ. Comparable results of umbilical cord blood and HLA-matched sibling donor hematopoietic stem cell transplantation after reduced-intensity preparative regimen for advanced hodgkin lymphoma. Blood. 2006;107:3804–3807. doi: 10.1182/blood-2005-09-3827. [DOI] [PubMed] [Google Scholar]
- 17.Marcais A, Porcher R, Robin M, Mohty M, Michalet M, Blaise D, et al. Impact of disease status and stem cell source impact on the results of reduced intensity conditioning transplant for hodgkin lymphoma: A retrospective study from the french society of bone marrow graft transplantation and cellular therapy. Haematologica. 2013;98:1467–1475. doi: 10.3324/haematol.2012.080895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cutler C, Stevenson K, Kim HT, Brown J, McDonough S, Herrera M, et al. Double umbilical cord blood transplantation with reduced intensity conditioning and sirolimus-based GVHD prophylaxis. Bone Marrow Transplant. 2011;46:659–667. doi: 10.1038/bmt.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devetten MP, Hari PN, Carreras J, Logan BR, van Besien K, Bredeson CN, et al. Unrelated donor reduced-intensity allogeneic hematopoietic stem cell transplantation for relapsed and refractory hodgkin lymphoma. Biol Blood Marrow Transplant. 2009;15:109–117. doi: 10.1016/j.bbmt.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avivi I, Canals C, Vernant JP, Wulf G, Nagler A, Hermine O, et al. Matched unrelated donor allogeneic transplantation provides comparable long-term outcome to HLA-identical sibling transplantation in relapsed diffuse large B-cell lymphoma. Bone Marrow Transplant. 2014;49:671–678. doi: 10.1038/bmt.2014.4. [DOI] [PubMed] [Google Scholar]
- 21.Spellman S, Setterholm M, Maiers M. Advances in the selection of HLA-compatible donors: refinements in HLA typing and matching over the first 20 years of the National Marrow Donor Program Registry. Biol Blood Marrow Transplant. 2008;14(9-Suppl):37–44. doi: 10.1016/j.bbmt.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-intensity conditioning regimen workshop: Defining the dose spectrum. report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15:367–369. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204–17. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 24.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–8. [PubMed] [Google Scholar]
- 25.Zhang X, Loberiza FR, Jr., Klein J, Zhang MJ. A SAS Macro For Estimation Of Direct Adjusted Survival Curves Based On A Stratified Cox Regression Model. Computer Methods and Programs in Biomedicine. 2007;88:95–101. doi: 10.1016/j.cmpb.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Loberiza FR, Klein JP, Zhang MJ. SAS macros for estimation of direct adjusted cumulative incidence curves under proportional subdistribution hazards models. Computer Methods and Programs in Biomedicine. 2011;101(1):87–93. doi: 10.1016/j.cmpb.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein JP, Moeschberger ML. Survival Analysis: Techniques for Censored and Truncated Data. 2nd Edition Springer Verlag; New York: 2003. [Google Scholar]
- 28.Hale GA, Shrestha S, Le-Rademacher J, Burns LJ, Gibson J, Inwards DJ, et al. Alternate donor hematopoietic cell transplantation (HCT) in non-hodgkin lymphoma using lower intensity conditioning: A report from the CIBMTR. Biol Blood Marrow Transplant. 2012;18:1036–1043. e1. doi: 10.1016/j.bbmt.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson SP, Canals C, Luang JJ, Tilly H, Crawley C, Cahn JY, et al. The outcome of reduced intensity allogeneic stem cell transplantation and autologous stem cell transplantation when performed as a first transplant strategy in relapsed follicular lymphoma: An analysis from the lymphoma working party of the EBMT. Bone Marrow Transplant. 2013;48:1409–1411. doi: 10.1038/bmt.2013.83. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez-Viña MA, Wang T, Lee SJ, Haagenson M, Aljurf M, Askar M, et al. Identification of a permissible HLA mismatch in hematopoietic stem cell transplantation. Blood. 2014;123(8):1270–1278. doi: 10.1182/blood-2013-10-532671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flomenberg N, Baxter-Lowe LA, Confer D, Fernandez-Vina M, Filipovich A, Horowitz M, et al. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. 2004;104(7):1923–1930. doi: 10.1182/blood-2004-03-0803. [DOI] [PubMed] [Google Scholar]
- 32.Pidala J, Wang T, Haagenson M, Spellman SR, Askar M, Battiwalla M, et al. Amino acid substitution at peptide-binding pockets of HLA class I molecules increases risk of severe acute GVHD and mortality. Blood. 2013;122(22):3651–3658. doi: 10.1182/blood-2013-05-501510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spellman SR, Eapen M, Logan BR, Mueller C, Rubinstein P, Setterholm MI, et al. A perspective on the selection of unrelated donors and cord blood units for transplantation. Blood. 2012;120(2):259–65. doi: 10.1182/blood-2012-03-379032. [DOI] [PMC free article] [PubMed] [Google Scholar]