Abstract
Two-color fluorescent in situ hybridization (FISH) is a widely used technique to compare relative gene expression patterns. Current two-color FISH protocols are not ideal for detecting weakly expressed transcripts or monitoring signal strength and background levels during the course of the reaction. Here, we describe an improved fluorescent in situ hybridization (FISH) protocol using the conventional highly sensitive chromogenic substrates NBT/BCIP and Vector Red in zebrafish embryos. This protocol substantially improves on existing FISH techniques by combining the advantages of long reactivity of alkaline phosphatase, chromogenic monitoring of both developing reactions, and the ability to perform subsequent high-resolution fluorescent imaging. While tested in zebrafish, a similar approach is expected to be applicable to ISH in any model organism.
Keywords: fluorescent in situ hybridization, FISH, two color, NBT, BCIP, Vector Red, zebrafish, confocal
In situ hybridization (ISH) is the method of choice for visualizing the distribution of transcripts in developing embryos. Simultaneous detection of two transcripts is commonly used to determine overlap in expression domains. Two-color ISH protocols have been reported in various organisms using simultaneous hybridization with digoxygenin (DIG)- and fluorescein (FL)-labeled probes, followed by sequential visualization with alkaline phosphatase (AP) chromogenic substrates including NBT/BCIP and Fast Red (1,2). The major advantage of chromogenic reactions is the ability to monitor the AP reaction to control signal strength and background levels. Furthermore, the AP reaction has a long productivity time to help detect weakly expressed transcripts. Nitro blue tetrazolium (NBT)/5-bromo-4-chloro-3-indolyl phosphate (BCIP), which produces a blue-purple precipitate is generally the substrate of choice for chromogenic ISH due to the strong signal and low background levels the reaction generates. However, with two-color ISH, the darker NBT/BCIP substrate often masks the lighter Fast Red substrate, making it difficult to determine if transcripts are co-expressed in the same cell.
To address this problem, multiplex FISH techniques that rely on horseradish peroxidase (POD) detection followed by sequential tyramide signal amplification (TSA) visualization have been developed (3-5). While these protocols allow for high-resolution imaging to examine overlapping expression in single cells, they do not allow for monitoring of the POD-TSA developing steps. Also, the tyramide substrate is fluorescent on its own and extensive washing is required to visualize signal. Additionally, because the enzymatic reaction lasts for only a short time, detecting weakly expressed transcripts can be challenging. A two-color FISH protocol for combined AP-Fast Blue and POD-TSA was recently described, however this protocol still requires one probe to be visualized without monitoring the developing step (6). We report here a two-color FISH protocol in zebrafish embryos that takes advantage of the fluorescent properties of the NBT/BCIP and Vector Red substrates. These substrates were chosen based on non-overlapping emission wavelengths; NBT/BCIP fluoresces in the near-infrared range (7), and Vector Red is visible with Texas Red or rhodamine filter sets, similar to Fast Red (6). Importantly, this protocol combines the advantages of long AP reactivity, chromogenic monitoring of both developing reactions, and the ability to perform subsequent high-resolution fluorescent imaging.
20 somite stage zebrafish embryos were fixed in 4% paraformaldehyde following standard procedures (8). They were dehydrated through an ethanol (EtOH) series and stored at -20°C. On day one, embryos were rehydrated, washed 3 times in PBT (1X PBS (3.7mM NaCl, 0.27mM KCl, 0.43mM Na2HPO4, 0.14mM KH2PO4) with 0.2% bovine serum albumin, 0.2% Tween 20), then incubated in prehybridization buffer (50% Formamide, 5X SSC (for 20X SSC stock: 3M NaCl, 0.3M sodium citrate, pH to 7.0), 50μg/ml heparin, 5mM EDTA, 0.5mg/ml torula yeast RNA, 0.1% Tween 20, pH 6.0 with citric acid) for 2 hours at 65°C. Embryos were incubated in probes diluted in prehybridization buffer at 65°C overnight. Two probes were applied simultaneously, and ideally the stronger probe is labeled with FL and the weaker probe with DIG. We tested the combination of myeloperoxidase (mpx)-DIG, a relatively weak probe which labels neutrophilic granulocytes and also exhibits variable expression levels in the inner cell mass (ICM) of zebrafish embryos (9), and either gata1-FL or alpha embryonic hemoglobin (hbae3)-FL, both of which are expressed in erythrocytes in the ICM (10,11). Both anti-DIG-AP and anti-FL-AP (Roche) were preabsorbed with acetone powder as previously described (8).
On day 2, embryos were washed with the following series of buffer washes at 65°C: 75%, 50%, and 25% prehybridization buffer diluted in 2X SSC for 15 minutes each, 2X SSC for 15 minutes, then twice in 0.2X SSC for 30 minutes each. Embryos were washed in a dilution series of 0.2X SSC:PBT (3:1, 1:1, 1:3, PBT) for 5 minutes each at room temperature. During the washes, the 10X anti-DIG-AP was spun down and the supernatant was diluted to 1X in 2% lamb serum in PBT. Embryos were incubated overnight at 4°C in anti-DIG-AP. We performed the DIG-AP reaction first, because the first AP reaction has higher sensitivity and provides more sensitive detection of the weaker probe.
On day 3, embryos were washed 6 times in PBT, once in AP buffer (100mM Tris pH 9.5, 100mM NaCl, 50mM MgCl2, 0.10% Tween 20) and developed in 400 μl of developing buffer (4.5 μl of 50 mg/ml NBT, 3.5 μl of 50 mg/ml BCIP per 1 ml of AP buffer). Once desired signal intensity was reached, the reaction was stopped with several PBT washes. The anti-DIG-AP was inactivated by fixing in 4% PFA for one hour at room temperature. Embryos were similarly processed for anti-FL-AP, except that AP buffer was replaced with 0.2M Tris pH 8.5 with 0.1% Tween 20. The FL probe was developed using Vector Red substrate (Vector Labs) according to manufacturer's instructions.
Dehydrating embryos in EtOH overnight after developing reduced background during fluorescent imaging, particularly for the NBT/BCIP substrate. Imaging is ideally performed within a few days after processing for the highest signal-to-noise ratio. Embryos were mounted in 0.6% low melting point agarose overlaid with embryo media. Confocal images were acquired using a Nikon A1R si STORM inverted microscope equipped with an Apo LWD 40×/1.15 NA microscope objective (Nikon Instruments Inc., USA). NBT/BCIP fluorescence was excited using a 647 nm diode laser set to 100 mW intensity and detected with a 740 nm long pass filter then imaged with a high-quantum efficiency (QE) bi-alkali photomultiplier tube with 10% QE at 800nm, similar to previously reported (7). Vector Red fluorescence was imaged with an excitation wavelength of 561 nm and detected with a 595/50 emission filter.
Volume reconstructions of confocal Z-stacks show that the expression domains of mpx clearly overlaps with both gata1 and hbae3 (Fig. 1A, B). In confocal sections, individual cells expressing mpx, gata1 or hbae3 could be clearly identified in the ICM region, with very little background fluorescence (Fig. 1C-F). There was definitive overlap of mpx and gata1 expression in the majority of mpx-expressing cells (Fig. 1C, E, G) as previously reported (12). Some cells had strong expression of mpx and weaker expression of gata1, but with the ability to monitor both developing reactions, following by fluorescent imaging, clear overlap can be seen. Only a subset of hbae3-positive cells also express mpx (Fig. 1D, F, H), indicating Vector Red is not being detected by the NBT/BCIP filter.
Figure 1. Fluorescent imaging of NBT/BCIP and Vector Red ISH.
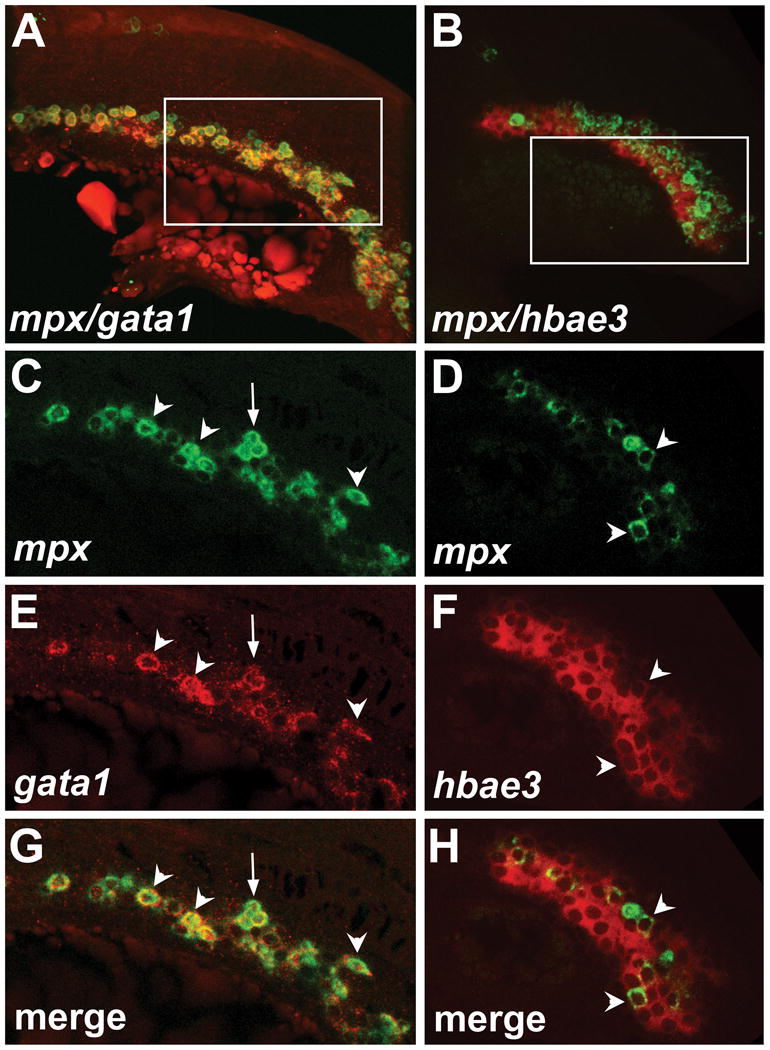
(A) Volume reconstruction of mpx (green) and gata1 (red) expression. (B) Volume reconstruction of mpx (green) and hbae3 (red) expression (C, D) mpx transcript localization in the ICM visualized by NBT/BCIP. (E) gata1 transcript localization in erythrocyte precursors visualized by Vector Red. (F) hbae3 transcript localization in erythrocytes visualized by Vector Red. (G) Merged channels show overlapping expression of mpx and gata1 in the majority of cells. (H) Merged channels show overlapping expression of mpx and hbae3 in a subset of hbae3-positive cells. Volume reconstructions were performed using Imaris software (Bitplane). Images in (C-H) are single confocal slices. Arrowheads indicate cells with expression of both transcripts. Arrows indicate cells with lower expression of gata1. All embryos are at the 20 somite stage, anterior to the left.
In conclusion, this FISH protocol allows researchers to examine the distribution and overlap of two gene transcripts using the highly sensitive AP substrates NBT/BCIP and Vector Red. The ability to monitor both developing reactions and higher sensitivity of NBT/BCIP and Vector Red substrates will substantially improve the ability to visualize weak or variably expressed genes.
Method Summary.
We describe an improved fluorescent in situ hybridization (FISH) protocol using the conventional highly sensitive chromogenic substrates NBT/BCIP and Vector Red in zebrafish embryos. This protocol substantially improves on existing FISH techniques by combining the advantages of long reactivity of alkaline phosphatase, chromogenic monitoring of both developing reactions, and the ability to perform subsequent high-resolution fluorescent imaging.
Acknowledgments
Research was supported by Cincinnati Children's Research Foundation and NIH R01 HL107369 award to S.S. J.A. Schumacher was supported by NIH T32HL00738 fellowship and E.J. Zhao was supported by AHA-Great Rivers Affiliate Summer Undergraduate Research Fellowship 12UFEL9990000.
Footnotes
Author contributions: J.A.S and E.J.Z performed experiments, J.A.S., E.J.Z. and S.S analyzed data, M.J.K. assisted with confocal imaging, J.A.S and S.S prepared the manuscript.
Competing interests: The authors declare no competing interests.
References
- 1.Jowett T, Lettice L. Whole-mount in situ hybridizations on zebrafish embryos using a mixture of digoxigenin- and fluorescein-labelled probes. Trends Genet. 1994;10:73–74. doi: 10.1016/0168-9525(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 2.Hauptmann G, Gerster T. Two-color whole-mount in situ hybridization to vertebrate and Drosophila embryos. Trends Genet. 1994;10:266. doi: 10.1016/0168-9525(90)90008-t. [DOI] [PubMed] [Google Scholar]
- 3.Brend T, Holley SA. Zebrafish whole mount high-resolution double fluorescent in situ hybridization. J Vis Expt. 2009;25:e1229. doi: 10.3791/1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clay H, Ramakrishnan L. Multiplex fluorescent in situ hybridization in zebrafish embryos using tyramide signal amplification. Zebrafish. 2005;2:105–111. doi: 10.1089/zeb.2005.2.105. [DOI] [PubMed] [Google Scholar]
- 5.Kosman D, Mizutani CM, Lemons D, Cox WG, McGinnis W, Bier E. Multiplex detection of RNA expression in Drosophila embryos. Science. 2004;305:846. doi: 10.1126/science.1099247. [DOI] [PubMed] [Google Scholar]
- 6.Lauter G, Soll I, Hauptmann G. Two-color fluorescent in situ hybridization in the embryonic zebrafish brain using differential detection systems. BMC Dev Biol. 2011;11:43. doi: 10.1186/1471-213X-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trinh le A, McCutchen MD, Bonner-Fraser M, Fraser SE, Bumm LA, McCauley DW. Fluorescent in situ hybridization employing the conventional NBT/BCIP chromogenic stain. BioTechniques. 2007;42:756–759. doi: 10.2144/000112476. [DOI] [PubMed] [Google Scholar]
- 8.Jowett T. Double in situ hybridization techniques in zebrafish. Methods. 2001;23:345–358. doi: 10.1006/meth.2000.1147. [DOI] [PubMed] [Google Scholar]
- 9.Bennett CM, Kanki JP, Rhodes J, Liu TX, Paw BH, Kieran MW, Langenau DM, Delahaye-Brown A, et al. Myelopoiesis in the zebrafish, Danio rerio. Blood. 2001;98:643–651. doi: 10.1182/blood.v98.3.643. [DOI] [PubMed] [Google Scholar]
- 10.Detrich HW, 3rd, Kieran MW, Chan FY, Barone LM, Yee K, Rundstadler JA, Pratt S, Ransom D, Zon LI. Intraembryonic hematopoietic cell migration during vertebrate development. Proc Natl Acad Sci USA. 1995;92:10713–10717. doi: 10.1073/pnas.92.23.10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brownlie A, Hersey C, Oates AC, Paw BH, Falick AM, Witkowska HE, Flint J, Higgs D, et al. Characterization of embryonic globin genes of the zebrafish. Dev Biol. 2003;255:48–61. doi: 10.1016/s0012-1606(02)00041-6. [DOI] [PubMed] [Google Scholar]
- 12.Glenn NO, Schumacher JA, Kim HJ, Zhao EJ, Skerniskyte J, Sumanas S. Distinct regulation of the anterior and posterior myeloperoxidase expression by Etv2 and Gata1 during primitive Granulopoiesis in zebrafish. Dev Biol. 2014;393:149–159. doi: 10.1016/j.ydbio.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


