Abstract
Many patients with angina and signs of myocardial ischemia on stress testing have no significant obstructive epicardial coronary disease. There are many potential coronary and non-coronary mechanisms for ischemia without obstructive epicardial coronary disease, and prominent among these is coronary microvascular and/or endothelial dysfunction. Patients with coronary microvascular and/or endothelial dysfunction are often at increased risk of adverse cardiovascular events, including ischemic events and heart failure despite preserved ventricular systolic function. In this article, we will review the diagnosis and treatment of coronary microvascular and endothelial dysfunction, discuss their potential contribution to heart failure with preserved ejection fraction, and highlight recent advances in the evaluation of atherosclerotic morphology in these patients, many of whom have non-obstructive epicardial disease.
Introduction: Microvascular Coronary Dysfunction and Ischemia Heart Disease
It is established that a mismatch between myocardial substrate supply and demand is the proximate mechanism responsible for myocardial ischemia. Based upon pathologic observations, clinicians initially thought that symptoms (eg, angina pectoris) and signs (eg, transient ST segment shifts, perfusion abnormalities, and/or wall motion abnormalities) of myocardial ischemia required a flow-limiting epicardial coronary stenosis. Although it was generally accepted that an occasional patient with severe aortic stenosis, severe hypertension, hypertrophic cardiomyopathy, and some other disorders (thyrotoxicosis, severe anemia, amyloidosis, Anderson Fabry Disease, etc.) could have such findings without a flow-limiting coronary stenosis, these were very infrequent cases. In 1967, Likoff et al. described a cohort of patients with angina pectoris and electrocardiographic abnormalities of myocardial ischemia but normal-appearing epicardial coronaries on angiography, and first suggested a possible coronary microvascular disorder.1 In a 1973 editorial, Kemp first used the term “Syndrome X” when commenting on group X in a study of patients with angina and normal coronary angiograms.2 In the years to follow, important work accrued to demonstrate that dysfunction of the microvasculature likely contributed to signs and symptoms of myocardial ischemia in many such patients with angina who did not have obstructive epicardial coronary stenosis, and the term “microvascular angina” was recommended by Cannon and Epstein.3 While the ability of abnormal microvascular function to contribute to myocardial ischemia was long debated, microvacular disease leading to tissue injury has long been an accepted mechanism in other organ systems. For example, microvascular disease in the kidney caused by hypertension and diabetes has been shown to contribute to glomerular injury and nephrosclerosis4 as well as retina injury. It has become clear that there is no consensus in the literature regarding the definition of cardiac syndrome X5, so we strongly recommend abandoning the use of the term.
In the current era, patients with symptoms and signs of ischemia, referred for invasive coronary evaluation, increasingly appear without obstructive epicardial coronary artery disease (CAD).6–8 Symptomatic patients with non-obstructive CAD have an elevated risk of adverse outcomes compared with cohorts without symptoms and/or signs of ischemic heart disease.7 These individuals consume medical resources rivaling those for patients with obstructive CAD.9 At least half of such patients have quantifiable coronary vascular dysregulation (endothelial and/or non-endothelial dependent macro- or microvascular dysfunction) capable of causing ischemia with provocative testing.6,10 This is now usually referred to as microvascular coronary dysfunction (MCD). MCD is increasingly recognized as an essential component in the spectrum of ischemic heart disease, particularly its prognosis.11–14 (Figure 1)
Figure 1.
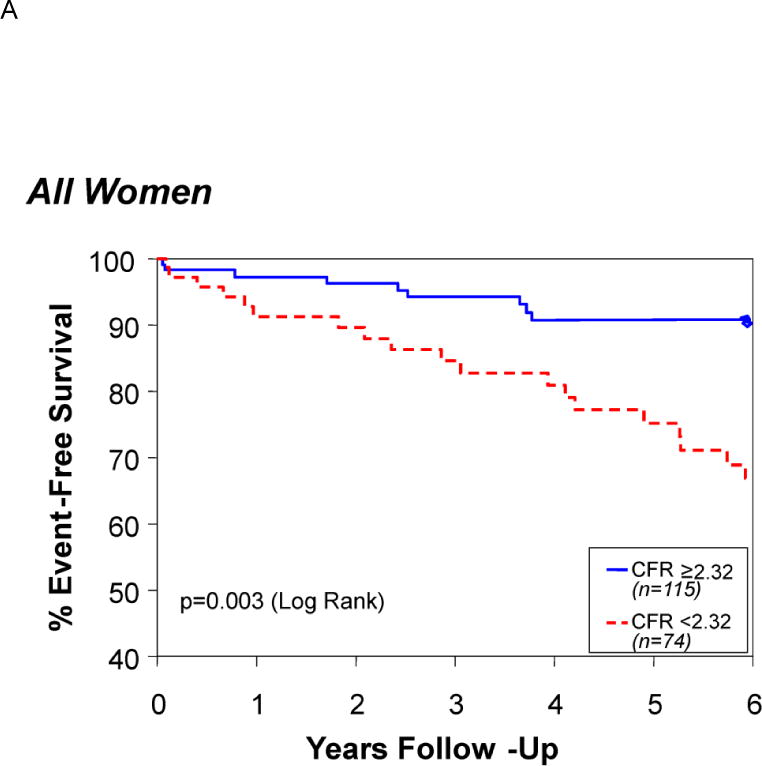
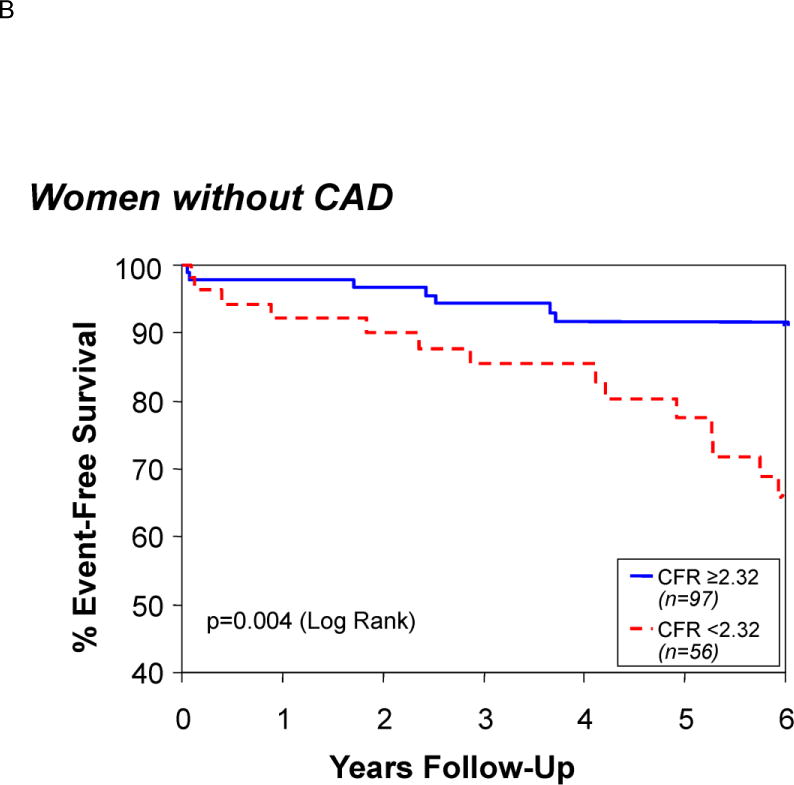
Survival free of death, myocardial infarction, stroke, or heart failure hospitalization in those patients with a coronary flow reserve above or below a receiver operating curve–determined optimal cut-off value of 2.32. Reprinted with permission from Pepine et al. J Am Coll Cardiol. 2010;55:2825–2832.11
Compared with healthy patients, patients with MCD more frequently have traditional risk factors for coronary disease, including hypertension and diabetes.15 However, in patients with angina and no obstructive epicardial coronary disease the prevalence of these traditional risk factors is not much different comparing those with or without quantifiable MCD.10,11 It is also likely that angiography referral-bias, related to the selection of patients thought to have high atherosclerosis, contributes to this risk factor prevalence. Additionally, MCD was long thought to be a disorder of women, but more recent reports indicate that the prevalence of MCD may be as high in men.14
Diagnosis of Microvascular Coronary Dysfunction
Many invasive and non-invasive techniques are available for assessment of MCD. Although not visualized by standard angiography, the coronary microcirculation may be indirectly assessed from the speed of radiographic contrast material movement through the coronary artery. This measure can be quantified as the TIMI frame count. This simple, objective, continuous contrast “transit-time” index is accurate, reproducible, highly correlated with Doppler blood flow measurements, and provides information for risk stratification.16 (Figure 2) The microcirculation can be directly assessed, in the absence of flow-limiting stenoses upstream, by measuring coronary flow reserve (CFR). The CFR can be invasively determined by a Doppler-tipped guide wire in a coronary artery and measuring blood flow velocity at baseline and after inducing hyperemia with a vasoactive agent. Typically the non–endothelium-dependent microvascular dilator adenosine (or regadenoson) is given by intracoronary or intravenous infusion. Dipyridamole may also be used intravenously to inhibit phosphodiesterases that break down cAMP (increasing cellular cAMP levels) and also block cellular reuptake of adenosine with increase in extracellular adenosine concentration.
Figure 2.
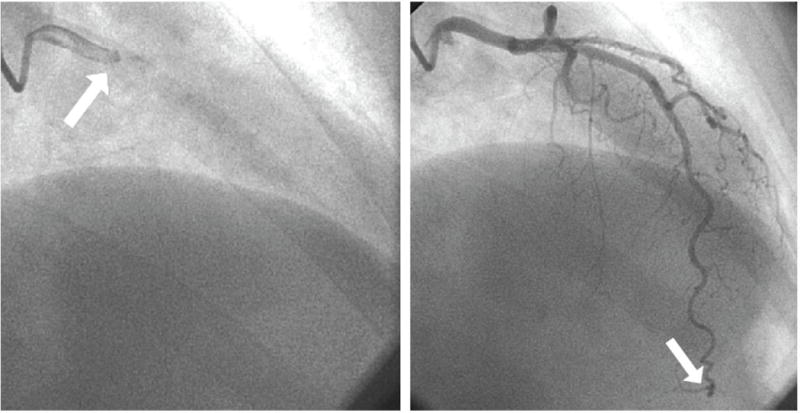
TIMI Frame Count: The first frame used to determine the TIMI Frame Count is the frame in which dye fully enters the artery of interest (left, arrow). The last frame that is counted is the frame when dye enters the distal landmark branch (right, arrow). Reprinted with permission from Petersen et al. PLoS One. 2014;9:e96630.16
The coronary microcirculatory response to adenosine is typically considered abnormal if the blood flow increase is less than 2.5 times that at baseline. We have shown that blood flow velocity, in women with suspected MCD, closely approximates volumetric flow, likely due to the fact that most of these patients also have endothelial dysfunction.17 Using velocity alone to determine the coronary flow velocity reserve (CFVR) simplifies the method since the cross-sectional area of the vessel, necessary for calculation of volumetric flow, is not required.
Fractional flow reserve (FFR) has emerged as an invasive method to measure the functional significance of obstructive epicardial disease. It is critically important to recognize that the presence of MCD may obscure information on functional epicardial stenosis severity derived from coronary pressure measurements (eg, FFR) alone. Recently, others have noted discordance between CFVR and FFR in a large proportion of cases.18 Patients with normal FFR but abnormal CFVR had significantly higher rates of adverse cardiovascular events compared with those patients who had a normal FFR and normal CFVR. Therefore, considering evaluation of microvascular function remains important even after invasive measures suggest no evidence of impaired flow in the epicardial coronary.
Invasive techniques are also used to evaluate endothelial-dependent mechanisms of vascular dysregulation. Intracoronary acetylcholine is commonly given to evaluate for lack of dilation or even paradoxical epicardial coronary constriction indicative of epicardial coronary endothelial dysfunction.12 When epicardial diameter reduction is excessive (usually defined as a reduction in post nitroglycerin coronary diameter of >70%) and associated with signs and/or symptoms of ischemia, epicardial spasm is considered present. More recently, higher doses of intracoronary acetylcholine have been used to evaluate for evidence of microvascular coronary spasm. The latter is suggested when ischemia, such as angina with abnormal ECG or LV wall motion, is precipitated in the absence of evidence for epicardial spasm.6
Non-invasive methods, such as positron emission tomography (PET), Doppler echocardiography, and gadolinium perfusion cardiac magnetic resonance imaging (MRI), are also increasingly being used to evaluate for the presence of MCD. Advanced, but well validated, techniques are available with PET to determine absolute myocardial blood flow reserve/gram of LV muscle.14 Transthoracic Doppler echocardiography provides assessment of coronary blood flow velocity in the left anterior descending (LAD) coronary that can be used to determine CFR after hyperemia. Doppler echocardiography–derived measures of CFR have been shown to correlate significantly with invasive measures of CFR.19 In contrast with PET, Doppler echocardiography does not require radiation exposure and is available at most centers. A limitation of transthoracic Doppler echocardiography–determined CFR is the feasibility of detecting LAD flow. Studies have reported that as few as 34% and as many as 96% of patients included in various cohorts have had successful evaluation of LAD flow.20 Contrast agents can enhance the Doppler signal and have led to improvement in measuring LAD flow responses.19 Finally, cardiac MRI is being used to record the first pass of intravenous gadolinium through the circulation. Given the high spatial resolution of cardiac MRI, relative perfusion is able to be determined in all layers of all of the ventricular segments and expressed as myocardial perfusion index. Previous work has shown a decrease in subendocardial perfusion in patients with suspected microvascular angina.21
Myocardial Ischemia and Ventricular Dysfunction
Many have questioned if the angina present in patients with MCD is due to true myocardial ischemia. Numerous studies have proven that objective evidence for myocardial ischemia is present in patients with angina who have no obstructive epicardial coronary disease.22 It remains unclear if the ischemia present in patients with MCD is caused strictly from isolated MCD or if other mechanisms contribute to simultaneously impair the myocardial substrate supply-demand relationship. Multiple mechanisms are likely to coexist in the same patent. For example, abnormal systemic vascular function is also known to contribute to impaired myocardial perfusion. Nichols et al. demonstrated that older women with angina and no obstructive epicardial CAD had findings suggestive of stiff central vessels with an associated increase in determinants of LV afterload.23 This would be expected to increase LV demand and could accentuate ischemia in patients who have MCD and an exhausted microvascular reserve.
Most patients with MCD have normal global measures of ventricular systolic function. However, diastolic dysfunction is the earliest functional abnormality documented in patients with ischemia caused by both obstructive coronary stenosis and in those patients with ischemia unrelated to obstructive stenosis.24 Previous work has demonstrated a high incidence of left ventricular diastolic dysfunction in patients with endothelial dysfunction.25 Therefore, it is not surprising that ventricular diastolic dysfunction and heart failure with preserved ejection fraction (HFpEF) is common in patients with MCD.14,26 Interestingly, myocardial perfusion has also been shown to be abnormal in patients with stress-induced cardiomyopathy who have no evidence of significant epicardial coronary obstruction.27
Treatment of Microvascular Coronary Dysfunction
Unfortunately, because of lack of evidence-based results of treatment on patient outcomes, management of symptomatic patients with MCD is often frustrating for the patients and their physicians. Standard anti-angina therapy is considered initially. Short acting nitrate therapy has been reported to relieve symptoms in only about half of patients with symptomatic MCD.28 Maintenance therapy for angina commonly includes β-blockers and calcium antagonists. β-blockers are particularly considered for patients with evidence of a high sympathetic tone, such as a high resting heart rate, and in a small study were superior to both nitrates and calcium antagonists in the control of angina symptoms.29
More novel anti-angina therapies also offer hope for the treatment of patients with MCD. Ivabradine specifically lowers heart rate by blocking the If current of the sinoatrial node. In patients with stable CAD, treatment with ivabradine has led to improved CFR, even after controlling for heart rate.30 In a study of 46 patients with normal coronary angiograms and MCD, treatment with ivabradine led to improvements in reported angina severity.31 Ranolazine is another newer anti-angina therapy that may help patients with symptomatic MCD. The anti-ischemic mechanism of ranolazine is unknown, but some propose that ranolazine blocks detrimental calcium overload in ischemic myocytes by blocking the late INa channel.32 Ranolazine has been shown to improve symptoms in patients with significant epicardial coronary disease and angina.32 More recently, small studies have shown that in patients without obstructive CAD and angina, ranolazine has led to significant improvements in symptoms.33 Additionally, in patients with MCD, treatment with ranolazine led to more significant improvement in exercise duration without ischemia compared with treatment with ivabradine.31
In addition to anti-angina therapy, there are many other medications offered to patients with symptomatic MCD. Angiotensin converting enzyme (ACE) inhibitors stimulate the synthesis of nitric oxide which contributes significantly to vasodilation, improved vascular elasticity, and inhibition of vascular hypertrophy.34 Additionally, ACE inhibitors are known to reduce ventricular pressure and attenuate an increased sympathetic tone. For these reasons ACE inhibitors are commonly considered in the treatment of symptomatic MCD.35 We have shown that the ACE inhibitor quinapril improves CFR and angina symptoms assessed by the validated Seattle Angina Questionnaire over 16 weeks in patients with MCD.36 This improved angina was linked with improved CFR. Statins have also been shown to improve endothelial function and promote vasodilation, and are commonly considered for patients with MCD who also benefit from stabilization of non-obstructive atherosclerotic disease.37 Statins combined with ACE inhibitors may offer the most benefit.
Epicardial Coronary Disease in Patients with Microvascular Coronary Disease
As previously discussed, many patients with angina without epicardial obstructive atherosclerotic disease are shown to have abnormal microvascular dysfunction and endothelial dysfunction of the epicardial coronary arteries.6 However, the poor outcomes seen in these patients may also relate to their epicardial atherosclerotic disease as more recent investigation has highlighted the true burden of epicardial coronary atherosclerosis in these patients who do not have significant luminal obstruction. In 100 patients with angina and normal standard angiography, intra-vascular ultrasound (IVUS) revealed that approximately 80% had atherosclerosis in their epicardial coronaries.38 (Figure 3) These non-obstructive atherosclerotic plaques can certainly lead to unstable coronary events.39 In women presenting with myocardial infarction but no obstructive epicardial coronary disease, IVUS revealed that nearly 40% had disruption of non-obstructive coronary plaques that included plaque rupture and plaque ulceration.40 Therefore, aggressive intervention aimed at stabilization of these plaques, with statins for example, are likely important for prevention of future events.
Figure 3.
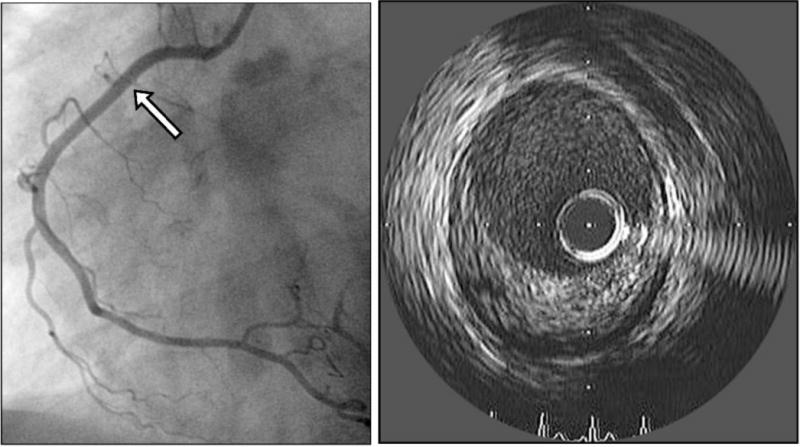
Patient with normal lumen area on right coronary angiography (left) but significant plaque of the right coronary artery seen on intravascular ultrasound (right).
Conclusions and Future Directions
Patients with angina but no significant obstructive epicardial coronary disease on standard coronary angiography are at increased risk of adverse cardiovascular events compared with people without angina. Many of these patients have myocardial ischemia caused by MCD and/or endothelial dysfunction of the epicardial coronaries that can be quantified using invasive and non-invasive methods.
Future effort is necessary to refine non-invasive methods for evaluation of microvascular and endothelial function. PET perfusion, MRI perfusion, CT perfusion, and contrast-enhanced Doppler echocardiography offer significant promise.
These patients have a high prevalence of LV diastolic dysfunction and heart failure that is likely caused by myocardial ischemia. Given the significant prevalence of HFpEF, appropriate identification and successful treatment of vascular dysfunction could have a significant impact on outcomes in patients with HFpEF.
Future study should define the prevalence of vascular dysfunction in patients with HFpEF, and should evaluate the response of ventricular function and clinical outcomes in patients treated for vascular dysfunction.
Treatment of MCD remains sub-optimal. Standard anti-angina therapy is appropriate, but more novel treatment offers promise.
Future study should continue to investigate the impact of novel anti-angina therapies, such as ivabradine and ranolazine, on clinical outcomes in patients with MCD.
Additionally, IVUS has revealed that while these patients do not have obstructive lesions in their epicardial coronaries, they do have a high burden of epicardial atherosclerosis that can become unstable leading to acute coronary events.
Future study should utilize invasive and non-invasive techniques to establish the burden and morphology of atherosclerosis in patients with angina and “normal” coronary angiograms. Future study will need to evaluate the improvement in clinical outcomes in patients who are treated with medications aimed at stabilization of non-obstructive atherosclerotic disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures
None.
References
- 1.Likoff W, Segal BL, Kasparian H. Paradox of normal selective coronary arteriograms in patients considered to have unmistakable coronary heart disease. N Engl J Med. 1967;276:1063–6. doi: 10.1056/NEJM196705112761904. [DOI] [PubMed] [Google Scholar]
- 2.Kemp HG. Left ventricular function in patients with the anginal syndrome and normal coronary arteriograms. Am J Cardiol. 1973;32:375–6. doi: 10.1016/s0002-9149(73)80150-x. [DOI] [PubMed] [Google Scholar]
- 3.Cannon RO, Epstein SE. “Microvascular angina” as a cause of chest pain with angiographically normal coronary arteries. Am J Cardiol. 1988;61:1338–43. doi: 10.1016/0002-9149(88)91180-0. [DOI] [PubMed] [Google Scholar]
- 4.Luke RG. Hypertensive nephrosclerosis: pathogenesis and prevalence. Essential hypertension is an important cause of end-stage renal disease. Nephrol Dial Transplant. 1999;14:2271–8. doi: 10.1093/ndt/14.10.2271. [DOI] [PubMed] [Google Scholar]
- 5.Vermeltfoort IA, Raijmakers PG, Riphagen II, Odekerken DA, Kuijper AF, Zwijnenburg A, et al. Definitions and incidence of cardiac syndrome X: review and analysis of clinical data. Clin Res Cardiol. 2010;99:475–81. doi: 10.1007/s00392-010-0159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ong P, Athanasiadis A, Borgulya G, Mahrholdt H, Kaski JC, Sechtem U. High Prevalence of a Pathological Response to Acetylcholine Testing in Patients With Stable Angina Pectoris and Unobstructed Coronary Arteries The ACOVA Study (Abnormal COronary VAsomotion in patients with stable angina and unobstructed coronary arteries) J Am Coll Cardiol. 2012;59:655–62. doi: 10.1016/j.jacc.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 7.Jespersen L, Hvelplund A, Abildstrøm SZ, Pedersen F, Galatius S, Madsen JK, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33:734–44. doi: 10.1093/eurheartj/ehr331. [DOI] [PubMed] [Google Scholar]
- 8.Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS Focused Update of the Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease : A Report of the American College of Cardiology/American Heart Association. Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2014 Jul 28; doi: 10.1161/CIR.0000000000000095. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Shaw LJ, Merz CN, Pepine CJ, Reis SE, Bittner V, Kip KE, et al. The economic burden of angina in women with suspected ischemic heart disease: results from the National Institutes of Health–National Heart, Lung, and Blood Institute–sponsored Women’s Ischemia Syndrome Evaluation. Circulation. 2006;114:894–904. doi: 10.1161/CIRCULATIONAHA.105.609990. [DOI] [PubMed] [Google Scholar]
- 10.Reis SE, Holubkov R, Conrad Smith AJ, Kelsey SF, Sharaf BL, Reichek N, et al. WISE Investigators Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J. 2001;141:735–41. doi: 10.1067/mhj.2001.114198. [DOI] [PubMed] [Google Scholar]
- 11.Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55:2825–32. doi: 10.1016/j.jacc.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Mering GO, Arant CB, Wessel TR, McGorray SP, Bairey Merz CN, Sharaf BL, et al. National Heart, Lung, and Blood Institute Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109:722–5. doi: 10.1161/01.CIR.0000115525.92645.16. [DOI] [PubMed] [Google Scholar]
- 13.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–40. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 14.Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129:2518–27. doi: 10.1161/CIRCULATIONAHA.113.008507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merz CN, Kelsey SF, Pepine CJ, Reichek N, Reis SE, Rogers WJ, et al. The Women’s Ischemia Syndrome Evaluation (WISE) study: protocol design, methodology and feasibility report. J Am Coll Cardiol. 1999;33:1453–61. doi: 10.1016/s0735-1097(99)00082-0. [DOI] [PubMed] [Google Scholar]
- 16.Petersen JW, Johnson BD, Kip KE, Anderson RD, Handberg EM, Sharaf B, et al. TIMI frame count and adverse events in women with no obstructive coronary disease: a pilot study from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) PLoS One. 2014;9:e96630. doi: 10.1371/journal.pone.0096630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reis SE, Holubkov R, Lee JS, Sharaf B, Reichek N, Rogers WJ, et al. Coronary flow velocity response to adenosine characterizes coronary microvascular function in women with chest pain and no obstructive coronary disease. Results from the pilot phase of the Women’s Ischemia Syndrome Evaluation (WISE) study. J Am Coll Cardiol. 1999;33:1469–75. doi: 10.1016/s0735-1097(99)00072-8. [DOI] [PubMed] [Google Scholar]
- 18.van de Hoef TP, van Lavieren MA, Damman P, Delewi R, Piek MA, Chamuleau SA, et al. Physiological basis and long-term clinical outcome of discordance between fractional flow reserve and coronary flow velocity reserve in coronary stenoses of intermediate severity. Circ Cardiovasc Interv. 2014;7:301–11. doi: 10.1161/CIRCINTERVENTIONS.113.001049. [DOI] [PubMed] [Google Scholar]
- 19.Caiati C, Montaldo C, Zedda N, Montisci R, Ruscazio M, Lai G, et al. Validation of a new noninvasive method (contrast-enhanced transthoracic second harmonic echo Doppler) for the evaluation of coronary flow reserve: comparison with intracoronary Doppler flow wire. J Am Coll Cardiol. 1999;34:1193–200. doi: 10.1016/s0735-1097(99)00342-3. [DOI] [PubMed] [Google Scholar]
- 20.Dimitrow PP. Transthoracic Doppler echocardiography – noninvasive diagnostic window for coronary flow reserve assessment. Cardiovasc Ultrasound. 2003;1:4. doi: 10.1186/1476-7120-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panting JR, Gatehouse PD, Yang GZ, Grothues F, Firmin DN, Collins P, et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med. 2002;346:1948–53. doi: 10.1056/NEJMoa012369. [DOI] [PubMed] [Google Scholar]
- 22.Buchthal SD, den Hollander JA, Merz CN, Rogers WJ, Pepine CJ, Reichek N, et al. Abnormal myocardial phosphorus-31 nuclear magnetic resonance spectroscopy in women with chest pain but normal coronary angiograms. N Engl J Med. 2000;342:829–35. doi: 10.1056/NEJM200003233421201. [DOI] [PubMed] [Google Scholar]
- 23.Nichols WW, Denardo SJ, Johnson BD, Sharaf BL, Bairey Merz CN, Pepine CJ. Increased wave reflection and ejection duration in women with chest pain and nonobstructive coronary artery disease: ancillary study from the Women’s Ischemia Syndrome Evaluation. J Hypertens. 2013;31:1447–54. doi: 10.1097/HJH.0b013e3283611bac. discussion 1454–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maseri A, Mimmo R, Chierchia S, Marchesi C, Pesola A, L’Abbate A. Coronary artery spasm as a cause of acute myocardial ischemia in man. Chest. 1975;68:625–33. [Google Scholar]
- 25.Tschöpe C, Bock CT, Kasner M, Noutsias M, Westermann D, Schwimmbeck PL, et al. High prevalence of cardiac parvovirus B19 infection in patients with isolated left ventricular diastolic dysfunction. Circulation. 2005;111:879–86. doi: 10.1161/01.CIR.0000155615.68924.B3. [DOI] [PubMed] [Google Scholar]
- 26.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–71. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 27.Galiuto L, De Caterina AR, Porfidia A, Paraggio L, Barchetta S, Locorotondo G, et al. Reversible coronary microvascular dysfunction: a common pathogenetic mechanism in Apical Ballooning or Tako-Tsubo Syndrome. Eur Heart J. 2010;31:1319–27. doi: 10.1093/eurheartj/ehq039. [DOI] [PubMed] [Google Scholar]
- 28.Kaski JC, Rosano GM, Collins P, Nihoyannopoulos P, Maseri A, Poole-Wilson PA. Cardiac syndrome X: clinical characteristics and left ventricular function. Long-term follow-up study. J Am Coll Cardiol. 1995;25:807–14. doi: 10.1016/0735-1097(94)00507-M. [DOI] [PubMed] [Google Scholar]
- 29.Lanza GA, Colonna G, Pasceri V, Maseri A. Atenolol versus amlodipine versus isosorbide-5-mononitrate on anginal symptoms in syndrome X. Am J Cardiol. 1999;84:854–6. doi: 10.1016/s0002-9149(99)00450-6. A858. [DOI] [PubMed] [Google Scholar]
- 30.Skalidis EI, Hamilos MI, Chlouverakis G, Zacharis EA, Vardas PE. Ivabradine improves coronary flow reserve in patients with stable coronary artery disease. Atherosclerosis. 2011;215:160–5. doi: 10.1016/j.atherosclerosis.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 31.Villano A, Di Franco A, Nerla R, Sestito A, Tarzia P, Lamendola P, et al. Effects of ivabradine and ranolazine in patients with microvascular angina pectoris. Am J Cardiol. 2013;112:8–13. doi: 10.1016/j.amjcard.2013.02.045. [DOI] [PubMed] [Google Scholar]
- 32.Chaitman BR. Ranolazine for the treatment of chronic angina and potential use in other cardiovascular conditions. Circulation. 2006;113:2462–72. doi: 10.1161/CIRCULATIONAHA.105.597500. [DOI] [PubMed] [Google Scholar]
- 33.Mehta PK, Goykhman P, Thomson LE, Shufelt C, Wei J, Yang Y, et al. Ranolazine improves angina in women with evidence of myocardial ischemia but no obstructive coronary artery disease. JACC Cardiovasc Imaging. 2011;4:514–22. doi: 10.1016/j.jcmg.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shahin Y, Khan JA, Samuel N, Chetter I. Angiotensin converting enzyme inhibitors effect on endothelial dysfunction: a meta-analysis of randomised controlled trials. Atherosclerosis. 2011;216:7–16. doi: 10.1016/j.atherosclerosis.2011.02.044. [DOI] [PubMed] [Google Scholar]
- 35.Kaski JC, Rosano G, Gavrielides S, Chen L. Effects of angiotensin-converting enzyme inhibition on exercise-induced angina and ST segment depression in patients with microvascular angina. J Am Coll Cardiol. 1994;23:652–7. doi: 10.1016/0735-1097(94)90750-1. [DOI] [PubMed] [Google Scholar]
- 36.Pauly DF, Johnson BD, Anderson RD, Handberg EM, Smith KM, Cooper-DeHoff RM, et al. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with improved microvascular function: A double-blind randomized study from the National Heart, Lung and Blood Institute Women’s Ischemia Syndrome Evaluation (WISE) Am Heart J. 2011;162:678–84. doi: 10.1016/j.ahj.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tiefenbacher CP, Friedrich S, Bleeke T, Vahl C, Chen X, Niroomand F. ACE inhibitors and statins acutely improve endothelial dysfunction of human coronary arterioles. Am J Physiol Heart Circ Physiol. 2004;286:H1425–32. doi: 10.1152/ajpheart.00783.2003. [DOI] [PubMed] [Google Scholar]
- 38.Khuddus MA, Pepine CJ, Handberg EM, Bairey Merz CN, Sopko G, Bavry AA, et al. An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease: a substudy from the National Heart, Lung and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) J Interv Cardiol. 2010;23:511–9. doi: 10.1111/j.1540-8183.2010.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, et al. PROSPECT Investigators A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–35. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 40.Reynolds HR, Srichai MB, Iqbal SN, Slater JN, Mancini GB, Feit F, et al. Mechanisms of myocardial infarction in women without angiographically obstructive coronary artery disease. Circulation. 2011;124:1414–25. doi: 10.1161/CIRCULATIONAHA.111.026542. [DOI] [PMC free article] [PubMed] [Google Scholar]


