Abstract
Staphylococcus aureus is a common cause of prosthetic joint infection (PJI). The prevalence of superantigens (SAgs) among PJI-associated S. aureus is unknown. Eighty-four S. aureus isolates associated with PJI isolated between 1999 and 2006 were studied. SAg genes, sea, seb, sec, sed, see, seg, seh, sei and tst, were assayed by PCR. Seventy-eight (92.9%) isolates carried at least one SAg gene studied, with 61 (72.6%) harboring more than one. seg was most commonly (70.2%) and seh was least frequently (4.8%) detected. tst-positive isolates were associated with early infection and increased ESR at diagnosis (P = 0.006 and P = 0.021, respectively). seg and sei were associated with methicillin resistance (P = 0.008 and 0.002, respectively). SAg genes are prevalent in S. aureus causing PJI; a majority of PJI-associated isolates produce biologically active SAgs in both planktonic and biofilm growth modes.
Keywords: S. aureus, superantigen, prosthetic joint infection
1. Introduction
With the increasing numbers of primary arthroplasty surgeries being performed, complications associated with prosthetic joints are becoming increasingly frequent (Kim, 2008). Of the post-operative complications associated with prosthetic joints, prosthetic joint infection (PJI) is the most detrimental (Harris & Sledge, 1990). PJI is often caused by staphylococci, including coagulase-negative staphylococci (CNS) and Staphylococcus aureus (Zimmerli, Trampuz, & Ochsner, 2004). PJI-associated bacteria grow as biofilms on prosthetic joints (Gallo, Kolar, Novotny, Rihakova, & Ticha, 2003), and as a result, management of PJI requires a combination of antimicrobial agents and surgery.
Clinical features and outcomes of staphylococcal PJI, including associated symptomatology, chronicity, tendency to relapse and even mortality, may be influenced by virulence factors, such as exotoxins, produced by the infecting organisms (Cunningham, Cockayne, & Humphreys, 1996). Among the exotoxins of S. aureus, staphylococcal enterotoxins (SEs) and toxic shock syndrome toxin (TSST-1) are well known superantigens (SAgs). They cause robust activation of T-cells expressing certain T cell receptor β chain variable region fragments (TCR Vβ), irrespective of antigenic specificity. SAgs may also contribute to establishing S. aureus infection by causing immune evasion or immune subversion (Gaus, Miethke, Wagner, & Heeg, 1994; Kawabe & Ochi, 1990; Llewelyn & Cohen, 2002; Novick, 2003; O’Hehir & Lamb, 1990; Taylor & Llewelyn, 2010).
The prevalence of SAgs have been mostly investigated among S. aureus strains isolated from diseases such as septic shock (Ferry, et al., 2005), infective endocarditis (Nienaber, et al., 2011) and toxic shock syndrome (DeVries, et al., 2011), in which clinical features reflect immune activation. To our knowledge, the prevalence of SAgs in S. aureus associated with PJI has not been investigated. Staphylococcal SAgs may help in establishment of PJI and contribute to its clinical features. We investigated the prevalence of SAgs in S. aureus associated with PJI and related the presence or absence of SAgs to clinical findings. We also examined whether S. aureus in in vitro biofilms produces functional SAgs and correlated the presence of SAgs with methicillin resistance.
2. Materials and Methods
2.1 Collection of bacterial isolates
A collection of 84 S. aureus isolates from patients diagnosed with PJI at Mayo Clinic (Rochester, MN) from 1999 to 2006 were studied. S. aureus were isolated from periprosthetic tissues, synovial fluid or the explanted prosthetic joints themselves (Trampuz, et al., 2007). Medical records of corresponding subjects were retrospectively reviewed for demographic characteristics, clinical course and outcome. This study was approved by the Mayo Clinic Institutional Review Board.
2.2 Clinical definitions
PJI was defined using diagnostic criteria outlined by the Infectious Diseases Society of America (Osmon, et al., 2013). Timing of infection was classified according to time since the most recent prosthesis implantation, defined as early (<3 months), delayed (3–12 months) and late (>12 months). Duration of symptoms before admission was categorized by one month intervals. Treatment strategies were categorized as chronic suppression, débridement and implant retention, resection and reimplantation, permanent resection, and disarticulation. Diagnosis of recurrence was confirmed by re-isolation of S. aureus from the same joint after a treatment strategy had been applied.
2.3 Preparation of genomic DNA and PCR
S. aureus was grown on sheep blood agar, and genomic DNA extracted using the DNeasy blood & tissue kit (Qiagen, Hilden, Germany). Genes for staphylococcal enterotoxins A, B, C, D, E, G, H, and I and TSST-1 were assayed by PCR using a Veriti® Thermal Cycler (Applied Biosystems, CA). Primers were synthesized by Integrated DNA Technologies®, (IA); primer sequences and PCR conditions are shown in Table 1 (Blaiotta, et al., 2004; Johnson, et al., 1991; Letertre, Perelle, Dilasser, & Fach, 2003; Lovseth, Loncarevic, & Berdal, 2004).
Table 1.
Nucleotide sequences of primers and references
| Gene | Primer name | Oligonucleotide sequence (5′-3′) | Amplicon size (base pair) | Ta(°C)a | Reference strain (control) | References |
|---|---|---|---|---|---|---|
| sea | SEA-1 SEA-2 |
GCA GGG AAC AGC TTT AGG C GTT CTG TAG AAG TAT GAA ACA CG |
521 | 55 | IDRL-7971b | (Lovseth, Loncarevic, & Berdal, 2004) |
| seb | SEB-1 SEB-2 |
TCG CAT CAA ACT GAC AAA CG GCA GGT ACT CTA TAA GTG CC |
478 | 55 | RN6734, pRN7114c | (Johnson, et al., 1991) |
| sec | SA-U SEC-2 |
TGT ATG TAT GGA GGT GTA AC AAT TGT GTT TCT TTT ATT TTC ATA A |
102 | 55 | ATCC 19095d | (Letertre, Perelle, Dilasser, & Fach, 2003) |
| sed | SED-1 SED-2 |
CTA GTT TGG TAA TAT CTC CT TAA TGC TAT ATC TTA TAG GG |
317 | 45 | ATCC 23235 | (Johnson, et al., 1991) |
| see | SA-U SEE-2 |
TGT ATG TAT GGA GGT GTA AC GCC AAA GCT GTC TGA G |
213 | 50 | ATCC 27664 | (Letertre, Perelle, Dilasser, & Fach, 2003) |
| seg | SEG-1 SEG-2 |
TGC TAT CGA CAC ACT ACA ACC CCA GAT TCA AAT GCA GAA CC |
704 | 53 | ATCC 19095d | (Blaiotta, et al., 2004) |
| seh | SA-U SEH-2 |
TGT ATG TAT GGA GGT GTA AC TCT CTA GGA GTT TTC ATA TC |
245 | 53 | ATCC 51811 | (Letertre, Perelle, Dilasser, & Fach, 2003) |
| sei | SEI-1 SEI-2 |
GAC AAC AAA ACT GTC GAA ACT G CCA TAT TCT TTG CCT TTA CCA G |
630 | 52 | ATCC 19095d | (Blaiotta, et al., 2004) |
| tst | TSST-1 TSST-2 |
GCT TGC GAC AAC TGC TAC AG TGG ATC CGT CAT TCA TTG TTA T |
559 | 55 | ATCC 51651 | (Lovseth, Loncarevic, & Berdal, 2004) |
PCR was performed using a total volume of 20 μl and 35 to 40 cycles: denaturation for 2 min at 94°C, annealing for 2 min, and primer extension for 1 min at 72°C. Platinum® PCR SuperMix (Invitrogen, NY, USA) was used. Ta represents annealing temperature.
Staphylococcus aureus IDRL-7971, isolated from human nares, was confirmed by ELISA to produce staphylococcal enterotoxin A (SEA) and staphylococcal enterotoxin B (SEB).
RN6734, pRN7114 is a generous gift from Richard Novick (New York Medical Center, NY), and is known to produce only SEB.
S. aureus ATCC 19095 is known to have both sec and the enterotoxin gene cluster (egc).
2.4 Preparation of culture supernatants and quantitation of planktonic and biofilm cultures
For planktonic cultures, S. aureus was grown to 108 CFU/ml in trypticase soy broth (TSB) for 24 hours. After centrifugation at 4000 rpm for 5 min, supernatants were collected, filtered through a 0.22 μm syringe filter (MILLEX®GP; Millipore, MA) and stored at −80ºC until further analysis. Biofilms were grown on Teflon® discs in 2 stages. During the first stage, the discs were placed in 24-well flat bottom plates with 2 ml of TSB containing a 1×106 CFU/ml inoculum. After 24 hours of incubation, each disc was removed, rinsed with sterile saline to remove planktonic cells and transferred to new 24-well flat bottom plates, with each well containing 2 ml of TSB containing 4 μg/ml of vancomycin (to inhibit the planktonic growth). After incubation for an additional 24 hours, culture media was collected, filtered through 0.22 μm syringe filters and frozen at −80ºC until further testing was performed. Quantitative cultures of biofilms on the discs were performed in duplicate after the initial incubation stage without vancomycin and following incubation with vancomycin. Prior to culture, biofilms were dislodged and disaggregated using vortexing and sonication, as previously described (del Pozo, et al., 2009). Quantitative culture results were expressed as the average biofilm density (log10CFU/cm2) or, for planktonic cells, log10CFU/ml for comparison of the biologic activity of supernatants from biofilm and planktonic cultures, respectively. To specifically investigate the correlation between methicillin susceptibility and biofilm growth in the presence of vancomycin, a convenience sample of 8 MSSA and 7 MRSA isolates was first grown on Teflon discs as described above. Then, during the second stage, the discs were cultured in TSB with and without vancomycin. After 24 hours of reincubation, the colony counts were determined.
2.5 T-cell proliferation assay with HLA-DR3 transgenic mouse splenocytes
To measure the biological function of SAgs produced by S. aureus grown in planktonic as well as biofilm states, a splenocyte proliferation assay was performed using HLA-DR3 transgenic mice. HLA-DR3 mice expressing functional HLA-DRA1*0101 and HLA-DRB1*0301 transgenes on a MHC class II-deficient background (AE°) have been previously described (Rajagopalan, et al., 2003). Splenocytes harvested from naive HLA-DR3 mice were cultured with 100 μl of serial twofold dilutions of the culture supernatant (from 1:2 to 1:256) prepared as above. Supernatants from isogenic S. aureus strains, RN6734 containing pRN5543::seb (pRN7114) and RN6734 containing pRN5543::seb(b.2) (pRN7116), which produce SEB only or no SAgs (generous gifts from Richard Novick, New York University Medical Center, New York, NY), were used as positive and negative controls, respectively (Vojtov, Ross, & Novick, 2002). Splenocytes were cultured at 105 cells per well in 100 μl of HEPES-buffered RPMI 1640 containing 5% fetal calf serum, serum supplement, and streptomycin and penicillin. After 48 hours of incubation, 1 μg of tritiated [3H] thymidine was added to the splenocytes and incubated for an additional 17 hours. The extent of proliferation was determined by measuring incorporated radioactivity using standard procedures. The dilution concentration that induced maximal proliferation among the varying concentrations was defined and data associated with that dilution concentration taken to mitogenic activity for comparative purposes. Mean radioactivity counts per minute (CPM) with standard deviation was recorded in triplicate wells. Supernatants from planktonic and biofilm cultures of a convenience sample of 38 isolates were tested by this method. Among those 38 isolates, 15 isolates were further tested using splenocytes from AE° (MHC class II-deficient) mice to determine whether there was nonspecific mitogenic activity.
2.6 Statistical analysis
Statistical analysis was performed using SPSS software (version 21; SPSS, Chicago, IL, USA). Continuous scaled data were compared using the Student’s t test or Mann-Whitney U test and categorical variables were compared using Fisher’s exact test. Probability of recurrence was calculated by Kaplan-Meier analysis and compared by log rank test. All tests were 2 sided; p-values less than 0.05 were considered statistically significant.
3. Results
3.1 Clinical characteristics
Of the 84 PJI isolates, medical records from 83 corresponding subjects were available for review. Demographics and clinical characteristics are shown in Table 2. Of the 83 subjects, 50 had a knee prosthesis, 31 had a hip prosthesis and 2 had a shoulder prosthesis. All had localized symptoms involving the prosthetic joint, with only 20 having fever at the time of diagnosis. The most common treatment strategy was resection and reimplantation, which was done following a two-stage procedure in 41 subjects. Another 22 subjects underwent resection arthroplasty, but without prosthesis reimplantation (i.e., permanent resection). Chronic suppression, débridement and implant retention, and disarticulation were performed in 2, 14 and 4 subjects, respectively. The mean follow up period after diagnosis of PJI was 23 months. Ten subjects had recurrence at an average of 8.5 months after intervention, with S. aureus alone re-isolated in nine and multiple organisms, including S. aureus, isolated in the tenth.
Table 2.
Characteristics of 83 subjects with Staphylococcus aureus prosthetic joint infection
| Characteristics | N = 83 (%) | |
|---|---|---|
| Age | Range (mean) | 32–98 (68) |
| Gender | ||
| Male | 48 (57.8) | |
| Female | 35 (42.2) | |
| Infection site | ||
| Hip | 31 (37.3) | |
| Knee | 50 (60.2) | |
| Shoulder | 2 (2.4) | |
| Timing of infection | ||
| Early (<3 months) | 19 (22.9) | |
| Delayed (3–12 months) | 8 (9.6) | |
| Late (>1 year) | 56 (67.4) | |
| Duration of symptoms | ||
| <1 month | 40 (48.2) | |
| ≥1 month | 43 (51.8) | |
| Clinical presentation | ||
| Fever | 20 (24.1) | |
| Local symptoms only | 63 (75.9) | |
| Treatment | ||
| Chronic suppression | 2 (2.4) | |
| Débridement and implant retention | 14 (16.9) | |
| Resection and reimplantation | 41 (49.4) | |
| Permanent resection | 22 (26.5) | |
| Disarticulation | 4 (4.8) | |
| Recurrence of infection | ||
| Recurrence | 10 (12.0) | |
| No recurrence | 73 (88.0) | |
| Laboratory finding (mean)a | ||
| WBC (/mm3) | 10639 | |
| ESR (mm/hour) | 38 | |
| CRP (mg/dL) | 32 | |
WBC, ESR and CRP denote white blood cell, erythrocyte sedimentation rate and C-reactive proteins, respectively. The reference ranges of WBC, ESR and CRP are 3500~10,500/mm3, 0~22 mm/hour and 0.02–0.8 mg/dL, respectively.
3.2 Distribution and prevalence of superantigen genes by PCR
Of the 84 S. aureus isolates tested by PCR, 78 (92.9%) had at least one SAg gene and 61 (72.6%) had more than one (Table 3). seg was most frequently found (70.2%), followed by sei (47.6%), sea (27.4%), tst (17.9%), seb (9.5%), sec (9.5%), sed (9.5%), see (7.1%) and seh (4.8%). The most common combination of genes were seg and sei (20.2%) followed by sea and seg (8.3%). Four isolates harbored four SAg genes. Among the 61 isolates with either seg or sei, 37 (60.7%) isolates had both together and of the remaining 24 isolates which had seg or sei but not both, 22 had seg only and 2 sei only.
Table 3.
Distribution of superantigen genes in 84 Staphylococcusaureus prosthetic joint infection isolates
| Superantigen genes | N = 84 (%) | |
|---|---|---|
| Overall | ||
| at least 1 gene | 78 (92.9) | |
| more than 1 genes | 61 (72.6) | |
| sea | 23 (27.4) | |
| seb | 8 (9.5) | |
| sec | 8 (9.5) | |
| sed | 8 (9.5) | |
| see | 6 (7.1) | |
| seg | 59 (70.2) | |
| seh | 4 (4.8) | |
| sei | 40 (47.6) | |
| tst | 15 (17.9) | |
| Profile of combination a | ||
| seg+sei | 17 (20.2) | |
| sea+seg | 7 (8.3) | |
| sed+seg+sei | 5 (6.0) | |
| sea+seg+sei | 4 (4.8) | |
| seg+tst | 4 (4.8) | |
| seg+sei+tst | 3 (3.6) | |
| sea+sec | 2 (2.4) | |
| sec+seg+sei | 2 (2.4) | |
| sed+seg+sei+tst | 2 (2.4) | |
| sea+sec+seg | 2 (2.4) | |
| see+seg+sei+tst | 2 (2.4) | |
Profile of combination is listed in order of frequency; combinations identified in single isolates are not shown.
3.3 Association of superantigen genes with clinical characteristics
Timing of infection and systemic symptoms were similar among subjects irrespective of their SAg status (data not shown). Surrogate markers of inflammation, including WBC, ESR and CRP, were similar in both groups (data not shown). However, tst-positive isolates were associated with earlier infection compared to tst-negative isolates (7/15 vs 12/68, P = 0.006), and subjects with tst-positive isolates were more likely to have an elevated ESR than were those with tst-negative isolates (mean 76.4 vs 54.6 mm/hr, P = 0.021). The presence of SAgs was not associated with duration of symptoms or recurrence (data not shown). Fever was more prevalent in subjects with sed-positive than those with sed-negative isolates (5/8 vs 15/75, P = 0.018). However, the clinical significance of this result is unclear since the total number of sed-positive isolates was small.
3.4 Association of superantigen genes and biofilm growth
Biofilms were grown on coupons following the two-stage protocol described in the methods section. There was no difference in the biofilm density (log10CFU/cm2) between the SAg gene-positive and -negative isolates during the first stage of biofilm growth (data not shown). The average biofilm density of all isolates was higher during the second compared to first stage [6.56±0.77 (mean±SD) at the end of first stage and 7.26±0.77 (mean±SD) at the end of second stage following 24-hour reincubation in 4 μg/ml of vancomycin (P <0.001)]. We next analyzed the association between the presence of SAg genes and heightened growth during the second stage in the presence of vancomycin. Overall, the colony counts of SAg-positive, but not SAg-negative, isolates were greater in the second stage biofilm culture (P <0.001 and P = 0.439 for SAg-positive and -negative isolates, respectively, Figure 1A). In a subgroup analysis, sec-, sed-, seg-, sei- and tst-positive isolates grew more abundantly in the second stage, and seb-, see- and seh-positive isolates did not (Figure 1A). For example, the average biofilm densities of seg-positive (7.39±0.75) and sei-positive (7.59±0.69) isolates were higher than seg-negative (6.95±0.72) and sei-negative (6.96±0.74) isolates on discs reincubated with vancomycin (P = 0.017 and P <0.001, respectively, Figure 1B). In contrast, sea-positivity (n = 23) was negatively correlated with biofilm density (P = 0.001, Figure 1B).
Figure 1. Correlation between SAg genes and biofilm growth.
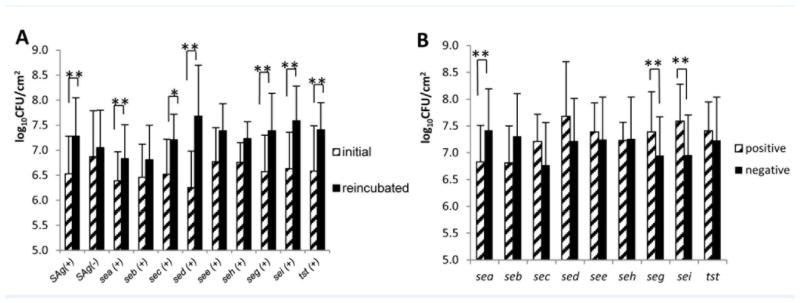
Teflon discs were grown in trypticase soy broth for 24 hours (initial biofilm), rinsed in normal saline and reincubated in trypticase soy broth with 4 μg/ml of vancomycin for 24 hours (reincubated biofilm). Panel A shows the changes in bacterial biofilm quantities between initial and reincubated biofilms for each of SAg gene-positive isolates. P-values were <0.001 for any SAg gene-positive, 0.430 for all SAg gene-negative, 0.010 for sea, 0.190 for seb, 0.022 for sec, 0.005 for sed, 0.183 for see, 0.145 for seh, <0.001 for seg, <0.001 for sei and 0.003 for tst. Panel B shows bacterial biofilm quantities after 24 hours incubation in 4 μg/ml vancomycin between each of SAg gene-positive and -negative isolates. P-values for the difference of biofilm densities are as follows: 0.001 for sea, 0.090 for seb, 0.768 for sec, 0.243 for sed, 0.593 for see, 0.868 for seh, 0.017 for seg, <0.001 for sei and 0.451 for tst. Error bars depict the standard deviation of means. *P <0.05 and ** P <0.01, by Student t-test.
3.5 Association of superantigen genes with methicillin susceptibility
All 84 isolates were vancomycin-susceptible; 24 were methicillin-resistant S. aureus (MRSA) and 60 were methicillin-susceptible S. aureus (MSSA), as determined by oxacillin susceptibility testing using agar dilution. While sed, seg and sei positive isolates had a statistically significant association with methicillin resistance (P = 0.039, P = 0.008 and P = 0.002, respectively), the other SAgs were not associated with methicillin susceptibility (Figure 2A).
Figure 2. Relationship between bacterial biofilm quantity, methicillin resistance and enterotoxin gene cluster (seg, sei).
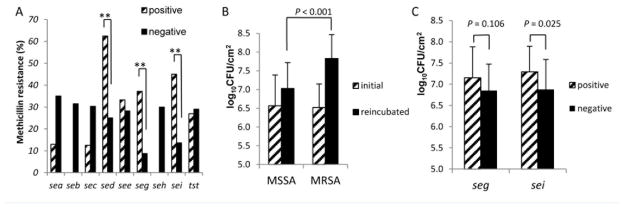
All 84 PJI isolates were tested for methicillin susceptibility by agar dilution. Panel A shows the distribution of methicillin resistance among each of SAg gene-positive and -negative isolates. P -values for the difference of rates of methicillin resistance are as follows: 0.062 for sea, 0.098 for seb, 0.429 for sec, 0.039 for sed, 1.000 for see, 0.008 for seg, 0.321 for seh, 0.002 for sei and 1.000 for tst. Panel B shows the change in bacterial biofilm quantity after reincubation in 4 μg/ml vancomycin comparing MRSA (n = 24) and MSSA (n = 60) (P <0.001). Panel C shows the differences in reincubated biofilm density between seg and sei-positive (n = 37 & 22, respectively)and -negative (n = 23 & 38, respectively) isolates limited to the MSSA group (n = 60). *P <0.05 and **P <0.01, by Fischer’s exact test and Student t-test. Error bars denote the standard deviation of means.
3.6 Association of biofilm growth with methicillin resistance and vancomycin exposure
Both MSSA and MRSA showed enhanced biofilm growth in the presence of vancomycin. However, enhanced biofilm growth in the presence of vancomycin was more pronounced for MRSA than MSSA (P <0.001). For MRSA (n = 24), the biofilm density (log10CFU/cm2) was 6.52±0.63 (mean±SD) in the first stage and 7.83±0.64 (mean±SD) in the second stage (P <0.001), whereas for MSSA it was 6.57±0.82 in the first stage and 7.03±0.69 (P <0.001, Figure 2B). We also investigated the association between the most prevalent SAg genes, seg and sei, and enhanced biofilm growth in the presence of vancomycin. As shown in Fig 2A, these SAgs were also strongly associated with methicillin resistance. Therefore, to investigate the association between the presence of these SAgs and enhanced growth with vancomycin independent of methicillin resistance, we analyzed only the 60 MSSA isolates. While seg-positive (7.15±0.73) isolates had a tendency toward having a higher biofilm density than seg-negative (6.85±0.62) isolates (P = 0.106), sei-positive (7.29±0.60) isolates grew biofilms with significantly more cells than did sei-negative (6.88±0.71) isolates (P=0.025) (Figure 2C).
In all the biofilm culture experiments described above, vancomycin was always included in the second stage of culture (to prevent planktonic growth). Therefore, we next investigated whether vancomycin augments biofilm growth. For this, 15 isolates comprising of both MRSA (n = 7) and MSSA (n = 8), were grown without vancomycin during the first stage as usual. However, during the second stage, they were grown either in the presence or absence of vancomycin. Overall, the biofilm density of reincubated discs was paradoxically higher with 4 μg/ml of vancomycin (7.57±0.70) than without vancomycin (7.18±0.50) (P = 0.031). This effect was pronounced in the MRSA group (7.87±0.70 vs 7.14±0.65, with and without vancomycin, respectively, P = 0.005), but not MSSA group (7.31±0.64 vs 7.22±0.37, with and without vancomycin, respectively, P = 0.680).
3.7 T-cell proliferation assay
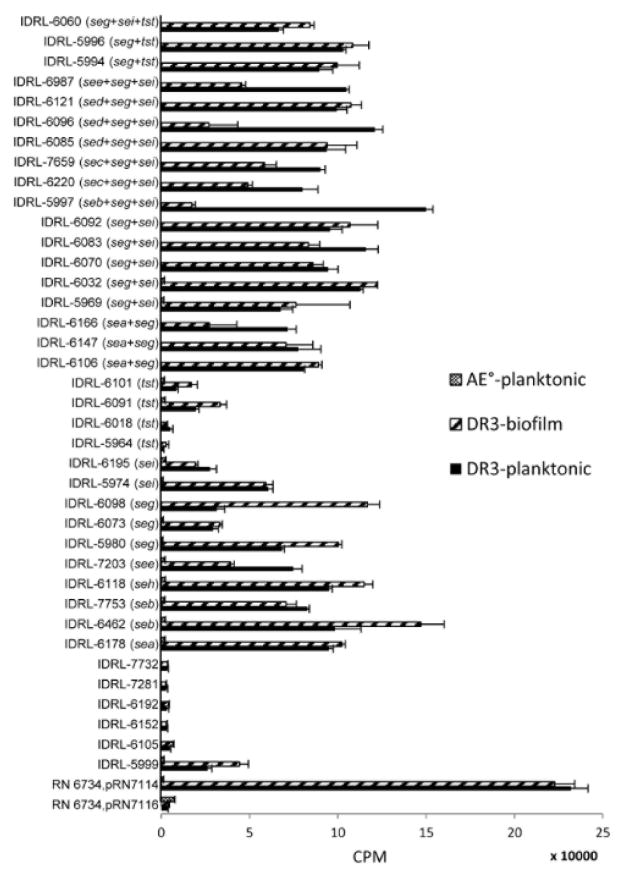
We next tested whether SAg-positive S. aureus PJI isolates produced functional SAgs in planktonic and biofilm growth modes. Culture supernatants from the SAg gene-positive isolates (which included 14 single SAg gene-positive isolates and 18 multiple SAg gene-positive isolates) induced robust proliferation of splenocytes from HLA-DR3 transgenic mice but not from AE° mice, which lack MHC class II molecules and hence cannot present SAgs (Figure 3). The supernatants from the six SAg gene-negative isolates tested, except IDRL-5999, had mitogenic activity similar to the supernatants from the SAg-negative control strain. The supernatants of IDRL-5999 had similar mitogenic activity to the SAg gene-positive isolates tested. IDRL-5999 may produce a SAg not included in the PCR assays studied. The tst only-positive isolates, IDRL-5964, IDRL-6018, IDRL-6091 and IDRL-6101 exhibited weaker mitogenic activities than other single SAg gene-positive isolates in both planktonic (P = 0.004) and biofilm (P = 0.01) culture. Overall, mitogenic activities were similar between planktonic and biofilm cultures for the 32 SAg gene-positive isolates (mean 75,432 vs 69,148 CPM, P = 0.506) tested.
Figure 3. Bioassay to test the mitogenicity of SAg produced by PJI isolates.
Culture supernatants from 38 PJI isolates were tested by T cell proliferation assay using splenocytes of transgenic HLA DR3 mice. Overall, there was no difference in mitogenic activity between the supernatants of planktonic and biofilm cultures. SAg genes present in each of isolates are noted in parentheses after the name of isolate. IDRL-5999, IDRL-6105, IDRL-6152, IDRL-6192, IDRL-7281 and IDRL-7732 were negative for all SAg genes tested. RN6734, pRN7116 was used as negative control; it is known to be negative for all SAg genes. RN6734, pRN7114, which produces SEB, was used as a positive control. A convenience sample subset of 14 planktonic supernatants from the 38 isolates was further tested using splenocytes of AE° mice (deficient in MHC class II molecules). CPM, counts per minute. Error bars; standard deviations.
4. Discussion
S. aureus is a common cause of PJI. It elaborates several exotoxins including SAgs; these toxins may contribute to its pathogenicity and virulence. Given their immune stimulating properties, associations between SAgs and acute diseases such as sepsis, toxic shock, endocarditis and pneumonia have been explored. Since S. aureus can produce SAgs in the biofilm state (Chung, et al., 2014), we hypothesized that S. aureus biofilms growing on prosthetic joints might elicit an inflammatory response through SAgs, impacting the pathogenesis of PJI. Therefore, we investigated the prevalence of SAg-producing S. aureus in PJI and correlated our findings with clinical features.
We found a 92.9% prevalence of SAgs in S. aureus associated with PJI isolates, which is similar to or higher than that reported in isolates from other disease states such as septic shock (Ferry, et al., 2005), infective endocarditis (Nienaber, et al., 2011) and diabetic foot ulcer (Vu, et al., 2014). While seg was the most common staphylococcal SAg gene associated with PJI (70.2%), close to two-thirds of the isolates tested had more than one SAg gene. The most common SAg gene combination was seg and sei, which coexist in enterotoxin gene cluster (egc) (Jarraud, et al., 2001). egc normally includes seg, sei, sem, sen, and seo together, but they are not always present due to egc polymorphisms (Blaiotta, Fusco, von Eiff, Villani, & Becker, 2006) and varying egc types (Collery, Smyth, Tumilty, Twohig, & Smyth, 2009).
In the present study, we found that subjects with tst-positive isolates had earlier onset of infection (P= 0.006), and elevated ESR (P = 0.021), a marker for inflammation, than those with tst-negative isolates. This suggests that TSST-1 may be actively produced and systemically absorbed in the early stages of PJI. However, the presence of other SAg genes was not associated with clinical findings of inflammation such as fever (except sed which was associated with fever, P=0.018). The lack of association between the presence of SAg genes and systemic findings of inflammation may be attributed to the small sample size studied. Also, even though the isolates studied had SAg genes, the actual production of SAgs may be repressed under the anaerobic conditions of PJI (Yarwood & Schlievert, 2000). Further, even if SAgs are produced, they may be confined to the fibrous zone in the setting of chronic PJI (Gristina, 1994). Finally, SAg activity may be limited by neutralizing anti-SAg antibodies (Holtfreter, et al., 2004; Parsonnet, et al., 2008).
There have been variable reports of an association between methicillin resistance and SAgs (Hu, et al., 2008; Yu, et al., 2012). Given that certain SAgs exist on mobile genetic elements, SAg genes can be transferred horizontally among the S. aureus strains irrespective of methicillin resistance. The association of sed and other SAg genes of the egc cluster with methicillin resistance in our study may be due to clonality among the MRSA isolates tested. An interesting observation is that S. aureus biofilms continued to grow in the presence of 4 μg/ml of vancomycin even though all of the isolates were vancomycin-susceptible. Vancomycin has been shown to promote biofilm formation in some strains of S. aureus, including MRSA, through several mechanisms (Abdelhady, et al., 2014; Abdelhady, et al., 2013; Boles & Horswill, 2008; Hsu, et al., 2011; Pozzi, et al., 2012; Sakoulas, et al., 2003). From a clinical viewpoint, it is important to recognize that after standard dosing, the concentration of vancomycin in periprosthetic tissues would be similar to or slightly higher than the 4 μg/ml used to inhibit planktonic growth in our study (Luzzati, et al., 2000). Another interesting finding of our study is the relationship between SAgs and biofilm growth in general. seg- and sei-positive isolates showed high biofilm densities. Therefore, egc may influence biofilm growth; there may be a clonal association between egc and the accessory gene regulator (agr) phenotype, impacting biofilm growth (Cafiso, et al., 2007; Vuong, Saenz, Gotz, & Otto, 2000). Overall, our finding that vancomycin paradoxically enhances biofilm growth of S. aureus, especially MRSA and that this is associated with the presence of certain SAgs may have clinical significance. Given that SAgs can also cause repression of other exotoxins (Vojtov, et al., 2002), SAgs might influence the growth of S. aureus through bacteria-intrinsic, non-immune mechanisms.
In the setting of prosthetic joints, wherein nutrition and essential elements for growth are depleted, S. aureus likely grows as biofilms rather than in the planktonic growth mode. Therefore, the biofilm growth conditions used in the present study may be a model of PJI. In addition to demonstrating the presence of SAg genes, we performed biological assays to demonstrate their functionality, which is a major strength of our study. However, there are some limitations. First, we did not test for the presence of all reported staphylococcal SAgs (Xu & McCormick, 2012). Nonetheless, the supernatants from most SAg gene-negative isolates had no mitogenic activity in our HLA-DR3 splenocyte proliferation assay. Second, we reviewed medical records retrospectively, which may impose bias in analyzing clinical characteristics. Third, we did not investigate the clonality of the S. aureus isolates studies, which may have resulted in sampling error in reporting prevalence and showing an epidemiologic linkage of MRSA with egc. Fourth, we used splenocytes of transgenic HLA-DR3 mice instead of human T-cells for functional assay of SAgs. Even though splenocytes from HLA class II transgenic mice respond robustly to SAgs, they may still be less responsive to SAgs than are human T cells due to differences in the repertoire of T cells expressing SAg-reactive T cell receptors (as shown by the low proliferative responses to TSST-1 in our study).
In conclusion, we showed a high prevalence of SAgs in S. aureus associated with PJI. A prospective study involving large number of subjects and an in-depth investigation into various systemic and local immunological/inflammatory markers is needed to determine whether there is an association between the presence of SAgs and PJI outcome.
Highlights.
Staphylococcus aureus is a frequent cause of prosthetic joint infection (PJI).
The prevalence of superantigens (SAgs) among PJI-associated S. aureus is unknown.
Close to 90% of S. aureus PJI isolates carried at least one SAg gene.
seg and seh were the most and least common superantigens, respectively.
Most SAg-carrying isolates produced biologically active SAgs in biofilms in vitro.
Acknowledgments
This study was supported by grants of the National Institute of Health [AI101172 to G.R., AI68741 to G.R. and C.S.D., R01 AR056647 to R.P., and R01 AI91594 to R.P.].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelhady W, Bayer AS, Seidl K, Moormeier DE, Bayles KW, Cheung A, Yeaman MR, Xiong YQ. Impact of vancomycin on sarA-mediated biofilm formation: Role in persistent endovascular infections due to methicillin-resistant Staphylococcus aureus. J Infect Dis. 2014 doi: 10.1093/infdis/jiu007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelhady W, Bayer AS, Seidl K, Nast CC, Kiedrowski MR, Horswill AR, Yeaman MR, Xiong YQ. Reduced vancomycin susceptibility in an in vitro catheter-related biofilm model correlates with poor therapeutic outcomes in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2013;57:1447–1454. doi: 10.1128/AAC.02073-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaiotta G, Ercolini D, Pennacchia C, Fusco V, Casaburi A, Pepe O, Villani F. PCR detection of staphylococcal enterotoxin genes in Staphylococcus spp. strains isolated from meat and dairy products. Evidence for new variants of seG and seI in S. aureus AB-8802. J Appl Microbiol. 2004;97:719–730. doi: 10.1111/j.1365-2672.2004.02349.x. [DOI] [PubMed] [Google Scholar]
- Blaiotta G, Fusco V, von Eiff C, Villani F, Becker K. Biotyping of enterotoxigenic Staphylococcus aureus by enterotoxin gene cluster (egc) polymorphism and spa typing analyses. Appl Environ Microbiol. 2006;72:6117–6123. doi: 10.1128/AEM.00773-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boles BR, Horswill AR. Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog. 2008;4:e1000052. doi: 10.1371/journal.ppat.1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafiso V, Bertuccio T, Santagati M, Demelio V, Spina D, Nicoletti G, Stefani S. agr-Genotyping and transcriptional analysis of biofilm-producing Staphylococcus aureus. FEMS Immunol Med Microbiol. 2007;51:220–227. doi: 10.1111/j.1574-695X.2007.00298.x. [DOI] [PubMed] [Google Scholar]
- Chung JW, Karau MJ, Greenwood-Quaintance KE, Ballard AD, Tilahun A, Khaleghi SR, David CS, Patel R, Rajagopalan G. Superantigen profiling of Staphylococcus aureus infective endocarditis isolates. Diagn Microbiol Infect Dis. 2014;79:119–124. doi: 10.1016/j.diagmicrobio.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collery MM, Smyth DS, Tumilty JJ, Twohig JM, Smyth CJ. Associations between enterotoxin gene cluster types egc1, egc2 and egc3, agr types, enterotoxin and enterotoxin-like gene profiles, and molecular typing characteristics of human nasal carriage and animal isolates of Staphylococcus aureus. J Med Microbiol. 2009;58:13–25. doi: 10.1099/jmm.0.005215-0. [DOI] [PubMed] [Google Scholar]
- Cunningham R, Cockayne A, Humphreys H. Clinical and molecular aspects of the pathogenesis of Staphylococcus aureus bone and joint infections. J Med Microbiol. 1996;44:157–164. doi: 10.1099/00222615-44-3-157. [DOI] [PubMed] [Google Scholar]
- del Pozo JL, Rouse MS, Mandrekar JN, Sampedro MF, Steckelberg JM, Patel R. Effect of electrical current on the activities of antimicrobial agents against Pseudomonas aeruginosa, Staphylococcus aureus, and Staphylococcus epidermidis biofilms. Antimicrob Agents Chemother. 2009;53:35–40. doi: 10.1128/AAC.00237-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AS, Lesher L, Schlievert PM, Rogers T, Villaume LG, Danila R, Lynfield R. Staphylococcal toxic shock syndrome 2000–2006: epidemiology, clinical features, and molecular characteristics. PLoS One. 2011;6:e22997. doi: 10.1371/journal.pone.0022997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry T, Thomas D, Genestier AL, Bes M, Lina G, Vandenesch F, Etienne J. Comparative prevalence of superantigen genes in Staphylococcus aureus isolates causing sepsis with and without septic shock. Clin Infect Dis. 2005;41:771–777. doi: 10.1086/432798. [DOI] [PubMed] [Google Scholar]
- Gallo J, Kolar M, Novotny R, Rihakova P, Ticha V. Pathogenesis of prosthesis-related infection. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2003;147:27–35. doi: 10.5507/bp.2003.004. [DOI] [PubMed] [Google Scholar]
- Gaus H, Miethke T, Wagner H, Heeg K. Superantigen-induced anergy of V beta 8+ CD4+ T cells induces functional but non-proliferative T cells in vivo. Immunology. 1994;83:333–340. [PMC free article] [PubMed] [Google Scholar]
- Gristina AG. Implant failure and the immuno-incompetent fibro-inflammatory zone. Clin Orthop Relat Res. 1994:106–118. [PubMed] [Google Scholar]
- Harris WH, Sledge CB. Total hip and total knee replacement (1) N Engl J Med. 1990;323:725–731. doi: 10.1056/NEJM199009133231106. [DOI] [PubMed] [Google Scholar]
- Holtfreter S, Bauer K, Thomas D, Feig C, Lorenz V, Roschack K, Friebe E, Selleng K, Lovenich S, Greve T, Greinacher A, Panzig B, Engelmann S, Lina G, Broker BM. egc-Encoded superantigens from Staphylococcus aureus are neutralized by human sera much less efficiently than are classical staphylococcal enterotoxins or toxic shock syndrome toxin. Infect Immun. 2004;72:4061–4071. doi: 10.1128/IAI.72.7.4061-4071.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CY, Lin MH, Chen CC, Chien SC, Cheng YH, Su IN, Shu JC. Vancomycin promotes the bacterial autolysis, release of extracellular DNA, and biofilm formation in vancomycin-non-susceptible Staphylococcus aureus. FEMS Immunol Med Microbiol. 2011;63:236–247. doi: 10.1111/j.1574-695X.2011.00846.x. [DOI] [PubMed] [Google Scholar]
- Hu DL, Omoe K, Inoue F, Kasai T, Yasujima M, Shinagawa K, Nakane A. Comparative prevalence of superantigenic toxin genes in meticillin-resistant and meticillin-susceptible Staphylococcus aureus isolates. J Med Microbiol. 2008;57:1106–1112. doi: 10.1099/jmm.0.2008/002790-0. [DOI] [PubMed] [Google Scholar]
- Jarraud S, Peyrat MA, Lim A, Tristan A, Bes M, Mougel C, Etienne J, Vandenesch F, Bonneville M, Lina G. egc, a highly prevalent operon of enterotoxin gene, forms a putative nursery of superantigens in Staphylococcus aureus. J Immunol. 2001;166:669–677. doi: 10.4049/jimmunol.166.1.669. [DOI] [PubMed] [Google Scholar]
- Johnson WM, Tyler SD, Ewan EP, Ashton FE, Pollard DR, Rozee KR. Detection of genes for enterotoxins, exfoliative toxins, and toxic shock syndrome toxin 1 in Staphylococcus aureus by the polymerase chain reaction. J Clin Microbiol. 1991;29:426–430. doi: 10.1128/jcm.29.3.426-430.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe Y, Ochi A. Selective anergy of V beta 8+,CD4+ T cells in Staphylococcus enterotoxin B-primed mice. J Exp Med. 1990;172:1065–1070. doi: 10.1084/jem.172.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. Changes in surgical loads and economic burden of hip and knee replacements in the US: 1997–2004. Arthritis Rheum. 2008;59:481–488. doi: 10.1002/art.23525. [DOI] [PubMed] [Google Scholar]
- Letertre C, Perelle S, Dilasser F, Fach P. Detection and genotyping by real-time PCR of the staphylococcal enterotoxin genes sea to sej. Molecular and Cellular Probes. 2003;17:139–147. doi: 10.1016/s0890-8508(03)00045-8. [DOI] [PubMed] [Google Scholar]
- Llewelyn M, Cohen J. Superantigens: microbial agents that corrupt immunity. The Lancet Infectious Diseases. 2002;2:156–162. doi: 10.1016/s1473-3099(02)00222-0. [DOI] [PubMed] [Google Scholar]
- Lovseth A, Loncarevic S, Berdal KG. Modified multiplex PCR method for detection of pyrogenic exotoxin genes in staphylococcal isolates. J Clin Microbiol. 2004;42:3869–3872. doi: 10.1128/JCM.42.8.3869-3872.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzati R, Sanna A, Allegranzi B, Nardi S, Berti M, Barisoni D, Concia E. Pharmacokinetics and tissue penetration of vancomycin in patients undergoing prosthetic mammary surgery. J Antimicrob Chemother. 2000;45:243–245. doi: 10.1093/jac/45.2.243. [DOI] [PubMed] [Google Scholar]
- Nienaber JJ, Sharma Kuinkel BK, Clarke-Pearson M, Lamlertthon S, Park L, Rude TH, Barriere S, Woods CW, Chu VH, Marin M, Bukovski S, Garcia P, Corey GR, Korman T, Doco-Lecompte T, Murdoch DR, Reller LB, Fowler VG., Jr Methicillin-susceptible Staphylococcus aureus endocarditis isolates are associated with clonal complex 30 genotype and a distinct repertoire of enterotoxins and adhesins. J Infect Dis. 2011;204:704–713. doi: 10.1093/infdis/jir389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. Mobile genetic elements and bacterial toxinoses: the superantigen-encoding pathogenicity islands of Staphylococcus aureus. Plasmid. 2003;49:93–105. doi: 10.1016/s0147-619x(02)00157-9. [DOI] [PubMed] [Google Scholar]
- O’Hehir RE, Lamb JR. Induction of specific clonal anergy in human T lymphocytes by Staphylococcus aureus enterotoxins. Proc Natl Acad Sci U S A. 1990;87:8884–8888. doi: 10.1073/pnas.87.22.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:e1–e25. doi: 10.1093/cid/cis803. [DOI] [PubMed] [Google Scholar]
- Parsonnet J, Goering RV, Hansmann MA, Jones MB, Ohtagaki K, Davis CC, Totsuka K. Prevalence of toxic shock syndrome toxin 1 (TSST-1)-producing strains of Staphylococcus aureus and antibody to TSST-1 among healthy Japanese women. J Clin Microbiol. 2008;46:2731–2738. doi: 10.1128/JCM.00228-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi C, Waters EM, Rudkin JK, Schaeffer CR, Lohan AJ, Tong P, Loftus BJ, Pier GB, Fey PD, Massey RC, O’Gara JP. Methicillin resistance alters the biofilm phenotype and attenuates virulence in Staphylococcus aureus device-associated infections. PLoS Pathog. 2012;8:e1002626. doi: 10.1371/journal.ppat.1002626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan G, Smart MK, Cheng S, Krco CJ, Johnson KL, David CS. Expression and function of HLA-DR3 and DQ8 in transgenic mice lacking functional H2-M. Tissue Antigens. 2003;62:149–161. doi: 10.1034/j.1399-0039.2003.00088.x. [DOI] [PubMed] [Google Scholar]
- Sakoulas G, Eliopoulos GM, Moellering RC, Jr, Novick RP, Venkataraman L, Wennersten C, DeGirolami PC, Schwaber MJ, Gold HS. Staphylococcus aureus accessory gene regulator (agr) group II: is there a relationship to the development of intermediate-level glycopeptide resistance? J Infect Dis. 2003;187:929–938. doi: 10.1086/368128. [DOI] [PubMed] [Google Scholar]
- Taylor AL, Llewelyn MJ. Superantigen-induced proliferation of human CD4+CD25- T cells is followed by a switch to a functional regulatory phenotype. J Immunol. 2010;185:6591–6598. doi: 10.4049/jimmunol.1002416. [DOI] [PubMed] [Google Scholar]
- Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, Mandrekar JN, Cockerill FR, Steckelberg JM, Greenleaf JF, Patel R. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007;357:654–663. doi: 10.1056/NEJMoa061588. [DOI] [PubMed] [Google Scholar]
- Vojtov N, Ross HF, Novick RP. Global repression of exotoxin synthesis by staphylococcal superantigens. Proc Natl Acad Sci U S A. 2002;99:10102–10107. doi: 10.1073/pnas.152152499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu BG, Stach CS, Salgado-Pabon W, Diekema DJ, Gardner SE, Schlievert PM. Superantigens of Staphylococcus aureus from patients with diabetic foot ulcers. J Infect Dis. 2014 doi: 10.1093/infdis/jiu350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong C, Saenz HL, Gotz F, Otto M. Impact of the agr quorum-sensing system on adherence to polystyrene in Staphylococcus aureus. J Infect Dis. 2000;182:1688–1693. doi: 10.1086/317606. [DOI] [PubMed] [Google Scholar]
- Xu SX, McCormick JK. Staphylococcal superantigens in colonization and disease. Front Cell Infect Microbiol. 2012;2:52. doi: 10.3389/fcimb.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarwood JM, Schlievert PM. Oxygen and carbon dioxide regulation of toxic shock syndrome toxin 1 production by Staphylococcus aureus MN8. J Clin Microbiol. 2000;38:1797–1803. doi: 10.1128/jcm.38.5.1797-1803.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Li T, Huang X, Xie J, Xu Y, Tu J, Qin Z, Parsons C, Wang J, Hu L, Wang L. Virulence gene profiling and molecular characterization of hospital-acquired Staphylococcus aureus isolates associated with bloodstream infection. Diagn Microbiol Infect Dis. 2012;74:363–368. doi: 10.1016/j.diagmicrobio.2012.08.015. [DOI] [PubMed] [Google Scholar]
- Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]