Abstract
Chloride intracellular channel 1 (CLIC1) has been shown to be up-regulated in various malignancies but its exact function remains unclear. Here, it is revealed that CLIC1 is critical for the stability of invadopodia in endothelial and tumor cells embedded in a 3-dimensional (3D) matrix of fibrin. Invadopodia stability was associated with the capacity of CLIC1 to induce stress fiber and fibronectin matrix formation following its β3 integrin (ITGB3)-mediated recruitment into invadopodia. This pathway, in turn, was relevant for fibrin colonization as well as slug (SNAI2) expression and correlated with a significant role of CLIC1 in metastasis in vivo. Mechanistically, a reduction of myosin light chain kinase (MYLK) in CLIC1- as well as β3 integrin-depleted cells suggests an important role of CLIC1 for integrin-mediated actomyosin dynamics in cells embedded in fibrin. Overall, these results indicate that CLIC1 is an important contributor to tumor invasion, metastasis and angiogenesis.
Implications
This study uncovers an important new function of CLIC1 in the regulation of cell-extracellular matrix interactions and ability of tumor cells to metastasize to distant organs.
Keywords: Metastasis, CLIC1, fibrin, fibronectin, integrin αvβ3
INTRODUCTION
The central function of the clotting cascade is to sustain hemostasis; however, clotting factors also make important contributions to inflammation, wound healing, angiogenesis and cancer (1–4). In the course of the clotting process, soluble fibrinogen is converted into insoluble fibrin which provides a provisional adhesive matrix for inflammatory, endothelial and tumor cells (5). In the case of tumor metastasis, a key function of fibrin is to protect circulating tumor cells from the cytotoxic activity of natural killer cells (4). However, adhesion of tumor cells to 3-dimensional (3D) matrices of fibrin or fibrin-fibronectin also directly affects critical pro-metastatic functions such as invasion, survival and colony formation (6, 7). In addition, 3D fibrin has been shown to provide a permissive environment for the maintenance and proliferation of tumor-initiating cells (8). Together, these data underscore that a thorough understanding of the adhesive interactions between tumor cells and blood clot could lead to novel strategies to inhibit tumor metastasis.
Fibrin and fibrinogen are specifically recognized by β3 integrins and it appears that the expression of activated integrin αvβ3 is required for the interaction of tumor cells with clotted plasma (6, 7). Moreover, paralleling its positive effects on invadopodia and colony formation in clot-embedded tumor cells, integrin αvβ3 has been shown to support tumor metastasis in a mechanism that depends on the generation of fibrin-fibronectin complexes (7). Fibronectin is critical for αvβ3-mediated functions in clot-embedded tumor cells in at least two ways: upstream of integrins as an activating stimulus for αvβ3 and downstream as an αvβ3 ligand that provides the necessary matrix rigidity for tumor cells to generate stress fibers, form colonies and induce epithelial-mesenchymal transition (EMT), e.g. through expression of the EMT master regulator, Slug (6, 7). Interestingly, stress fiber formation is also associated with αvβ3-dependent maintenance and proliferation of tumor initiating cells in fibrin suggesting that mechanotransduction mediated by integrin αvβ3 is an important stimulus for expression of metastasis-related genes in clot-embedded tumor cells (8).
In search of factors that mediate the interaction of tumor cells with clotted plasma, we became interested in chloride intracellular channel 1 (CLIC1), which functions as an internalizing receptor for the clot-binding peptide CLT1 on endothelial cells (9). CLIC1 is overexpressed in the tumor vasculature and frequently up-regulated in patients with cancers originating from the breast, lung and liver (10–12). Increasing concentrations of CLIC1 have also been detected in the serum of patients with aggressive ovarian cancer suggesting that CLIC1 could be useful as a tumor marker (13). CLIC1 has been shown to induce invasion and proliferation of tumor as well as endothelial cells, but the mechanism behind this function is not clear (14, 15). We previously observed that ligation of integrin αvβ3 leads to redistribution of CLIC1 into lamellipodia of endothelial cells suggesting that CLIC1 membrane recruitment is relevant for cell spreading (9). Based on the established role of integrin αvβ3 for clot invasion and the apparent cooperation between αvβ3 and CLIC1, we aimed to analyze the role of CLIC1 for invadopodia formation in fibrin-embedded endothelial and tumor cells.
MATERIALS AND METHODS
3-Dimensional Cell Culture
Human umbilical venous endothelial cells (HUVEC) were purchased from Lonza, human 786-0 kidney cancer and HT1080 fibrosarcoma cells were purchased from ATCC. Primary human tumor cells were isolated from kidney tumors of 2 patients with metastatic and 2 patients with localized RCC as previously described (6). The cells were designated as M1/2 (metastatic) and L1/2 (localized) accordingly. M1 was derived from a female (70–79 years) with metastasis to the lung (pT3bN0M1), while M2 was derived from a male (50–59 years) with metastasis to the lung, liver and lymph node (pT3bN2Mx). The tumor stage of L1 (male, 40–49 years) and L2 (male, 40–49 years) was in each case pT1aNxMx. Cells were cultured at 37°C under a humidified, 5% CO2 atmosphere according to manufacturers’ specifications. For 3D culture, cells were mixed with 2 mg/ml fibrinogen (Enzyme Research Laboratories, Inc.) in the presence of 2 mM CaCl2 and 25 µg/ml FXIII (Enzyme Research Laboratories) to generate fibrin gels as previously described (6). Clotting was induced with 2.5 U/ml thrombin (Sigma), and 15 µl suspensions were pipetted onto tissue culture plates and inverted at room temperature for 10 minutes to solidify. Embedded cells were incubated with media supplemented with FBS at 37C under a humidified, 5% CO2 atmosphere.
Invadopodia Analysis
Clots were analyzed for invadopodia formation 24 hours after embedding at designated areas by phase contrast microscopy (Nikon Eclipse TS100; 10× magnification). Invadopodia formation was classified as complete (i.e. elongated or stellate shape) or incomplete (i.e. round shape with or without rudimentary invadopodia) and calculated for completely spread cells as % of the total cell number of a microscopy field. Colonization was measured by counting the total number of clot-embedded cells per microscopy field after 48 hours. In addition, we analyzed invadopodia formation over time by live cell imaging. To this end, fibrin embedded 786-0 cells were transferred to a BioStation IM (Nikon) pre-equilibrated to 37°C, 5% CO2. Phase contrast images were captured every 10 minutes for two hours and then every 30 minutes for the next 22 hours to track invadopodia. Images and videos were prepared using the BioStation IM software. Using these images, Image J software was used to measure the length of each invadopodium in each of 5 fields and an average was calculated for selected time points. Using the videos, the life time of each invadopodium was measured by determining the time points at which an invadopodium first formed and then disappeared. Average invadopodia life time was calculated from videos of 5 optical fields.
Confocal Microscopy
Fibrin-embedded cells were fixed in 4% paraformaldehyde, permeabilized with 0.2% triton ×100 and incubated with anti-fibronectin (Millipore), anti-CLIC1 (Abcam), anti-β3 integrin (Abcam), anti-pMLC (Cell Signaling) or isotype control, followed by incubation with Alexa Fluor 488- or 546-conjugated secondary antibody (Invitrogen). To visualize the cytoskeleton and nuclei, cells were stained with Alexa Fluor 546-phalloidin (Invitrogen) and Draq5 (eBioscience), respectively. The myosin light chain kinase (MLCK) inhibitor ML-7 was added at 10 µM where indicated. Fibrin-embedded cells were analyzed using a confocal microscope (Leica TCSSL) and images were processed with Adobe Photoshop. To quantify fibronectin matrix formation, fibrin-embedded cells were stained for fibronectin as described except that the cells were not permeabilized. 20× confocal images were analyzed for the % area of fibronectin staining using ImageJ software. ImageJ was also used to assess CLIC1 expression in invadopodia and the cell periphery, which were identified using F-actin staining. To this end, we measured the average CLIC1 fluorescence intensities in the cell periphery/invadopodia compared to the cell interior (without the nucleus) and calculated the ratio between the two compartments.
Gene Silencing
HUVEC were grown for 24 hours prior to transfection with 25 nM CLIC1 (Dharmacon On-TARGETplus SMARTpool L-009530-00) or non-targeting control (Dharmacon On-TARGETplus D-001810-10) siRNA. SMARTpool siRNA contains a pool of four siRNA sequences directed against the target gene. Cells were transfected in Opti-MEM medium (Invitrogen) using Lipofectamine 2000 reagent (Invitrogen) according to manufacturer instructions. After 6 hours, cells were placed in normal culture medium and grown for an additional 42 hours. To achieve stable knockdown of CLIC1 and β3 integrin, we transduced 786-0 and HT1080 cells with 5 lentiviral shRNA vectors compared to a scrambled control shRNA (University of Pittsburgh Cancer Institute Vector Core Facility). For our experiments, we used the 2 clones with the highest knockdown efficiency for each target. Sub-confluent cells were treated with virus suspension containing 8 µg/ml polybrene for 19 hours at 32°C, 5% CO2. Then, after a recovery phase of 24 hours in complete medium at 37°C, cells were placed under puromycin selection for 2 weeks. Target knockdown was confirmed by western blot analysis and the two most efficiently knocked-down clones were selected for further experimentation (CLIC1-2: CCGGCCTGTTGCCAAAGTTACACATCTCGAGATGTGTAACTTTGGCAACAGGTTTT TTG; CLIC1-4: CCGGGTGGATGAAACCA GTGCTGAACTCGAGTTCAGCACTGGTTT CATCCACTTTTTTG; β3-3: CCGGCCACGTCTACCTTCACCAATACTCGAGTATTGGT GAAGGTAGACGTGGTTTTT; β3-5: CCGGGATGCAGTGAATTGTACCTATCTCGAGA TAGGTACAATTCACTGCATCTTTTT).
Western blot analysis
Cell pellets were lysed by adding 2× SDS sample buffer. Proteins were separated by SDS-PAGE, transferred onto nitrocellulose and stained with 0.05% Ponceau S (Sigma) to ensure equivalent protein loading. Immunoblots were blocked with 5% bovine serum albumin and probed overnight at 4°C with anti-CLIC1 (Abcam), anti-fibronectin (Millipore), anti-Slug (Cell Signaling), anti-MLCK (Sigma), anti-phospho-MLCK (Life Technologies), anti-β3 (BD Biosciences), and anti-β-Actin (Sigma) antibodies followed by incubation with peroxidase-conjugated anti-mouse or rabbit IgG antibody (Biorad). Lastly, the blots were incubated with enhanced chemiluminescence (Perkin Elmer) to visualize antibody binding.
Experimental Metastasis
To induce metastasis, 5×105 HT1080 cells were injected into the tail vein (i.v.) of female, 6–8 weeks old athymic nude mice (Charles Rivers). Three weeks after tumor cell injection, lungs were isolated and fixed in Bouin’s solution (Sigma). To assess tumor multiplicity, tumor nodules were counted on the surface of lungs using a stereo microscope (Zeiss Stemi 2000-C).
Statistical Analysis
Significance was determined using Student’s two-tailed t-tests or one-way ANOVA followed by the posthoc Tukey’s multiple comparisons test (GraphPad Prism 5) with p<0.05 considered significant. Error bars show mean ± SEM.
RESULTS
CLIC1 supports invadopodia formation in fibrin in vitro and metastasis in vivo
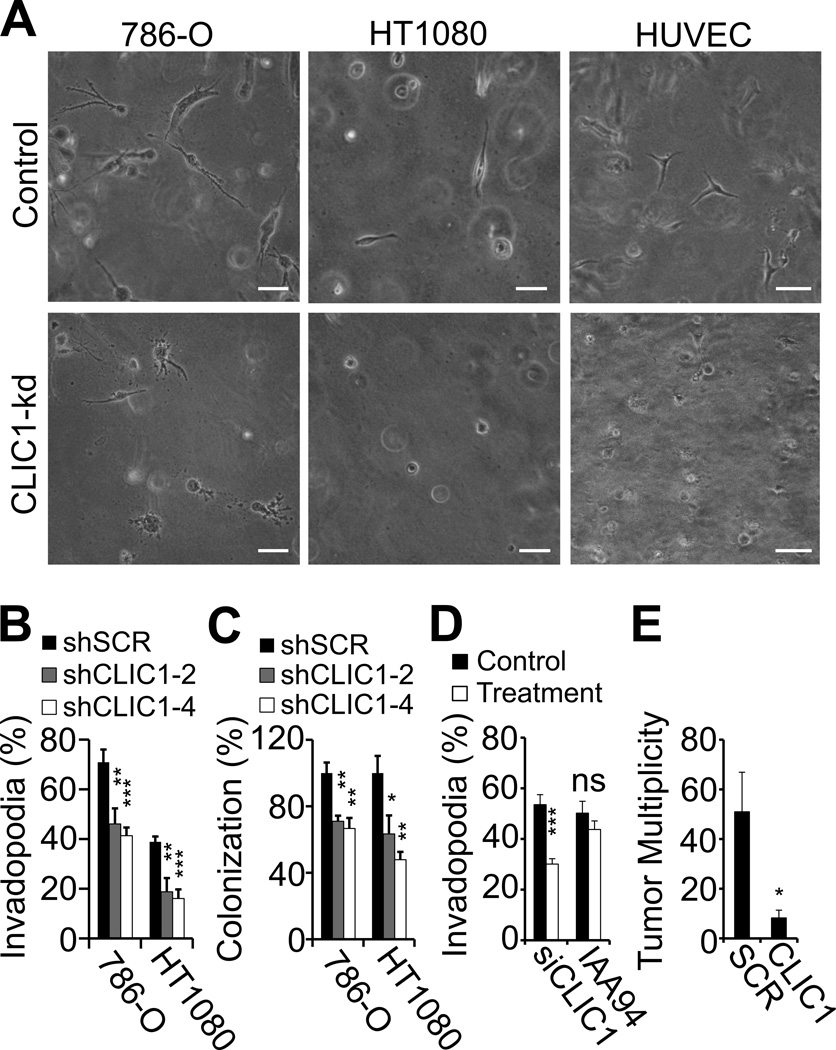
We previously identified CLIC1 as a target for the anti-angiogenic peptide CLT1 (9). This study implicated CLIC1 in regulating adhesive interactions between integrin αvβ3 and fibronectin, two adhesion proteins involved in the invasion of blood clot (6, 7). To determine if CLIC1 itself promotes clot invasion, we embedded CLIC1-depleted 786-0 kidney cancer and HT1080 fibrosarcoma cells in a 3-dimensional matrix of fibrin and scored clots for invadopodia-positive cells by phase contrast microscopy. Knockdown with CLIC1 (shCLIC1) compared to scrambled shRNA (shSCR) led to a marked decrease of CLIC1 protein expression in two independently isolated 786-0 and HT1080 clones, which translated into significant inhibition of invadopodia as well as colony formation, but had no effect on cell death in fibrin-embedded tumor cells (Fig. 1A–C; Supplementary Fig. S1). In addition, invadopodia formation was markedly reduced, when endothelial cells were treated with siRNA against CLIC1 (Fig. 1A, D; Supplementary Fig. S1). Treatment with IAA94, on the other hand, had no effect suggesting that the role of CLIC1 in invadopodia formation is independent of its function as an ion channel. To determine if CLIC1 is relevant for metastasis, we injected shCLIC1- and shSCR-HT1080 cells into the tail vein of athymic nude mice and analyzed lungs for tumor multiplicity 3 weeks later (Fig. 1E). Paralleling our in vitro results, we found that knockdown of CLIC1 significantly reduced experimental lung metastasis, suggesting that CLIC1-mediated functions are necessary for efficient tumor cell seeding in the lungs. Together, these results show that fibrin-embedded tumor and endothelial cells depend on CLIC1 for invadopodia and colony formation in vitro and lung metastasis in vivo.
Fig. 1. CLIC1 supports invadopodia formation in vitro and metastasis in vivo.
(A–D), 786-0, HT1080 and endothelial cells (HUVEC) were analyzed for invadopodia formation 24 hours after embedding in a 3D fibrin matrix by phase contrast microscopy. (A), representative images of fibrin-embedded HUVEC, 786-0 and HT1080 cells are shown (upper panel, controls; lower panel, CLIC1-knock down cells). Scale bars, 50 µm. (B–C), phase contrast microscopy images of fibrin clots were analyzed for percent invadopodia-positive 786-0 and HT1080 cells (B) and overall cell count (C) per optical field after CLIC1 silencing with two CLIC1 shRNA vectors (shCLIC1–2 and 4) compared to scrambled shRNA (shSCR). (D), invadopodia formation in fibrin-embedded HUVEC after transfection with CLIC1 siRNA (control, non-targeted siRNA) or after treatment with 200 µM IAA94 (control, DMSO). (E), the number of lung tumors (tumor multiplicity) was assessed 3 weeks after i.v. injection of 5 × 105 CLIC1 or SCR shRNA-transformed HT1080 cells. * p<0.05, ** p<0.01, *** p<0.001.
CLIC1 is upregulated in invadopodia of fibrin-embedded tumor and endothelial cells
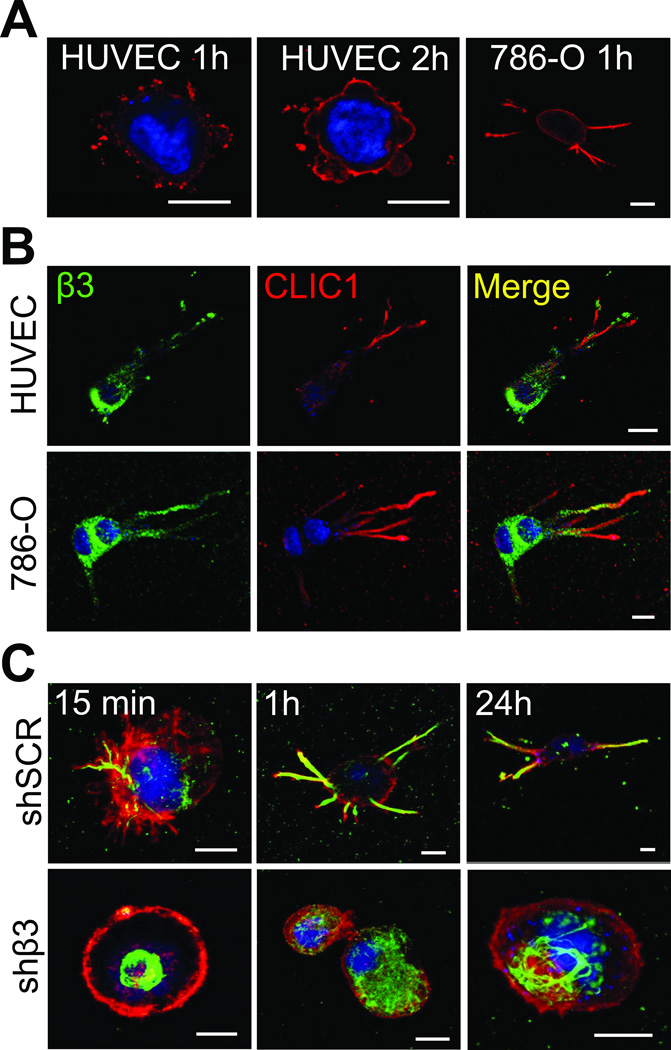
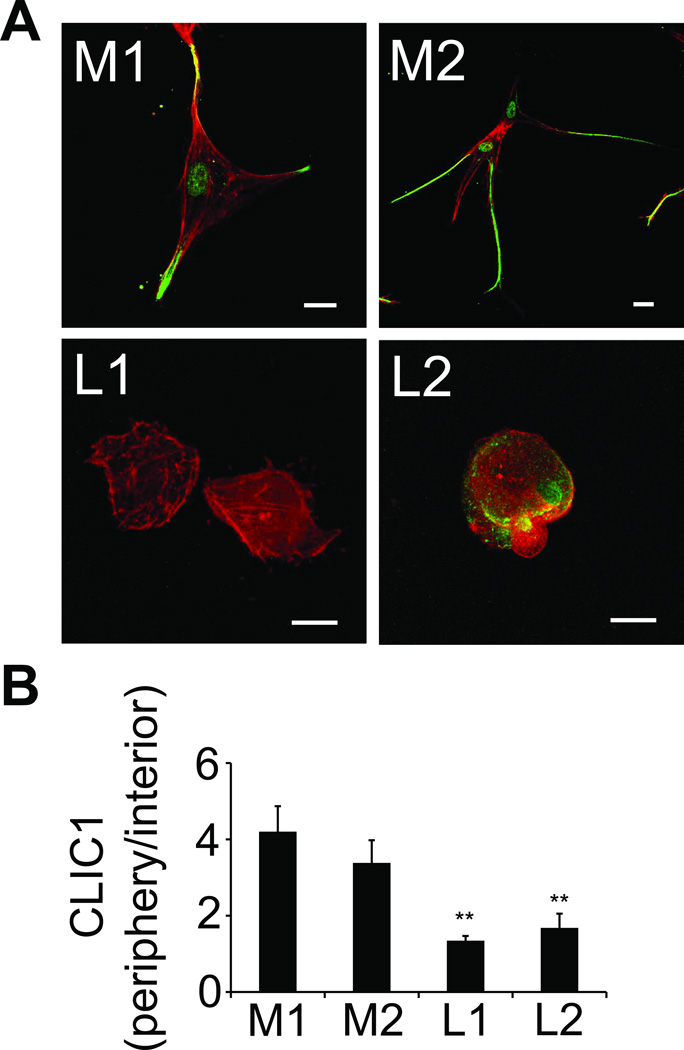
To determine the function of CLIC1 for invadopodia formation, we analyzed the subcellular localization of CLIC1 in fibrin-embedded HUVEC and 786-0 cells by confocal microscopy. This study revealed that CLIC1 expression was concentrated around nascent invadopodia first in a punctate pattern (after 1 hour) and then in form of a solid, cortical ring (after 2 hours) (Fig. 2A). From a largely cortical localization, CLIC1 shifted towards invadopodia, which developed in 786-0 within 1 hour and in HUVEC within 24 hours of embedding (Fig. 2A–B). After 24 hours, CLIC1 was almost exclusively localized in mature invadopodia of both HUVEC and 786-0, where it partially co-localized with β3 integrin (Fig. 2B). Conversely, we found that recruitment of CLIC1 towards the cell membrane or into invadopodia was largely impaired in β3-depleted 786-0 cells (shβ3) even though CLIC1 was expressed at similar protein levels (Fig. 2C; Supplementary Fig. S2). To determine the clinical relevance of CLIC1 expression and localization, we stained primary tumor cells from patients after embedding in fibrin with anti-CLIC1. Using confocal microscopy, we found a strong proclivity of CLIC1 for invadopodia in RCC cells from 2 patients with metastasis (Fig. 3). This was in sharp contrast to primary RCC cells from 2 patients without metastasis, which lacked the pronounced expression of CLIC1 at the cell membrane. These results show that the localization of CLIC1 in invadopodia correlates with expression of β3 integrin. Moreover they suggest that CLIC1 is highly enriched in invadopodia of metastatic tumor cells.
Fig. 2. CLIC1 is upregulated in invadopodia of fibrin-embedded tumor and endothelial cells.
(A), confocal microscopy images of HUVEC or 786-0 cells embedded in fibrin clots for 1 or 2 hours after immunostaining with anti-CLIC1 (red). Nuclei are stained with draq5 (blue). (B) Confocal microscopy images of 24 hour fibrin embedded HUVEC and 786-0 cells after immunostaining for β3 integrin (green) and CLIC1 (red). Nuclei are stained with draq5 (blue). (C), fibrin-embedded 786-0 cells transformed with scrambled (shSCR, top) or β3 shRNA (shβ3, bottom) were fixed after indicated times and probed for CLIC1 (green) and F-actin (red). Nuclei are stained with draq5 (blue). Scale bars, 10 µm.
Fig. 3. CLIC1 is upregulated in invadopodia of metastatic RCC.
(A), primary kidney tumor cells from 2 patients with metastasis (M1/2) and 2 without metastasis (L1/2) were embedded in fibrin clots for 24 hours. In preparation for confocal microscopy, the fibrin-embedded cells were fixed and co-stained with anti-CLIC1 (green) and phalloidin (red). Scale bars, 20 µm. (B), CLIC1 fluorescence intensity is depicted as a ratio of peripheral to interior CLIC1 in primary tumor cells from metastatic (M) and localized (L) kidney cancer to assess CLIC1 redistribtion to invadopodia at indicated times. **P < 0.01, L1 and 2 vs. M1 and P < 0.05, L1 and 2 vs. M2.
CLIC1 promotes tumor cell spreading through effects on myosin-light chain kinase
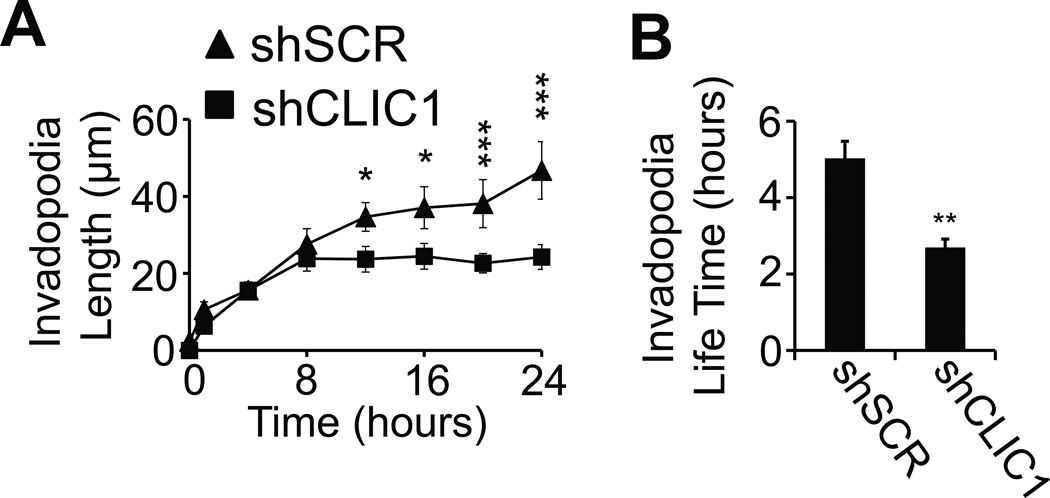
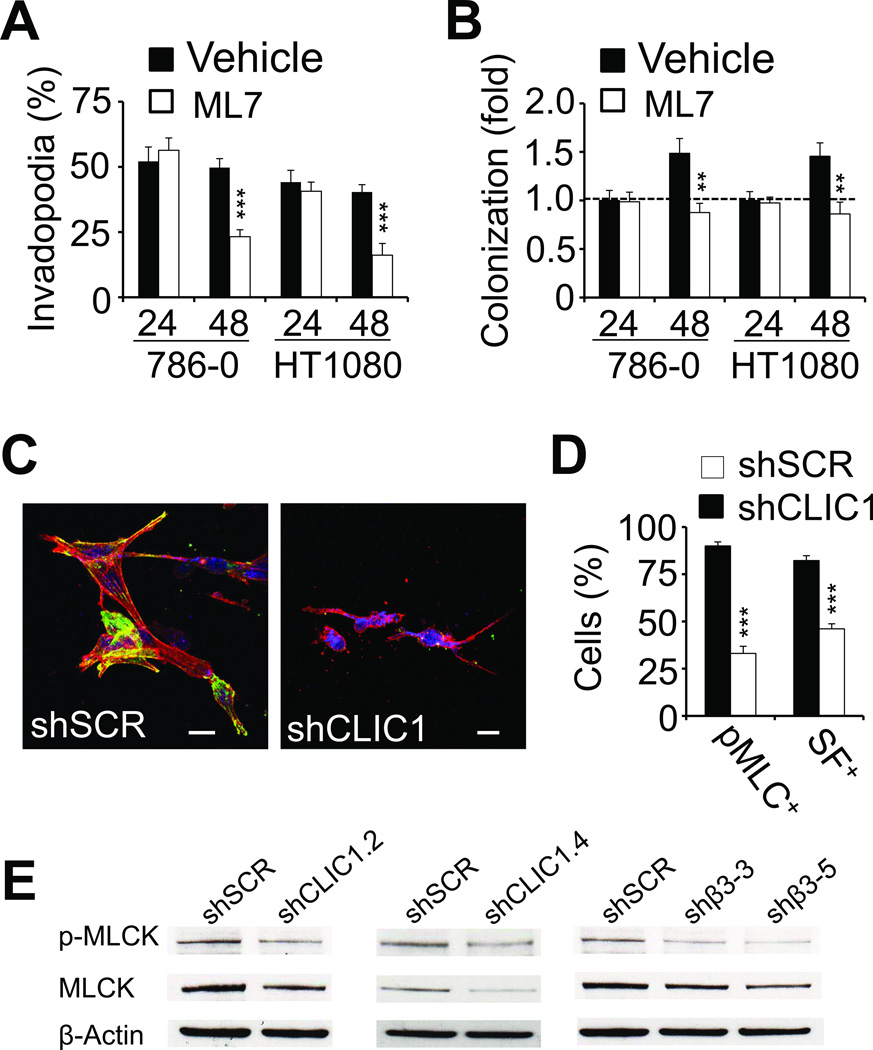
To establish a detailed account of the role of CLIC1 for tumor cell spreading, we performed live cell imaging with fibrin-embedded 786-0 cells. Video microscopy revealed that both 786-0-shCLIC1 and the control 786-0-shSCR cells were very similar in the early phase (0–8 hours), which is characterized by the formation of dynamic, rapidly extending and retracting invadopodia (Fig. 4A–B; supplementary movie 1 and 2). Then, after 8 hours, control cells began to stabilize and further lengthen their invadopodia as a prerequisite for spreading. Invadopodia in CLIC1 knockdown cells, in contrast, did not gain much length after 8 hours and, instead, destabilized at an increasing rate leaving membrane fragments behind. Interestingly, a similar effect was achieved when we inhibited cell spreading with the myosin-light chain kinase (MLCK) inhibitor ML7. While ML7 had no effect on the initial invadopodia outgrowth (i.e. within the first 24 hours), the inability of cells to generate stress fibers correlated with significantly reduced invadopodia stability and colony formation, indicating that myosin light chain kinase generates important signals for proliferation in 3D fibrin (Fig. 5A–B). To follow up on these results, we analyzed CLIC1-dependent actomyosin dynamics by staining 48 hour fibrin-embedded 786-0 cells with an antibody against phosphorylated myosin light chain (MLC) as well as phalloidin. Using confocal microscopy, we found that CLIC1 knockdown significantly impaired the capacity of fibrin-embedded tumor cells to activate MLC and generate stress fibers (Fig. 5C–D). Further upstream, we detected a marked reduction of phosphorylated and total myosin light chain kinase (MLCK) in 786-0-shCLIC1 while RhoA activity remained unaffected (Fig. 5E; Supplementary Fig. S3A). Interestingly, MLCK was also reduced in 786-0-shβ3, suggesting that CLIC1 regulates myosin light chain activity downstream of integrin αvβ3 (Fig. 5E). Together, our results indicate that CLIC1 promotes tumor cell spreading through effects on MLCK.
Fig. 4. CLIC1 stabilizes invadopodia.
(A–B), phase contrast images of 786-0 cells transformed with CLIC1 or scrambled shRNA (SCR) were captured at indicated times using BioStation IM live microscopy to assess invadopodia length (A) and life time (B) after embedding in fibrin. * p<0.05, ** p<0.01, *** p<0.001.
Fig. 5. CLIC1 promotes tumor cell spreading through effects on myosin-light chain kinase.
(A–B), 786-0 as well as HT1080 cells treated with 10 µM ML7 or vehicle were analyzed for invadopodia (A) and colony formation (B) 24 and 48 hours after embedding in fibrin using phase contrast microscopy. ** p<0.01, *** p<0.001. (C), confocal microscopy images of shSCR (left) and shCLIC1 (right) 786-0 embedded in fibrin (48 hours) and stained for phospho-MLC (green) as well as F-actin (red). Nuclei are stained with draq5 (blue). Scale bars, 20 µm. (D), fluorescence microscopy fields were scored for phospho-MLC- and stress fiber (SF)-positive 786-0 cells as percent of total after 48 hours embedding in fibrin. (E), extracts from 786-0 cells treated with CLIC1 and β3 shRNA (2 clones each) were probed for MLCK or phospho-MLCK. Controls were treated with scrambled shRNA (SCR). β-actin shows protein loading.
CLIC1 and MLCK are necessary for fibronectin matrix formation in 3D fibrin
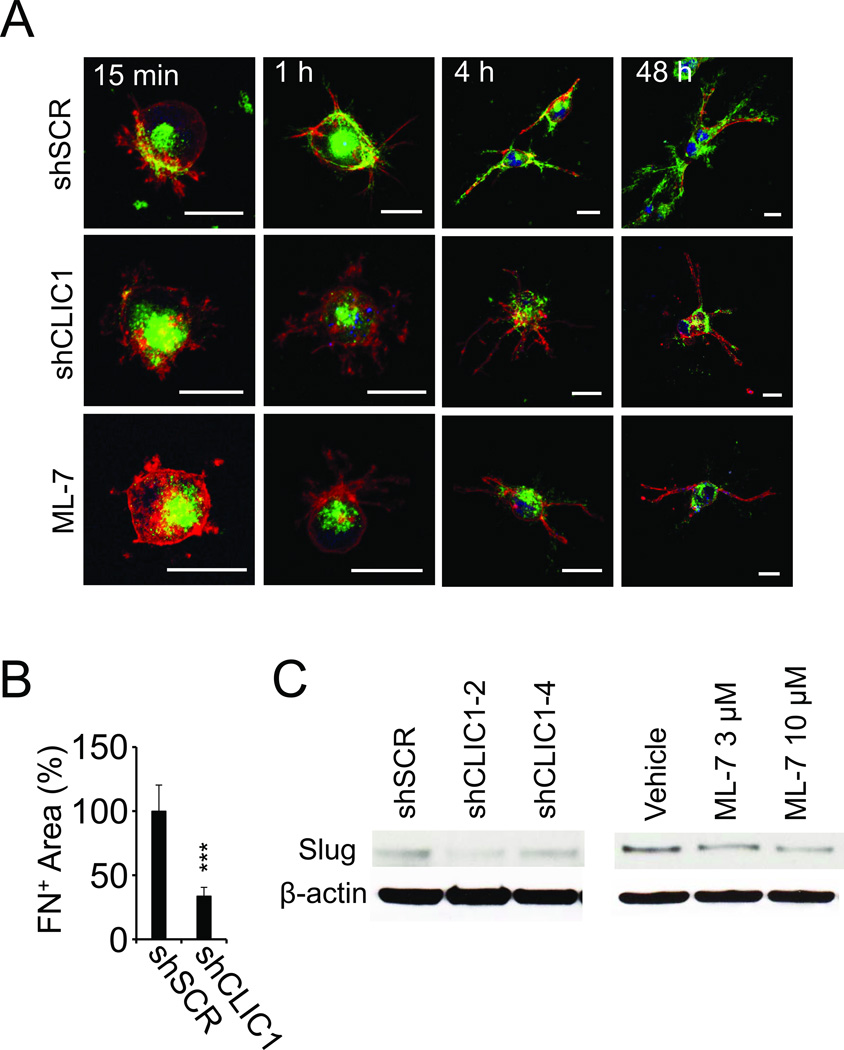
Actin-myosin interactions are a prerequisite for fibronectin fibril formation (16). Therefore, we decided to assess the effect of CLIC1 on fibronectin matrix assembly. Staining for fibronectin and F-actin showed that fibrin-embedded 786-0-shSCR control cells begin to develop invadopodia within 15 minutes after embedding in fibrin and that this process was accompanied by the relocation of intracellular fibronectin towards the cortical cytoskeleton adjacent to the nascent invadopodia (Fig. 6A). Between 1 and 4 hours, fibronectin co-localized increasingly with invadopodia while by 48 hours a mature extracellular matrix was visible in areas where invadopodia have retracted. Notably, this process of fibronectin assembly was impaired in shCLIC1-transformed as well as tumor cells treated with the MLCK inhibitor ML-7 resulting in a significantly diminished fibronectin matrix after 48 hours of embedding in fibrin (Fig. 6A–B; Supplementary Fig. S3B). We previously showed that fibronectin matrix assembly is necessary to maintain expression of the EMT master regulator Slug (6). Paralleling these data, we detected reduced Slug expression in 786-0-shCLIC1 cells as well as in 786-0 cells treated with ML-7 (Fig. 6C). Together these results indicate that both CLIC1 and MLCK are necessary for fibronectin matrix assembly and expression of the EMT master regulator Slug.
Fig. 6. CLIC1 promotes fibronectin matrix formation in 3D fibrin.
(A), fibrin-embedded 786-0 transformed with shSCR (upper panel), shCLIC1 (middle panel) or treated with ML-7 (10 µM; lower panel) were fixed after indicated times and probed for fibronectin (green) and F-actin (red) using confocal microscopy. Nuclei are stained with draq5 (blue). Scale bars, 20 µm. (B), fibronectin matrix formation was assessed from non-permeabilized 786-0 cells embedded in fibrin using confocal microscopy. (C), extracts from 786-0 cells treated with CLIC1 shRNA or exposed to ML-7 for 48 hours were probed for Slug. Controls were treated with scrambled shRNA (SCR) or vehicle, respectively. β-actin shows protein loading.
DISCUSSION
We previously identified CLIC1 as an internalizing receptor for the anti-angiogenic peptide CLT1 on proliferating endothelial cells (9). Here, we demonstrate that CLIC1 supports invadopodia stability and stress fiber formation in clot-embedded cells through effects on MLCK. This function was accompanied by relocation of CLIC1 into invadopodia, which in turn depended on the expression of β3 integrin. Through its effects on actomyosin, CLIC1 supports fibronectin matrix assembly as well as Slug expression and, therefore, mediates critical tumor cell functions such as fibrin invasion, colony formation and lung metastasis.
CLIC1 was originally identified as a nuclear chloride channel protein (NCC27) in a macrophage cell line, but since its discovery CLIC1 expression has been detected in many different tissues (17, 18). Despite its ubiquitous expression, CLIC1 has regularly been shown to be up-regulated in patients with malignant tumors of the brain, liver, lung, ovaries and gastro-intestinal tract and in many of these malignancies, CLIC1 expression correlates with aggressive disease and poor outcome (12, 19–22). In addition, CLIC1 has been shown to be over-expressed in the tumor vasculature indicating that CLIC1 plays an important role in the different aspects of tumor growth and metastasis (10). In agreement with this, CLIC1 was found to be involved in cell migration, invasion and proliferation but its function in these processes remains largely elusive (15, 23, 24). We previously demonstrated that tumor metastasis to the lungs correlates with the ability of tumor cells to generate invadopodia in clotted plasma and that this mechanism depends on integrin αvβ3 and fibronectin (6, 7, 25). Here, we established that CLIC1 cooperates with integrin αvβ3 and fibronectin to support invadopodia and colony formation in fibrin in vitro and that this function correlates with the capacity of CLIC1 to promote lung metastasis in vivo.
Our data indicate that CLIC1 controls invadopodia stability and cell spreading by regulating MLCK, which is known to mediate critical biological functions such as cell division and motility (26, 27). In addition, MLCK has important effects on vesicular transport as well as cell contractility, which is required for the assembly of single fibronectin molecules into fibrils (28, 29). The resulting fibronectin matrix in turn provides the necessary rigidity for cells to generate stress fibers in matrices as soft as fibrin (6, 30). Fibronectin has also been shown to contribute to myosin light chain activity via syndecan-dependent stimulation of the RhoA/ROCK pathway (31). However, this mechanism did not seem to play a role in CLIC1 knockdown cells, which exhibit levels of activated, GTP-loaded RhoA comparable to cells treated with a control vector. Instead, it appears that CLIC1 supports tumor colonization and metastasis through alternative effects on the actin cytoskeleton and that these effects include regulation of myosin-light chain kinase. This interpretation is in line with a previous report showing that MLCK promotes cell cycle entry of single dormant breast cancer cells (30). Similar to our results, proliferation and metastasis in this system depended on the capacity of tumor cells to spread and generate a fibronectin matrix in a 3-dimensional matrix of collagen.
CLIC1 is a metamorphic protein that can exist in at least two distinct forms, namely as a soluble monomer featuring structural characteristics of a glutathion transferase or as an insoluble, membrane-spanning oligomer with chloride channel function (32). To this end, CLIC1 plays an important role for the phagocytic function of macrophages, which appears to depend on CLIC1 chloride conductance for phagosome acidification (33). This function of CLIC1 is resembled by a close relative, CLIC4, which mediates acidification of vacuoles in endothelial cells (34). However, it is important to note that CLIC4 has a number of additional functions, which do not seem to depend on anion currents, including the stabilization of phospho-smad2/3 as well as the p65 subunit of NFκB (35, 36). The interaction of CLIC4 with NFκB in endothelial cells is significant for pulmonary hypertension as it leads to activation of HIF1α and induction of an overall mesenchymal phenotype with invadopodia and stress fibers (36). Another CLIC family member, CLIC3, in turn contributes to cell invasion through recycling of MT1-MMP and integrin α5β1 to the cell surface suggesting that stabilizing proteins involved in cell-extracellular matrix interactions is a common theme of CLIC family members (37, 38). CLIC1 fits neatly into this scheme as it functions to maintain MLCK expression, either directly or indirectly.
We previously showed that interaction of a tumor homing peptide CLT1 with CLIC1 and CLIC3 can cause endocytosis of fibronectin-CLT1 complexes (9, 39). A prerequisite of CLIC1-mediated internalization was the ligation of integrin αvβ3 with fibronectin, which caused CLIC1 cell surface expression (9). CLIC3-mediated internalization on the other hand depended on ligation of integrin α5β1 (39). Paralleling these data, our current study indicates that the process of relocating CLIC1 into invadopodia depends on β3 integrin. Interestingly, CLIC1 translocation into the cell membrane can be induced by NADPH oxidase, which in turn has been shown to be activated by the Rho GTPase Rac following integrin ligation (40, 41). Another key function of Rac is to mediate F-actin formation and, thus, to provide membrane binding sites for CLIC1 in invadopodia (42, 43). Notably, binding of CLIC1 to F-actin has been shown to inhibit CLIC1 chloride conductance, which is in agreement with our result that the CLIC1 channel blocker IAA94 had no effect on invadopodia formation (42).
Based on our results, we propose a model where β3 integrin-mediated recruitment of CLIC1 into invadopodia promotes expression of MLCK in clot-embedded tumor and, presumably, endothelial cells. The resulting contraction of the actin cytoskeleton is necessary for the generation of stress fibers as well as fibronectin fibrils in fibrin-embedded tumor cells and this in turn is a prerequisite for focal adhesion-dependent growth signaling (30, 44). Our previous results show that fibronectin expression plays an important role in maintaining expression of the EMT transcription factor Slug, which in turn promotes fibrin invasion and lung metastasis (6). In line with the concept that CLIC1 acts through stress fiber and fibronectin matrix formation, we show now that clot colonization, Slug expression and lung metastasis are impaired in CLIC1 knockdown cells. Moreover, antagonizing myosin light chain function with the MLCK inhibitor ML-7 also impairs invadopodia stability, fibronectin matrix assembly and Slug expression, strongly suggesting that the role of CLIC1 in clot colonization and Slug expression is a direct result of CLIC1’s effect on MLCK. Together, this study indicates an important function of CLIC1 in the regulation of cell-extracellular matrix interactions, which has direct effects on the ability of circulating tumor cells to colonize clot in vitro and to metastasize to distant organs in vivo. Thus, strategies to inhibit CLIC1 could be useful for the treatment of aggressive cancer.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Dr. Robert Sobol and Ashley Brown from the UPCI Vector Core Facility for constructing shRNA vectors. This project used the UPCI Cell and Tissue Imaging Facility, UPCI Animal Facility and the UPCI Vector Core Facility, which are supported by the UPCI Cancer Center Support Grant.
GRANT SUPPORT
This work was supported by National Institutes of Health grants CA134330 (JP), 5T32DK007774-14 (LAG), and P30CA047904 (UPCI CCSG).
Footnotes
The authors have no potential conflict of interest.
REFERENCES
- 1.Millien VO, Lu W, Shaw J, Yuan X, Mak G, Roberts L, et al. Cleavage of fibrinogen by proteinases elicits allergic responses through Toll-like receptor 4. Science. 2013;341:792–796. doi: 10.1126/science.1240342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van den Berg YW, van den Hengel LG, Myers HR, Ayachi O, Jordanova E, Ruf W, et al. Alternatively spliced tissue factor induces angiogenesis through integrin ligation. Proc Natl Acad Sci U S A. 2009;106:19497–19502. doi: 10.1073/pnas.0905325106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drew AF, Liu H, Davidson JM, Daugherty CC, Degen JL. Wound-healing defects in mice lacking fibrinogen. Blood. 2001;97:3691–3698. doi: 10.1182/blood.v97.12.3691. [DOI] [PubMed] [Google Scholar]
- 4.Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105:178–185. doi: 10.1182/blood-2004-06-2272. [DOI] [PubMed] [Google Scholar]
- 5.Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3:1894–1904. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 6.Knowles LM, Gurski LA, Engel C, Gnarra JR, Maranchie JK, Pilch J. Integrin alphavbeta3 and fibronectin upregulate Slug in cancer cells to promote clot invasion and metastasis. Cancer Res. 2013;73:6175–6184. doi: 10.1158/0008-5472.CAN-13-0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malik G, Knowles LM, Dhir R, Xu S, Yang S, Ruoslahti E, et al. Plasma fibronectin promotes lung metastasis by contributions to fibrin clots and tumor cell invasion. Cancer Res. 2010;70:4327–4334. doi: 10.1158/0008-5472.CAN-09-3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, Tan Y, Zhang H, Zhang Y, Xu P, Chen J, et al. Soft fibrin gels promote selection and growth of tumorigenic cells. Nat Mater. 2012;11:734–741. doi: 10.1038/nmat3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knowles LM, Malik G, Hood BL, Conrads TP, Pilch J. CLT1 targets angiogenic endothelium through CLIC1 and fibronectin. Angiogenesis. 2012;15:115–129. doi: 10.1007/s10456-011-9247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill JJ, Tremblay TL, Pen A, Li J, Robotham AC, Lenferink AE, et al. Identification of vascular breast tumor markers by laser capture microdissection and label-free LC-MS. J Proteome Res. 2011;10:2479–2493. doi: 10.1021/pr101267k. [DOI] [PubMed] [Google Scholar]
- 11.Li RK, Zhang J, Zhang YH, Li ML, Wang M, Tang JW. Chloride intracellular channel 1 is an important factor in the lymphatic metastasis of hepatocarcinoma. Biomed Pharmacother. 2012;66:167–172. doi: 10.1016/j.biopha.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Wang W, Xu X, Shao W, Li L, Yin W, Xiu L, et al. The expression and clinical significance of CLIC1 and HSP27 in lung adenocarcinoma. Tumour Biol. 2011;32:1199–1208. doi: 10.1007/s13277-011-0223-0. [DOI] [PubMed] [Google Scholar]
- 13.Tang HY, Beer LA, Tanyi JL, Zhang R, Liu Q, Speicher DW. Protein isoform-specific validation defines multiple chloride intracellular channel and tropomyosin isoforms as serological biomarkers of ovarian cancer. J Proteomics. 2013;89:165–178. doi: 10.1016/j.jprot.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng DL, Huang QL, Zhou F, Huang QJ, Lin JY, Lin X. PA28beta regulates cell invasion of gastric cancer via modulating the expression of chloride intracellular channel 1. J Cell Biochem. 2012;113:1537–1546. doi: 10.1002/jcb.24022. [DOI] [PubMed] [Google Scholar]
- 15.Tung JJ, Kitajewski J. Chloride intracellular channel 1 functions in endothelial cell growth and migration. J Angiogenes Res. 2010;2:23. doi: 10.1186/2040-2384-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwarzbauer JE, Sechler JL. Fibronectin fibrillogenesis: a paradigm for extracellular matrix assembly. Curr Opin Cell Biol. 1999;11:622–627. doi: 10.1016/s0955-0674(99)00017-4. [DOI] [PubMed] [Google Scholar]
- 17.Valenzuela SM, Martin DK, Por SB, Robbins JM, Warton K, Bootcov MR, et al. Molecular cloning and expression of a chloride ion channel of cell nuclei. J Biol Chem. 1997;272:12575–12582. doi: 10.1074/jbc.272.19.12575. [DOI] [PubMed] [Google Scholar]
- 18.Ulmasov B, Bruno J, Woost PG, Edwards JC. Tissue and subcellular distribution of CLIC1. BMC Cell Biol. 2007;8:8. doi: 10.1186/1471-2121-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang S, Wang XM, Yin ZY, Zhao WX, Zhou JY, Zhao BX, et al. Chloride intracellular channel 1 is overexpression in hepatic tumor and correlates with a poor prognosis. APMIS. 2013;121:1047–1053. doi: 10.1111/apm.12093. [DOI] [PubMed] [Google Scholar]
- 20.Chen CD, Wang CS, Huang YH, Chien KY, Liang Y, Chen WJ, et al. Overexpression of CLIC1 in human gastric carcinoma and its clinicopathological significance. Proteomics. 2007;7:155–167. doi: 10.1002/pmic.200600663. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, He S, Tu Y, Ji P, Zong J, Zhang J, et al. Elevated expression of chloride intracellular channel 1 is correlated with poor prognosis in human gliomas. J Exp Clin Cancer Res. 2012;31:44. doi: 10.1186/1756-9966-31-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang HY, Beer LA, Chang-Wong T, Hammond R, Gimotty P, Coukos G, et al. A xenograft mouse model coupled with in-depth plasma proteome analysis facilitates identification of novel serum biomarkers for human ovarian cancer. J Proteome Res. 2012;11:678–691. doi: 10.1021/pr200603h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang JW, Peng SY, Li JT, Wang Y, Zhang ZP, Cheng Y, et al. Identification of metastasis-associated proteins involved in gallbladder carcinoma metastasis by proteomic analysis and functional exploration of chloride intracellular channel 1. Cancer Lett. 2009;281:71–81. doi: 10.1016/j.canlet.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 24.Setti M, Savalli N, Osti D, Richichi C, Angelini M, Brescia P, et al. Functional role of CLIC1 ion channel in glioblastoma-derived stem/progenitor cells. J Natl Cancer Inst. 2013;105:1644–1655. doi: 10.1093/jnci/djt278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knowles LM, Malik G, Pilch J. Plasma Fibronectin Promotes Tumor Cell Survival and Invasion through Regulation of Tie2. J Cancer. 2013;4:383–390. doi: 10.7150/jca.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Komatsu S, Yano T, Shibata M, Tuft RA, Ikebe M. Effects of the regulatory light chain phosphorylation of myosin II on mitosis and cytokinesis of mammalian cells. J Biol Chem. 2000;275:34512–34520. doi: 10.1074/jbc.M003019200. [DOI] [PubMed] [Google Scholar]
- 27.Kunida K, Matsuda M, Aoki K. FRET imaging and statistical signal processing reveal positive and negative feedback loops regulating the morphology of randomly migrating HT-1080 cells. J Cell Sci. 2012;125:2381–2392. doi: 10.1242/jcs.096859. [DOI] [PubMed] [Google Scholar]
- 28.Zhong C, Chrzanowska-Wodnicka M, Brown J, Shaub A, Belkin AM, Burridge K. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol. 1998;141:539–551. doi: 10.1083/jcb.141.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neco P, Giner D, Viniegra S, Borges R, Villarroel A, Gutierrez LM. New roles of myosin II during vesicle transport and fusion in chromaffin cells. J Biol Chem. 2004;279:27450–27457. doi: 10.1074/jbc.M311462200. [DOI] [PubMed] [Google Scholar]
- 30.Barkan D, Kleinman H, Simmons JL, Asmussen H, Kamaraju AK, Hoenorhoff MJ, et al. Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res. 2008;68:6241–6250. doi: 10.1158/0008-5472.CAN-07-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saoncella S, Echtermeyer F, Denhez F, Nowlen JK, Mosher DF, Robinson SD, et al. Syndecan-4 signals cooperatively with integrins in a Rho-dependent manner in the assembly of focal adhesions and actin stress fibers. Proc Natl Acad Sci U S A. 1999;96:2805–2810. doi: 10.1073/pnas.96.6.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Littler DR, Harrop SJ, Fairlie WD, Brown LJ, Pankhurst GJ, Pankhurst S, et al. The intracellular chloride ion channel protein CLIC1 undergoes a redox-controlled structural transition. J Biol Chem. 2004;279:9298–9305. doi: 10.1074/jbc.M308444200. [DOI] [PubMed] [Google Scholar]
- 33.Jiang L, Salao K, Li H, Rybicka JM, Yates RM, Luo XW, et al. Intracellular chloride channel protein CLIC1 regulates macrophage function through modulation of phagosomal acidification. J Cell Sci. 2012;125:5479–5488. doi: 10.1242/jcs.110072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ulmasov B, Bruno J, Gordon N, Hartnett ME, Edwards JC. Chloride intracellular channel protein-4 functions in angiogenesis by supporting acidification of vacuoles along the intracellular tubulogenic pathway. Am J Pathol. 2009;174:1084–1096. doi: 10.2353/ajpath.2009.080625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shukla A, Malik M, Cataisson C, Ho Y, Friesen T, Suh KS, et al. TGF-beta signalling is regulated by Schnurri-2-dependent nuclear translocation of CLIC4 and consequent stabilization of phospho-Smad2 and 3. Nat Cell Biol. 2009;11:777–784. doi: 10.1038/ncb1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wojciak-Stothard B, Abdul-Salam VB, Lao KH, Tsang H, Irwin DC, Lisk C, et al. Aberrant chloride intracellular channel 4 expression contributes to endothelial dysfunction in pulmonary arterial hypertension. Circulation. 2014;129:1770–1780. doi: 10.1161/CIRCULATIONAHA.113.006797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Macpherson IR, Rainero E, Mitchell LE, van den Berghe PV, Speirs C, Dozynkiewicz MA, et al. CLIC3 controls recycling of late endosomal MT1-MMP and dictates invasion and metastasis in breast cancer. J Cell Sci. 2014 doi: 10.1242/jcs.135947. [DOI] [PubMed] [Google Scholar]
- 38.Dozynkiewicz MA, Jamieson NB, Macpherson I, Grindlay J, van den Berghe PV, von Thun A, et al. Rab25 and CLIC3 collaborate to promote integrin recycling from late endosomes/lysosomes and drive cancer progression. Dev Cell. 2012;22:131–145. doi: 10.1016/j.devcel.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knowles LM, Zewe J, Malik G, Parwani AV, Gingrich JR, Pilch J. CLT1 targets bladder cancer through integrin alpha5beta1 and CLIC3. Mol Cancer Res. 2013;11:194–203. doi: 10.1158/1541-7786.MCR-12-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Milton RH, Abeti R, Averaimo S, DeBiasi S, Vitellaro L, Jiang L, et al. CLIC1 function is required for beta-amyloid-induced generation of reactive oxygen species by microglia. J Neurosci. 2008;28:11488–11499. doi: 10.1523/JNEUROSCI.2431-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu P, Tan MJ, Huang RL, Tan CK, Chong HC, Pal M, et al. Angiopoietin-like 4 protein elevates the prosurvival intracellular O2(−):H2O2 ratio and confers anoikis resistance to tumors. Cancer Cell. 2011;19:401–415. doi: 10.1016/j.ccr.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 42.Singh H, Cousin MA, Ashley RH. Functional reconstitution of mammalian 'chloride intracellular channels' CLIC1, CLIC4 and CLIC5 reveals differential regulation by cytoskeletal actin. FEBS J. 2007;274:6306–6316. doi: 10.1111/j.1742-4658.2007.06145.x. [DOI] [PubMed] [Google Scholar]
- 43.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 44.Oakes PW, Beckham Y, Stricker J, Gardel ML. Tension is required but not sufficient for focal adhesion maturation without a stress fiber template. J Cell Biol. 2012;196:363–374. doi: 10.1083/jcb.201107042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.