Abstract
Progesterone and progesterone receptors (PR) are essential for the development and cyclical regulation of hormone-responsive tissues including the breast and reproductive tract. Altered functions of PR isoforms contribute to the pathogenesis of tumors that arise in these tissues. In the breast, progesterone acts in concert with estrogen to promote proliferative and pro-survival gene programs. In sharp contrast, progesterone inhibits estrogen-driven growth in the uterus and protects the ovary from neoplastic transformation. Progesterone-dependent actions and associated biology in diverse tissues and tumors are mediated by two progesterone receptor isoforms, PR-A and PR-B. These isoforms are subject to altered transcriptional activity or expression levels, differential cross-talk with growth factor signaling pathways, and distinct post-translational modifications and cofactor binding partners. Herein, we summarize and discuss the recent literature focused on progesterone and PR isoform-specific actions in breast, uterine, and ovarian cancers. Understanding the complexity of context-dependent PR actions in these tissues is critical to developing new models that will allow us to advance our knowledge base with the goal of revealing novel and efficacious therapeutic regimens for these hormone-responsive diseases.
Keywords: progesterone, progestin, progesterone receptor, isoforms, breast cancer, endometrial cancer, uterine, ovarian cancer
Introduction
Progesterone and Progesterone Receptors (PR) are increasingly gaining attention for their emerging role as critical regulators of breast and gynecological cancers. With this newfound spotlight on PR action, there is an urgent need to define the mechanisms by which progesterone and progestins exert their effects upon tumor types in different PR+ tissues and bring clarity to areas of confusion in the field. Much of the difficulty lies in the nuanced context-dependent actions of PR, the different isoform-specific actions of PR-A relative to PR-B (PR isoforms are not measured separately in the clinic), the differential or potential off-target actions of synthetic progestins (i.e. used clinically) versus natural progesterone, and the seemingly paradoxical biological effects that progesterone exerts on the breast versus gynecological tissues. In the breast, progesterone is proliferative and works in concert with estrogens and Estrogen Receptors (ER) to induce expansion of glandular structures during development (reviewed in (Brisken and O'Malley 2010)). Progesterone is a key mediator of mammary gland stem cell expansion (Brisken 2013). In the presence of estrogen, ER-induced expression of PR is required to induce proliferation by both autocrine and paracrine mechanisms (Brisken 2013); PR-target genes include secreted factors (wnt4) that act on nearby PR-negative cells. In contrast to ER and PR cooperative actions in the breast, progesterone opposes ER actions in the ovary and endometrium, and acts in an antiproliferative manner to induce tissue regression (Kim and Chapman-Davis 2010). Our goal herein is to examine the relevant literature, clarify the rhetoric, and identify the gaps in our knowledge that require further inquiry.
Progesterone is a steroid hormone that is produced primarily by the corpus luteum in the ovaries during the second half of the menstrual cycle or luteal phase. Progesterone is also produced, to a lesser extent, in the adrenal glands and, during pregnancy, the placenta. Thus, cyclical hormone exposure beginning at menarche and ending in menopause occurs monthly and regulates the growth and differentiation of specialized tissues within the reproductive tract and breast tissues (Graham and Clarke 1997; Lydon, et al. 1995). Pregnancy interrupts this process and is characterized by high progesterone levels, which are required for fetal development, breast development for lactation, maintenance of uterine/placental integrity, and myometrial quiescence (Mendelson 2009).
PR expression in responsive tissues is driven by estrogen bound ER, therefore ER is permissive for the actions of PR and progesterone. As a result, one experimental challenge researchers face in determining the actions of PR is differentiating them from those of ER. Elegantly designed mouse models and transplant studies have delineated the developmental processes attributed to each receptor (reviewed in (Brisken and O'Malley 2010)). Briefly, PR-B is the predominant isoform required for mammary gland development and expansion, whereas PR-A is necessary for proper uterine development and reproductive actions (Conneely, et al. 2001). PR expression is limited to 10-15% of mammary luminal cells and primarily signals in a paracrine manner to induce proliferation of steroid receptor negative cells (Brisken, et al. 1998). PR-containing cells proliferate autonomously during periods of massive glandular expansion, such as pregnancy. PR is expressed in both the epithelial and stromal compartments of the breast and uterus and signals in both paracrine and autocrine manners to affect biology (Brisken 2013; Kim and Chapman-Davis 2010; Kim, et al. 2013). The actions of progesterone and its isoforms in normal physiology of the breast (see (Kariagina, et al. 2008)), uterus (see (Kim et al. 2013), and ovary (see (Modugno, et al. 2012)) have been extensively reviewed elsewhere.
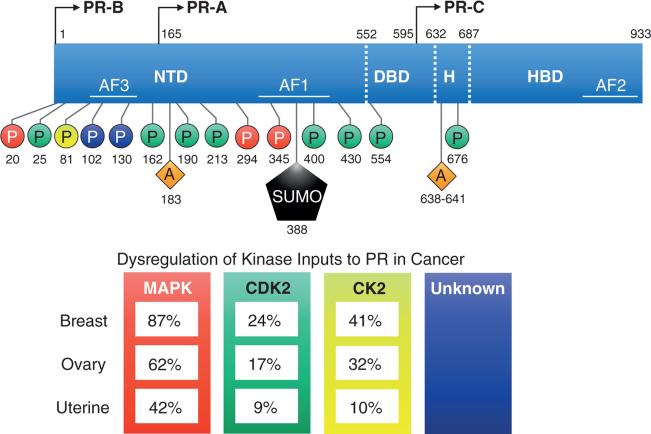
PRs are members of the steroid hormone family of nuclear receptors and as such are composed of a modular domain structure that includes an N-terminal domain (NTD), DNA binding domain (DBD), hinge region (H), and hormone binding domain (HBD) (Figure 1). There are three PR isoforms: full length PR-B, N-terminally truncated PR-A (-164 amino acids), and the non-functional PR-C, consisting of only the hinge region and HBD (Figure 1). PR-B and PR-A are typically expressed in equimolar ratios and function as ligand activated transcription factors, whereas PR-C expression is limited and may serve largely to sequester ligand, as it is incapable of binding DNA (Condon, et al. 2006).
Figure 1. The post-translational modifications of progesterone receptors.
17 post-translational modification sites that impact PR-mediated transcriptional action. PR-B, but not PR-A, includes 164 additional amino acids in the NTD (called B upstream segment) where the third activation function domain and multiple phosphorylation sites are located. PR-B and PR-A are transcribed from the same gene and their protein isoforms are identical from amino acids 165-993. The protein tertiary structure results in a folding at the hinge region between the DBD and HBD. Post-translational modifications (phosphorylation, acetylation, and SUMOylation) can occur basally or in response to ligand binding and affect PR transcriptional activity. In particular, activated protein kinase pathways input to PR via phosphorylation and these pathways are heavily altered in breast, ovarian, and uterine carcinomas. Numbering reflects amino acid residue positions. The color of phosphorylation sites is associated with the following: red = MAPK; green = CDK2; yellow = CK2; purple = unknown kinases. PR, progesterone receptor protein isoforms A, B, or C; NTD, N-(amino)-terminal domain; DBD, DNA binding domain; H, hinge region; HBD, hormone binding domain; AF, activation function 1-3; P, phosphorylation; A, acetylation; SUMO, small ubiquitin-like modifier (SUMOylation). Dysregulation of Kinase Inputs to PR in Cancer: The percent of TCGA (The Cancer Genome Atlas) tumors containing alterations in MAPK, CDK2, or CK2 components were identified using the cBioPortal.org analysis tool. For analysis of dysregulated kinases: MAPK includes canonical c-Raf, Mek, and Erk signaling pathway genes: RAF1, MAP3K1, MAP3K2, MAPK3, MAPK1; CKD2, cyclin-dependent kinase 2; CK2, casein kinase 2, alpha 1 polypeptide, CSNK2A1.
Progesterone diffuses through the lipid membrane and interacts with the HBD of PR-A or PR-B. This interaction alters the confirmation of PR favoring nuclear localization, dimerization (A:A, B:B, or A:B dimers are possible), and DNA binding. Classically, PR binds Progesterone Response Elements (PRE) in the DNA and recruits cofactors and transcriptional machinery to initiate gene transcription. PR can also participate in the transcriptional complexes of other DNA bound transcription factors to alter gene expression (Cicatiello, et al. 2004; Faivre, et al. 2008; Owen, et al. 1998; Stoecklin, et al. 1999). Non-classical or extranuclear signaling of PR involves PR direct binding to kinases complexed at the membrane with growth factor receptors (such as EGFR or IGF1R) to initiate rapid activation of downstream signaling cascades (Boonyaratanakornkit, et al. 2001; Migliaccio, et al. 1998). For example, progesterone induces rapid activation of ERK1/2 MAP kinase pathways, which function to activate a variety of transcription factors via phosphorylation events, including PR itself (Boonyaratanakornkit et al. 2001; Faivre et al. 2008; Migliaccio et al. 1998) (Figure 1). Notably, PR-B, but not PR-A, is capable of rapid signaling, likely in part owing to its relatively increased occupancy in the cytoplasm (Boonyaratanakornkit, et al. 2007). The regulation of gene programs driven by PR and progesterone is highly dependent on the local cellular environment and the intracellular signaling context. Thus, PRs act as ‘sensors’ of cell context, rapidly adjusting to hormonal fluctuation and integrating a variety of extracellular signals to enable tight control of developmental programs. The mechanisms by which PR selects and modulates genetic programs in response to variable external signals are discussed below.
Breast
Proliferative actions of PR in the breast
Progesterone, acting through PR, is a critical mediator of mammary gland tissue expansion during breast development after puberty. Mouse models lacking PR-B, but not PR-A, exhibit marked defects in mammary gland branching and alveologenesis (Conneely, et al. 2003), supporting the concept that PR-B is the predominant isoform required for mammary gland development and expansion. Interestingly, ER is also required for mammary gland proliferation during pubertal development (Daniel, et al. 1987). However, in the adult mammary gland, proliferation occurs via the actions of PR at its primary target genes, while ER is necessary for PR expression (Brisken 2013). In hormone ablation and replacement studies in adult mice, ovariectomy halts glandular proliferation. Exogenous estrogen alone provides a weak signal, whereas treatment with estrogen and progesterone restores glandular proliferation (Wang, et al. 1990). Tissue transplant studies in genetically modified mice demonstrated that ER and PR induce proliferation in mammary gland structures primarily via paracrine signaling (Brisken et al. 1998). PR is expressed in both the epithelial and stromal compartments of the breast and is limited to 10-15% of mammary luminal cells (Brisken 2013). Progestin stimulation of PR-positive mammary epithelial cells induces transcription and secretion of multiple mitogenic factors, including Wnts, Areg, HB-EGF, and RANKL that induce proliferation of neighboring PR-negative cells (Brisken 2013) (Figure 2). Recent evidence has implicated that these same PR signaling outputs (RANKL, WNT) are required for maintenance and expansion of the mammary gland stem cell compartment. Studies in mice blocking either PR or its downstream effector RANKL demonstrated a loss in mammary stem cell function and mammary cells expressing stem cell markers, respectively (Asselin-Labat, et al. 2006; Joshi, et al.). Furthermore, the importance of the PR-RANKL axis was confirmed in primary human tissue microstructures (Tanos, et al. 2013). Recently, bi-potent human mammary progenitor cells were shown to express PR, independent of ER (Hilton, et al. 2012). Additionally, progesterone treatment of human mammary epithelial cells cultured as multi-cellular acini structures increased the progenitor cell population (Graham, et al. 2009). These data, indicating that progesterone is a key source of self-renewal and replicative potential in the mammary gland, raise important questions about the contribution of PR and progesterone to the development of breast cancer and tumor progenitor cell maintenance.
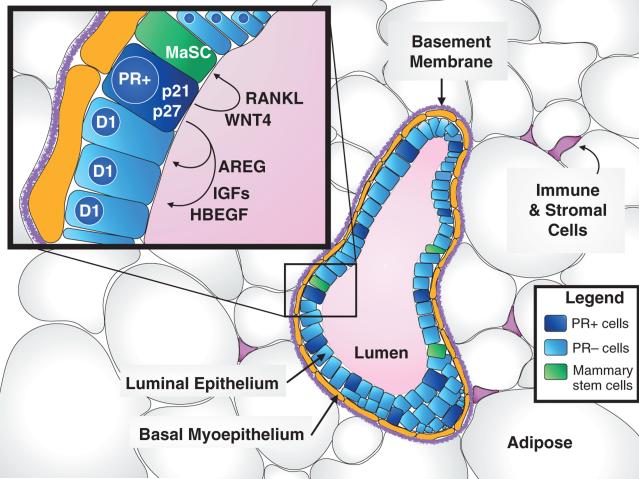
Figure 2. Progesterone receptor action in the normal mammary gland.
Pictured here in cross-section, alveoli are the primary glandular structures of the breast that form in groups (lobules) that are connected to the nipple through a network of ducts embedded within supporting stromal and adipose cells. Each alveolar unit contains a hollow lumen surrounded by a layer of apical luminal epithelium and basal myoepithelium (that are contractile and help with milk secretion during pregnancy). A basement membrane separates the epithelium from the surrounding adipose and stroma (that includes infiltrating immune cells, connective tissue, fibroblasts, and endothelium). The epithelium is derived and maintained from a population of self-renewing mammary stem cells. As illustrated in the inset, the majority of these mammary epithelial cells undergoing cell cycle progression (expressing cyclin D1) receive their proliferative signals via paracrine growth factor production (AREG, IGFs, and HBEGF) from nearby PR-positive cells. PR-positive cells also produce paracrine factors to maintain the mammary stem cell compartment, including WNT4 and RANKL. During early events in breast tumorigenesis, non-dividing PR+ cells (that express cell cycle inhibitors p21 and p27) may overcome cell cycle inhibition and actively begin proliferation via autocrine signaling. PR, progesterone receptor; AREG, amphiregulin; RANKL, receptor activator of nuclear factor kappa-B ligand; WNT4, wingless-type MMTV integration site family, member 4; IGF, insulin-like growth factor; HBEGF, heparin-binding EGF (epidermal growth factor)-like growth factor; D1, cyclin D1, CCND1; p21, cyclin-dependent kinase inhibitor 1A, CDKN1A; p27, cyclin-dependent kinase inhibitor 1B, CDKN1B; MaSC, mammary stem cell
Recent findings implicate progesterone as a key mediator of breast cancer progenitor cell plasticity. Exposure of ER+/PR+ breast cancer cultures to progesterone induces the emergence of cells expressing known progenitor and stem cell markers, such as CK5 and CD44. These cells possess properties that include therapy resistance and heightened mammosphere-forming potential (Cittelly, et al. 2013; Horwitz, et al. 2008). PR regulation of microRNAs (mIRs) facilitates the increase in stem-like phenotypes in breast cancer cell cultures. Downregulation of mIR-29a facilitates dedifferentiation by releasing the transcription factor KLF4 to alter gene programs (Cittelly et al. 2013), while mIR-141 repression by PR prevents downregulation of PR itself and STAT5, which is known to control progenitor cell phenotypes (Yamaji, et al. 2009). Notably, evidence is mounting in favor of the prevailing hypothesis that Hormone Replacement Therapies (HRT), which include progestins, induce a greater incidence in breast cancer by the expansion of pre-malignant stem cell populations (Horwitz and Sartorius 2008).
Indeed, epidemiological evidence and clinical findings have demonstrated that synthetic progestins, whether given in HRT as postmenopausal treatments or as hormonal contraceptives in pre-menopausal women, confer a greater breast cancer risk. Progestin-containing contraception is linked to elevated risk of developing breast cancer in multiple epidemiological studies (Collaborative Group on Hormonal Factors in Breast 1996; Hunter, et al. 2010; Li, et al. 2012; Soini, et al. 2014). Similarly, other epidemiological studies indicate that greater exposure to progesterone throughout an individual's lifetime leads to greater likelihood of breast cancer (reviewed in (Knutson and Lange 2014)). Large-scale clinical trials, including the Women's Health Initiative (hazard ratio 1.26; 95% CI 1.02-1.55) (Chlebowski, et al. 2009), Million Women's Study (relative risk 2.00 [1.88-2.12], p<0·0001) (Beral 2003), E3N-EPIC cohort (relative risk 1.3 [1.1-1.5]) (Fournier, et al. 2005), and Finnish Cancer Registry case-controlled analysis (odds ratio 1.36; 95% CI 1.27-1.46). (Lyytinen, et al. 2010), demonstrate that women taking progestins added to estrogen therapy are in greater jeopardy of developing breast tumors. Unexpectedly, estrogen alone as a single-agent therapy for women who have had a hysterectomy confers protection against breast cancer (hazard ratio 0.77; 95% CI 0.62-0.95) (Chlebowski and Anderson 2012). Recently, a retrospective analysis of Finnish women using the levonorgestrel-releasing intrauterine system of contraception also demonstrated a heightened risk of breast cancer (standardized incidence ratio for one purchase 1.16; 95% 1.09-1.22. For users with two purchases: 1.40; 95% 1.24-1.57) (Soini et al. 2014). However the same regimen conferred protection from endometrial (for one purchase 0.50; 95% 0.35-0.70. For users with two purchases 0.25; 95% 0.05-0.73) and ovarian cancers (0.60; 95% 0.45-0.76) as well as lung (0.68; 95% 0.49-0.91) and pancreatic (0.50; 95% 0.28-0.81) (Soini et al. 2014). In studies comparing HRT containing synthetic and natural progestins (albeit with smaller cohort sizes) natural progestins did not significantly increase breast cancer risk (Fournier, et al. 2008; Fournier et al. 2005). Importantly, the relative instability of natural progesterone may account for the differential biological outcomes compared to long-lived synthetic progestins, raising interesting questions about the duration and level of exposure to PR activators (reviewed in (Brisken 2013)). Alternatively, synthetic progestins may elicit off-target effects on other steroid receptors that may also contribute to their deleterious or protective effects (reviewed in (Knutson and Lange 2014)). Together, these epidemiological and clinical findings support the notion that uncontrolled PR action in pre-neoplastic breast tissue contributes to breast cancer development. This data is corroborated by an expansive body of literature demonstrating in both in vivo and in vitro models of luminal breast cancer that exposure to progestins increases proliferation and promotes pro-survival and progression of malignant breast cells (reviewed in (Daniel, et al. 2011)). Interestingly, while approximately 70% of newly diagnosed breast tumors are ER+/PR+ (luminal type tumors) approximately 40% and 25% of luminal tumors exhibit loss of heterozygosity (LOH) at the PGR or ER locus, respectively (Knutson and Lange 2014). Generally, ER and PR LOH are positively correlated. However, interestingly, despite this genetic loss, ER and PR mRNA levels remain very similar to that of diploid luminal tumors (Knutson and Lange 2014), suggesting that other compensatory factors may exist in these tumors to maintain ER and PR expression.
Context dependent PR activation
The gene programs driven by PR are determined by a diverse array of cellular conditions that modify the receptor and its cofactors, which serve to direct transcriptional complexes to specific promoters. Not surprisingly, progesterone binding produces a dramatic shift in PR mediated gene selection. PR remains bound to and regulates expression (both activation and repression) of a multitude of genes in the unliganded state (Daniel, et al. 2014; Dressing, et al. 2014; Knutson, et al. 2012b), whereas upon ligand binding PR relocates to a subset of progesterone responsive genes. These two broad categories of PR driven genes, unliganded and liganded gene sets, are further regulated by the convergence of particular kinase pathway outputs (Figure 1), in the form of direct phosphorylation of PR and its cofactors (reviewed in (Hagan and Lange 2014)). For example, phospho-S294 PR, in response to MAPK or CDK2 activation, regulates an overlapping yet distinct set of gene targets in the presence of progesterone compared to phospho-S81 PR (via activated CK2), and the same (i.e. sensitivity of selected genes to phosphorylated PR) is true for unliganded target genes (Daniel, et al. 2007; Daniel and Lange 2009; Hagan, et al. 2011b; Knutson, et al. 2012a). To date, post-translational modifications identified on PR that alter its transcriptional activity include: phosphorylation (S294, S345, S81, S400), SUMOylation (K388), acetylation (K183, K638, K640, K641), and ubiquitinylation (Figure 1) (Beleut, et al. 2010; Chung, et al. 2014; Daniel et al. 2007; Daniel, et al., 2010; Daniel and Lange 2009; Dressing et al. 2014; Faivre et al. 2008; Hagan et al. 2011b; Knutson et al. 2012a; Lange, et al. 2000; Pierson-Mullany and Lange 2004b). PR transcriptional activity and promoter selection is thus dramatically altered by the activation state of mitogenic signaling pathways such as MAPK, AKT, CDK2, cAMP, and CK2 (Figure 1). In addition, the availability of particular cofactors and their post-translational modification states are also determinants of PR gene selectivity (Hagan and Lange 2014). In short, PR is capable of inducing diverse biological outcomes dependent on the cellular context as determined by the presence or absence of activated signaling pathways and the availability of cofactors. Studies probing the complexity of PR action thus require particular care in both the design of model systems and the interpretation of specific results. For example, breast cancer cells in culture respond differently to progestins depending on the culture conditions. Cells cultured in 2D (adherent to plastic dishes) elicit a biphasic response characterized by one or few rounds of cell cycle progression followed by growth arrest (Groshong, et al. 1997; Musgrove, et al. 1991), whereas in 3D culture conditions (such as soft agar) progesterone is clearly mitogenic and a mediator of cell survival (Faivre and Lange 2007). These data may reflect an alteration in signaling pathways and kinase activation that is dependent upon cell polarity and/or cellular junctions or “structural” communication that in turn informs PR gene selectivity and modulates the strength and duration of its transcriptional activity (i.e. aspects of PR action that are missed using reporter assays).
Notably, PR-A and PR-B are differentially susceptible to post-translational modifications in response to the same kinase signals. This complexity contributes to the distinctions between the genes they activate and ultimately the biological consequences for PR+ and nearby PR-null cells (i.e. responsive to PR-derived paracrine signals). For example, PR-B, but not PR-A, is robustly phosphorylated on Ser294 in response to MAPK activation. Ser294 phosphorylation is a major regulatory input for PR-B, controlling increased sensitivity to progestin, an increased rate of ubiquitinylation of PR (an activation step for several steroid receptors (Salghetti, et al. 2001)) required for degradation through the 26S proteasome pathway (Lange et al. 2000), decreased SUMOylation on K388 (Daniel et al. 2007), unliganded transcriptional activity (Daniel and Lange 2009), and altered promoter selectivity (Knutson et al. 2012b). Similarly, CUE domain containing 2 (CUEDC2), an ubiquitin-binding motif-containing protein, targets the K388 SUMOylation site for ubiquitinylation and degradation of PR, suggesting that PR ubiquitinylation may oppose SUMOylation via competition for the same required lysine residue (Zhang, et al. 2007). In contrast, modest (low to unmeasurable in intact cells) PR-A Ser294 phosphorylation confers less responsiveness of this isoform to kinase inputs and increased K388 SUMOylation (a transcriptionally repressive modification) (Daniel et al. 2007). The increased SUMOylation of PR-A relative to PR-B may account for the increased trans-repressive activity of this isoform (Abdel-Hafiz, et al. 2009). PR-A is known to repress the activities of PR-B, ER, AR, and GR (Abdel-Hafiz, et al. 2002). PR isoforms also participate in distinct complexes with cofactors, owing in part to differences in post-translational modifications, but also due to cofactor binding sites located in the PR-B N-terminus (Giangrande, et al. 2000). Differential transcriptional complex components aid in determining relative transcriptional activities (i.e. altered hormone sensitivity) and are responsible for directing receptor gene selectivity; PR-A and PR-B have distinct and over-lapping gene signatures in breast cancer cells (Richer, et al. 2002). Importantly, evaluation of endogenous genes to determine the impact of phosphorylation events on steroid receptor action is critical. Phosphorylation events have been shown to alter promoter selection rather than absolute transcriptional activity. Luciferase assays measure transcriptional activity, but fail to detect alterations in promoter selectivity. Thus, mutant PRs that appear to be fully functional in luciferase assays repeatedly fail to activate selected endogenous (native) promoters of genes in intact cells (Daniel, et al. 2009; Qiu and Lange 2003).
In breast cancer cell models and in clinical studies the ratio of PR-A to PR-B is a critical determinant of the biological or physiological response to progesterone (reviewed in (Mote, et al. 2007)). In normal tissues, PR-A and PR-B typically occur as a 1:1 ratio. However, unbalanced PR-A and PR-B expression occurs in the normal breast of women at high risk of developing breast cancer, while altered ratios in breast tumors are linked to endocrine resistance (Mote, et al. 2004; Venkitaraman 2002). Differential signaling and transcriptional activities of the isoforms as well as altered ability of PR-A to trans-repress other steroid receptors likely contribute to breast pathologies (Abdel-Hafiz et al. 2002). The mechanisms that drive imbalanced PR-A to PR-B ratios are still under investigation. We hypothesize that increased kinase activity in the pre-malignant or early malignant setting drives PR-B phosphorylation leading to its hyperactivity and subsequent rapid protein turnover (relative to PR-A) (Daniel et al. 2007; Lange et al. 2000). Thus, activated phospho-PR-B receptors exhibit an overall decreased steady state protein level relative to PR-A receptors (which are not appreciably phosphorylated on Ser294 in response to MAPK or CDK2 activation). In this setting, PR-B exhibits heightened transcriptional activity on selected target genes, yet is less detectable. PR-B is widely recognized as the more proliferative isoform (Faivre and Lange 2007) and as such, may primarily drive the dysplastic phenotypes seen in these tumors. In addition, loss of PR-A (the more repressive isoform) via promoter methylation (Pathiraja, et al. 2011), may lead to loss of its protective actions and provide an epigenetic “stepping stone” in tumor progression, an event that is similarly observed in endometrial tumors (discussed below). Unfortunately, in the clinic, total PR levels are still measured using antibodies that fail to distinguish between PR isoforms (primarily conducted by IHC). This represents a missed opportunity to gain a much better understanding of PR isoforms as distinct biomarkers of disease progression. Given the differential activities of the receptors and their known effects on breast cancer cell biology, measuring the isoforms individually is likely to provide valuable information relevant to the use of tailored endocrine therapies. In addition, examining PR-isoform-specific gene programs in tumors may further inform tumor biology and in turn drive treatment strategies targeting individual PR isoforms.
ER and PR crosstalk
An emerging paradigm in steroid receptor biochemistry is crosstalk between different receptor types, which allows receptors to modulate the signaling and transcriptional responses to non-cognate ligands. Recent studies have shown that steroid receptors, including PR, ER, AR, and GR, participate in complexes with each other to a degree that is much more extensive than previously thought (Daniel et al. 2014; Giulianelli, et al. 2012; Need, et al. 2012; Peters, et al. 2009). This crosstalk is critical to understanding breast cancer biology because ER and PR are capable of modulating the activities of each other, which has implications for endocrine therapy responses. Our recent studies showed that ER, PR-B, and the coactivator and signaling scaffold molecule, PELP1, are constitutively complexed in human breast tumor samples and cell lines (Daniel et al. 2014). The consequences of this interaction in the presence of estrogen in ER+/PR+ breast cancer cell models include: enhanced ER phosphorylation, altered ER promoter selectivity, increased cellular proliferation, and decreased sensitivity to tamoxifen treatment (Daniel et al. 2014). In similar studies, ER and PR complexes exhibited enhanced transcriptional and proliferative responses to progestins as well (Giulianelli et al. 2012). Ultimately, these studies demonstrated that breast cancer cells harboring both ER and PR-B might, in fact, be exquisitely sensitive to exposure of either hormone. Perhaps, in the case of endocrine resistance, steroid receptors can substitute for each other or utilize alternative ligands to drive proliferative gene programs and escape inhibition of one receptor type. Relevant to this concept, ER-alpha may be activated by thyroid hormone (T4) or by cholesterol metabolites (Tang, et al. 2004; Wu, et al. 2013), providing an easy “escape” for tumors under the selection pressure of aromatase inhibitors.
PRs “enable” signaling pathways via “feed-forward” cofactor expression
Our recent studies have elucidated mechanisms whereby PR acts as a sensor to integrate multiple signals (kinase pathway activation and hormone exposure) and ensure persistent activation of particular gene programs, in part via regulation of unique cofactor expression and by upregulation of signaling pathway components. For example, STAT5 is a PR target gene (Richer, et al. 1998), and these factors interact directly and cooperate at numerous PR/STAT5 target genes (Hagan, et al. 2013). Progesterone binding in the presence of high intracellular CK2 activity, a commonly activated kinase in cancer, initiates PR phosphorylation on Ser81 to induce robust STAT5 expression. PR then cooperates with STAT5 on selected target genes required for proliferation, stem cell maintenance, and inflammatory responses (Hagan et al. 2011b). In fact, we hypothesized that STAT5 functions as a pioneer factor recruiting S81 phosphorylated PR to specific chromatin loci (Hagan and Lange 2014). In other circumstances, namely during cell cycle progression through mitosis when both MAPK and CDK2 phosphorylation sites on PR are induced (Ser294, Ser345, and Ser400), cyclin D1 mRNA and protein is directly upregulated in response to progestin (Dressing et al. 2014). Phospho-S345 PR and cyclin D1 (acting as a coactivator of transcription) then cooperate as part of SP1-containing transcriptional complexes to enact a new genetic program in the cell, distinct from that of cells with little to no cyclin D1 expression (Dressing et al. 2014). This paradigm, whereby in response to progestin, PR induces the same pathway factors it requires to fulfill specific context-dependent biological outcomes is recapitulated in ovarian cells. In progesterone-treated ovarian cancer cell models, PR induces the increased expression of FOXO1, which in turn binds to PR in order to further modulate selected FOXO1/PR target genes required for progesterone-dependent induction of cellular senescence (Diep, et al. 2013) (discussed below). In sum, PR are exquisitely sensitive to the local signaling environment in addition to ligand availability and the presence of cofactors that when bound to PR, persistently direct or select highly specific genetic programs. The potential for distinct biological responses to transient versus persistent exposure to progestins is not considered clinically, during HRT, for example. The kinetics of feed-forward signaling events enacted by ligand-bound PRs are underappreciated and a topic for further study.
Uterus
Epidemiological role of progesterone in endometrial cancers
Continuous exposure to sex steroid imbalances, where there is insufficient progesterone or excessive estrogen acting upon endometrial tissue, can result in hyperplasia of the glandular epithelial tissue, with potential to progress to atypical hyperplasia and endometrial carcinoma (Kim et al. 2013; Yang, et al. 2011). Endometrial cancer is the most common gynecologic cancer and is classified into Type I and Type II carcinomas, each characterized by varied hormonal dependence, glandular/stromal architecture, progression and patient outcome (Samarnthai, et al. 2010). Type I endometrioid tumors represent 70-80% of all endometrial cancers; often estrogen dependent, presenting at a lower grade at an early stage with good patient prognosis. Type II non-endometrioid tumors are aggressive and rarely hormone-dependent; diagnosed at a later stage with poorer prognosis and higher recurrence rates. In the progression from low grade (well-differentiated cancers with clear glandular structures and stromal tissue) to high-grade (poorly differentiated cancers) loss of stromal tissue and myometrial invasion is common. Because of progesterone's ability to antagonize proliferation and promote atrophy of the endometrium (Charles 1964), progesterone and its derivatives (progestins) have been used successfully as therapeutics to treat endometrial hyperplasias and cancers. High response rates (70-90%) are often observed for women with pre-invasive atypical hyperplasia or early stages of endometrial cancers without myometrial invasion (Kaku, et al. 2001; Ushijima, et al. 2007). Yet, the efficacy of progestins declines to modest response rates (15-25%) when used for cases of advanced or recurrent cancer (Banno, et al. 2012) and more than 30% of patients with well-differentiated, hormone-dependent Type I tumors will fail to respond (Shao 2013). The mechanisms that result in the progression from progestin sensitivity to the hormone refractory state, or “progesterone resistance”, are poorly understood.
Unlike most mammals, the uterine endometrium of human and some non-human primates undergoes cyclical monthly changes that result in the growth, angiogenesis and differentiation of the functional (proliferative) endometrium (Clancy 2009; Ramsey, et al. 1976). Shifts in the synthesis and secretion of the ovarian steroids, estrogen and progesterone, during this menstrual cycle serve as the principal hormonal drivers for these changes. Rising circulating estradiol during the mid- to late follicular phase of the cycle promotes the proliferation of the functional endometrium (Figure 3); this most luminal portion of the endometrium regenerates each cycle from the basal endometrium and contains the glandular epithelial and stromal cells. Following ovulation, during the secretory luteal phase, rising circulating progesterone antagonizes these proliferative effects of estradiol and supports the differentiation of stromal cells and the decidualization of the endometrium (Figure 3).
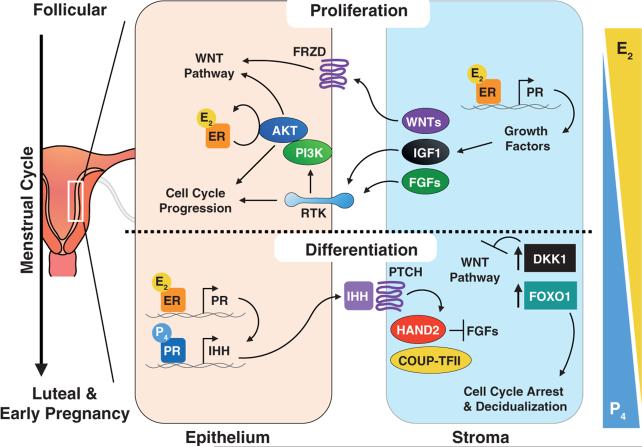
Figure 3. Epithelial-stromal interactions regulating proliferation and differentiation of the uterine endometrium.
The uterine endometrium is stylized in this figure, with predominant signaling pathways represented during the proliferative follicular phase of the menstrual cycle (above dotted line) and during the differentiation of the luteal phase (below dotted line). Arrows on the right indicate relative concentrations of circulating steroid hormone levels. During the follicular phase, the predominant steroid, estrogen (E2; estradiol), acts through its receptor (ER; expressed in epithelium and stroma) to activate the PI3K/Akt pathway and promote inhibitory phosphorylation of GSK-3β, leading to activation of Wnt signaling, regulation of cell cycle proteins and enhanced cell proliferation. E2 also can induce the expression of critical growth factors such as Wnt ligands, IGF1, and FGFs that are secreted by the epithelia and stroma, and which bind to epithelial membrane receptors (i.e. receptor tyrosine kinases, RTKs) to support proliferation. During the luteal phase and early pregnancy, progesterone (P4), as the predominant hormone, antagonizes E2-induced proliferation and promotes differentiation of the glandular epithelium. P4 acts through its receptor (PR) to induce expression of indian hedgehog (IHH) within the epithelium, which binds to Patched (PTCH) on the surface of the stromal cells and through the COUP-TFII and Hand2 complex inhibits expression of FGFs. In addition, P4 also appears to induce the stromal expression of the Wnt signaling antagonist, dickkopf-related protein 1 (DKK1) and the transcription factor, FOXO1, which leads to inhibition of Wnt signaling, inhibition of cell cycle progression, and expression of decidualization-specific genes for stromal cell differentiation. Frequent alterations in endometrial cancer include altered ER/PR expression, PTEN loss of function, activation of PI3K/AKT signaling, and mutations to FGFR; these events are predicted to impact PR actions in the context of tumorigenesis.
Epithelial-stromal interactions within the endometrium: PR isoform specificity
PR-A and PR-B are expressed in both the epithelial and stromal cells of the endometrium and their expression fluctuates during the menstrual cycle as well as during implantation and pregnancy. During the follicular phase of the cycle, both isoforms are expressed at high levels when the endometrium is proliferating, then decline after ovulation through the luteal phase (Mylonas, et al. 2007). In general, PR-A expression appears to be predominant in the stromal cells, declining less during the luteal phase whereas, in glandular epithelial cells, PR-B dominance is seen in the late secretory phase (Mote, et al. 1999). The antagonistic effects of progesterone on the estrogen-induced proliferation and growth of the functional endometrium occurs primarily during the luteal phase and are dependent on the presence of functional PR expression. The absence of PR results in unopposed estrogen-induced endometrial hyperplasia in PR knockout (PRKO) mice (Lydon et al. 1995). Tissue recombination studies with wild-type and PRKO uteri show that progesterone inhibits epithelial proliferation only in co-cultures with uteri expressing stromal PR (Kurita, et al. 1998). Such studies support the importance of stromal PR expression as the inhibitory mediator of anti-proliferative actions of progesterone. However, PR expression within the epithelia is still relevant since progesterone is unable to inhibit estradiol-induced endometrial proliferation or induce expression of important target genes encoding paracrine factors or cell cycle regulatory proteins in mice uteri lacking epithelial-specific PR expression (Franco, et al. 2012). Therefore, the interplay between the epithelial and stromal cells of the endometrium is essential, with both cell types playing a role in the actions of progesterone.
Similar to the breast (discussed above), each PR isoform can have very distinct target genes and biological functions, dependent on hormonal mileu and cellular context. In general, PR-B is viewed as the stronger transcriptional activator and PR-A functions as a transcriptional inhibitor of PR-B activity (Hovland, et al. 1998; Tora, et al. 1988; Vegeto, et al. 1993). Selective ablation of PR-A in mice results in a PR-B dependent gain of function, with enhanced estradiol-induced endometrial proliferation (Conneely et al. 2003; Mulac-Jericevic, et al. 2000). This unexpected observation suggests that PR-A is likely necessary for opposing the actions of both estradiol and progesterone in the endometrium, thereby limiting the proliferative effects of the PR-B receptor in this tissue. In addition, PR-A is also needed for progesterone-mediated changes during the luteal phase and implantation of the conceptus, since lack of PR-A results in impaired uterine implantation and little decidualization of the endometrial layer. This delicate balance of PR isoforms is further illustrated with transgenic mice overexpressing PR-A in glandular epithelium and stromal tissue. This experimental increase in the PR-A:PR-B ratio results in endometrial hyperplasia and atypia with enhanced expression of uterine epithelial growth factors such as amphiregulin known to be regulated by progesterone; these effects can be abolished by treatment with the anti-progestin, mifepristone (Fleisch, et al. 2009). These results show that progesterone can be either an anti- or pro-proliferative force on the endometrium depending on isoform expression.
Studies of the uterine myometrium highlight the fact that the ratio of PR isoforms may be naturally exploited to remove the inhibitory effects of progesterone on myometrial contractions, thus allowing for estrogen activation and the initiation of parturition. One mechanism for this functional progesterone withdrawal may be a shift in the PR-A:PR-B ratio expressed within the myometrium with a concomitant antagonism of PR-B mediated transcription (Merlino, et al. 2007; Mesiano, et al. 2002; Mesiano, et al. 2011; Pieber, et al. 2001). There is also evidence that a change in the PR-A:PR-B:PR-C isoform expression, specifically within the fundal myometrium (i.e. upper portion of the uterine body), could contribute to this process. Protein and mRNA expression of PR-C, as well as PR-B, increase during labor in women and is associated with NF-κB activation and cytokine-mediated transcriptional activation of the PR gene (Condon et al. 2006). The potential transcriptional consequences of such isoform shifts, as experimentally manipulated or observed in these studies, are evident in gene array studies with primary human stromal cells expressing exogenous PR-A, PR-B, or the combination, where distinct expression profiles are observed for each isoform as well as progesterone concentration-dependent efficacy that was both target gene and isoform specific (Yudt, et al. 2006). Overall, these results show that progesterone can be a positive or negative driver of cell processes such as endometrial proliferation or myometrial contractions depending on the isoform expression and downstream transcriptional and signaling activation. Misregulation of isoform expression, therefore, can lead to dysfunction and pre-neoplastic events.
Epithelial-stromal interactions within the endometrium: PR-driven paracrine communication
The regulation of paracrine factors and their signaling pathways by progesterone supports epithelial-stromal communication, is critical for normal uterine function and may play a role in endometrial cancer pathogenesis (Figure 3). Signaling via factors such as Indian hedgehog (Ihh) and Wnt ligands can be modulated by progesterone through regulation of the expression or activity of these paracrine factors or their downstream signaling molecules (Wetendorf and DeMayo 2012) (Figure 3). Within the hedgehog pathway, Ihh and dickkopf related protein 1 (DKK1) are PR target genes (Takamoto, et al. 2002). Activation of stromal PR results in induction of Ihh expression by the epithelia and subsequent stromal expression of patched homolog 1 (PTCH1) and nuclear receptor subfamily 2, group F, member 2 (NR2F2) (Figure 3). This can lead, in particular through NR2F2 (e.g. COUP-TFII), to activation of transcription factors such Hand2 and potential antagonism of mitogenic pathway activation by growth factors, such as fibroblast growth factors (FGFs) (Li, et al. 2011). This paracrine loop is thought to inhibit estrogen signaling and thereby halt uterine epithelial proliferation. The Wnt/β-catenin signaling pathway is critical for the control of stem cell/progenitor compartments and the balance between ‘stemness’ (e.g. proliferation with Wnt pathway active) and differentiation (e.g. inhibited Wnt pathway) in many tissues (Clevers 2006). In the endometrium, this pathway is also implicated in control of the proliferation-differentiation shift during the menstrual cycle and the actions of progesterone during the luteal phase may be through the inhibition of this pathway (Wang, et al. 2010). Exposure to estrogen during the proliferative phase of the cycle leads to activation of this pathway with enhanced expression of Wnt pathway components (i.e. Wnt4, Wnt5a, Frizzled 2 (Hou, et al. 2004)) and Wnt target genes such as IGF-1 (Wang, et al. 2009), a critical endometrial growth factor secreted by stromal cells (Cooke, et al. 1997; McCampbell, et al. 2006), as well as down-regulation of DKK1 (stromal) and FOXO1, Wnt/β-catenin signaling inhibitors (Talbi, et al. 2006; Wang et al. 2009) (Figure 3). Of note, Wnt4 is a paracrine effector for progesterone-induced expansion of the mammary stem cells (Joshi, et al. 2010). Crosstalk with the PI3K/Akt pathway is also involved since E2-induced Akt activation, via ERα, results in inhibition of GSK-3β, stabilization of β-catenin with enhanced transcription of Wnt target genes, ultimately leading to cell cycle progression (Tong and Pollard 1999). Progesterone antagonizes the Wnt/β-catenin pathway via enhanced transcription of DKK1 and FOXO1 genes, retention of active GSK-3β, and nuclear exclusion of cyclin D1 resulting in cell cycle arrest (Chen, et al. 2005; Kyo, et al. 2011; Wang et al. 2009; Ward, et al. 2008). Interestingly, blocking FOXO1 expression attenuates the ability of progesterone to inhibit epithelial cell growth whereas expression of a dominant negative AKT enhances the inhibitory effect of this hormone (Kyo et al. 2011). These studies emphasize the crosstalk between paracrine signaling and mitogenic pathways modulated by ER and PR in the homeostasis of endometrial growth. Notably, the dysregulation of the PI3K/Akt and Wnt/β-catenin, in particular, is one hallmark of endometrial cancer pathogenesis. It is tempting to speculate that early events such as activating mutations in these key signaling pathways lead to imbalanced hormone-dependent stromal and epithelial crosstalk that then predisposes to neoplastic transformation of endometrial tissue.
Mechanisms of progestin resistance in endometrial cancer
Misregulation of PR isoform expression, localization, and activity are common phenotypes observed in EC that could be involved and potentially targeted to improve sensitivity to progestin therapy. In general, hyperplasias express higher levels of PR-A and PR-B (Miyamoto, et al. 2004) and comparison of low to high grade endometrial cancers reveals reduced to absent expression of one or both isoforms in epithelia or stroma; these expression profiles are often associated with shorter progression-free survival and overall survival rates (Jongen, et al. 2009; Kreizman-Shefer, et al. 2014; Leslie, et al. 1997; Miyamoto et al. 2004; Sakaguchi, et al. 2004; Shabani, et al. 2007). This silencing of PR expression may be due to hypermethylation of CpG islands within the promoter or first exon regions of the PR gene or to the presence of associated deacetylated histones. These epigenetic modifications were observed in endometrial cancer cell lines as well as tumor samples and may be exclusive to PR-B (Ren, et al. 2007; Sasaki, et al. 2001; Xiong, et al. 2005). Treatment of such cells with DNA methyltransferase or histone deacetylase inhibitors can restore both PR-B expression and its regulation of target genes such as FOXO1, p21, p27 and cyclin D1 (Xiong et al. 2005; Yang, et al. 2014). Downregulation of PR via post-transcriptional mechanisms such as miRNAs could be another means of suppressing progesterone sensitivity, as observed in breast cancer cell lines via overexpression of miR-26a and miR-181a (Maillot, et al. 2009), but this remains to be examined in endometrial cancer models.
Post-translational modifications of PR, such as phosphorylation or SUMOylation, serve as input points for activated mitogenic pathways to regulate PR signaling (Dressing, et al. 2009; Hagan, et al. 2011a) and therefore may contribute to progesterone resistance. Studies with endometrial stromal cells have shown that activation of cAMP signaling can sensitize cells to progesterone by suppressing SUMOylation of the PR-A isoform leading to enhanced transcriptional activity and target gene induction, supporting normal endometrial decidualization (Jones, et al. 2006). Although the relevance of such PR modifications has not been extensively explored in the context of endometrial cancer, it is known that oncogenic activation of KRAS, PI3K, or AKT and/or loss of functional tumor suppressors such as PTEN are common genetic alterations observed in endometrial cancer (Hecht and Mutter 2006) (Figure 1). Janzen and colleagues have recently used an in vivo endometrial regeneration model to test how these common genetic alterations affect PR isoform expression and responsiveness to progestin therapy within epithelial and stromal compartments of the endometrium (Janzen, et al. 2013). Tumors generated from epithelial cells lacking PTEN were responsive to progesterone showing early decreased proliferation and later apoptosis, but co-administration of estrogen was necessary for tumor resolution as well as maintenance of stromal PR expression. Deletion of PR in stromal cells or combined epithelial-specific genetic mutations (i.e. PTEN loss and Kras activation) caused progesterone resistance, while overexpression of PR in stroma was able to resensitize tumors to therapy. Interestingly, tumors with the combined mutations showed depressed PR expression, especially stromal PR-A, due to epigenetic modifications; analysis of the PR-A promoter revealed multiple sites of hypermethylation. In addition to the function of stromal PR-A, studies have also highlighted the importance of PR-B where DNA methylation and decreased PR-B expression in endometrial cancer results in decreased FOXO1 and BIRC3 expression, enhancement of adhesion molecules, and cell cycle regulatory proteins. This ultimately lifts progesterone antagonism of estrogenic effects resulting in enhanced cell proliferation and survival (Shao 2013). These studies illustrate the importance of functional PR expression, uterine epithelial/tumor-stromal interactions, and hormonal milieu on PR signaling and therapeutic efficacy.
Ovary
Epidemiological role of progesterone in ovarian tumors
Ovarian cancer is the seventh most common cause of cancer-related deaths worldwide (Jemal, et al. 2011). As the deadliest of all gynecologic malignancies, ovarian cancer has a death rate of more than 50% due to late detection and diagnosis of the disease and intrinsic or acquired resistance to current therapeutic regimens. The identification of robust biomarkers for early detection will have a substantial impact on survival rates, while prognostic molecular markers may allow for efficacious targeted therapeutic strategies.
A considerable body of epidemiological data suggests that progesterone and progestins play a protective role against ovarian carcinogenesis. Progesterone deficiencies due to increasing age, infertility, or a genetic LOH at the PR gene locus are associated with increased ovarian cancer risk (Edmondson and Monaghan 2001; Gabra, et al. 1996). In contrast, elevated progesterone levels decrease ovarian cancer risk. The protective effect of pregnancy has been documented in Asian, European, and North American populations (Banks, et al. 1997); progesterone levels during pregnancy are 10-fold greater than luteal phase levels measured during the menstrual cycle. Similarly, hormonal oral contraceptive use has been consistently associated with a reduced risk. In an analysis of 20 epidemiological studies between 1970-1991, it was estimated that a 35% reduction in risk was associated with ever-use of oral contraceptives (Hankinson, et al. 1992). Additionally, the risk of ovarian cancer is correlated with duration of oral contraceptive use: 10-12% decrease in risk with one year of use and 50% decrease after 5 years of use in both nulliparous and parous women (Hankinson et al. 1992). Progesterone exerts a protective effect on ovarian cancer risk by reducing ovulation through elevated progesterone levels from oral contraceptive use or during pregnancy. Furthermore, PR expression, PR-B specifically (Akahira, et al. 2000; Akahira, et al. 2002; Lenhard, et al. 2012), in ovarian tumors is a favorable prognostic marker associated with longer progression-free survival (Akahira et al. 2000; Hempling, et al. 1998; Hogdall, et al. 2007; Lee, et al. 2005; Lindgren, et al. 2001; Munstedt, et al. 2000; Sinn, et al. 2011; Tangjitgamol, et al. 2009; Yang, et al. 2009).
BRCA1/2 mutations may alter the production and sensitivity to estrogen and progesterone as carriers have an increased risk for breast and ovarian cancer. Studies in mice carrying a BRCA1 mutation in ovarian granulosa (i.e. hormone producing) cells (Chodankar, et al. 2005; Hong, et al. 2010; Yen, et al. 2012) and in humans with either a BRCA1 or BRCA2 mutation (Widschwendter, et al. 2013) demonstrated that BRCA mutations confers higher serum (circulating) levels of both estrogen and progesterone. Moreover, serous tubal intra-epithelial carcinoma in the distal end of the fallopian tube was discovered in 10-15% of BRCA carriers who had prophylactic salpingo-oophorectomy (Folkins, et al. 2008; Norquist, et al. 2010). Ultimately, little mechanistic information exists related to the impact that hormones have on the prevention and/or pathogenesis of ovarian cancer. The evidence related to the pathophysiology of ovarian cancer suggests a strong connection with estrogen, progesterone, and, more recently, androgen actions in the development and progression of ovarian cancer. Steroid hormone action in ovarian cancer is grossly understudied, and there is an urgent need to focus on the early events related to the contribution of hormones in the context of altered signaling events (loss of p53 or PTEN, elevation of AKT signaling) that predisposes women including those with BRCA mutations to an increased risk of breast and ovarian cancer.
PR as a prognostic marker in ovarian tumors
Recent studies have revealed that ‘ovarian cancer’ is not a single disease, and a significant portion of ovarian tumors may not originate from ovarian tissue. At present, five major histopathological subtypes of epithelial ovarian cancer have been characterized and are phenotypically and molecularly distinct (2011): high-grade serous, low-grade serous, endometrioid, clear cell, and mucinous. Pathological and genomic studies indicate that cancers of these major subtypes are frequently derived from non-ovarian tissues that have metastasized and homed to the ovary (Figure 4). Clear cell and endometrioid ovarian cancers are derived either from the cervix or endometriosis, which itself is associated with retrograde menstruation from the endometrium (Obata, et al. 1998; Sato, et al. 2000) (Figure 4). Invasive mucinous ovarian cancers are metastases from the lower intestinal tract (e.g. stomach, colon, appendix) to the ovary (Khunamornpong, et al. 2006) (Figure 4). High-grade serous ovarian cancers are derived from the distal fallopian tubes (Folkins et al. 2008; Lee, et al. 2007) (Figure 4). A recent study demonstrated that ovulation, the release of hormones (e.g. estrogen and progesterone), growth factors, and inflammatory factors among others, promoted the migration of intrauterine-injected malignant cells towards the ovarian stromal compartment to form “ovarian” tumors (Yang-Hartwich, et al. 2014). Thus, it is plausible that the unique hormonal milieu provided by functional ovaries serves to attract pre-malignant and malignant cells that may remain dormant (i.e. under progesterone concentrations) or fully progress to tumors (i.e. postmenopausal contexts or upon loss of progesterone or functional PRs). Approximately 90% of ovarian cancers are detected in the ovary, with over 50% ovarian cancers diagnosed in post-menopausal women (American Cancer Society. Cancer Facts and Figures 2014. Atlanta).
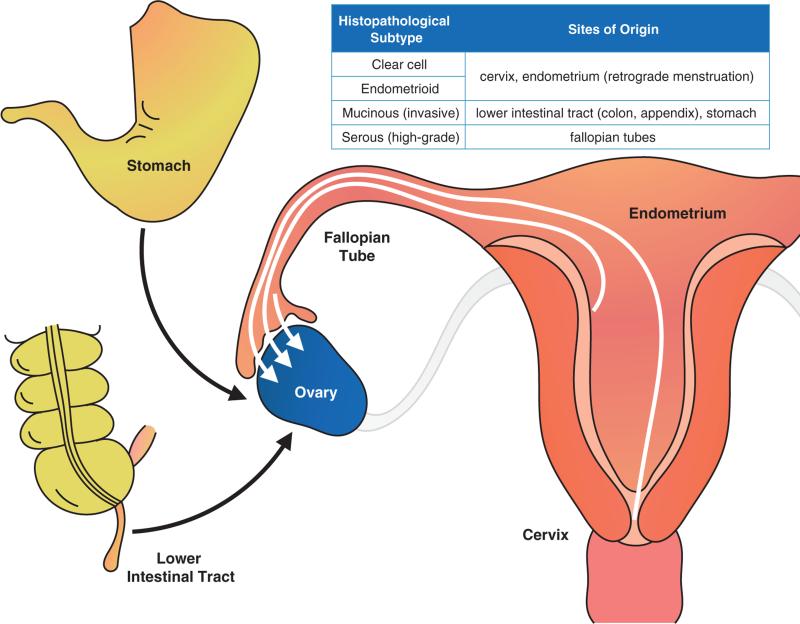
Figure 4. Cellular origins of ovarian cancer.
Ovarian cancer is a collective term for several distinct invasive diseases that originate in the peritoneal cavity. Inset, the known sites of origin associated with the major histopathological subtypes of ovarian cancer. Mucinous ovarian cancers are metastases on the ovary from the gastrointestinal tract, including the stomach, colon, or appendix. Endometrioid and clear cell ovarian cancers are derived either from the cervix or from the uterus via progression of endometriosis, which is linked to retrograde menstruation from the endometrium. High-grade serous ovarian cancers are either derived from metastases from the distal fallopian tube or from the surface of the ovary.
Until recently, little was known about the relative distribution of PR within the subtypes of epithelial ovarian tumors. In a cohort of 504 tumors, we reported that 35% of ovarian tumors are PR-positive, with the highest total PR expression in endometrioid (67%) and serous (35%; low grade serous, 64%) subtypes (Diep et al. 2013). In accordance with our study, the international Ovarian Tumor Tissue Analysis consortium examined the association of ER and PR expression with subtype-specific survival in nearly 3,000 invasive epithelial ovarian tumors reporting positive total PR expression in endometrioid (67%), low-grade serous (57%), and high-grade serous (31%) tumors (Sieh, et al. 2013). Additionally, the study confirmed the prognostic significance of PR expression in ovarian tumors strongly expressing PR (≥50% tumor cell nuclei staining). Strong PR expression in high-grade serous ovarian carcinomas was associated with a significant improvement in survival; positive PR expression (weak or strong) in endometrioid carcinomas was associated with significantly improved disease-specific survival independent of patient age and tumor grade, site, and stage. Of note, ER expression conferred a patient survival advantage in endometrioid ovarian tumors only. ER may contribute to the favorable prognosis in endometrioid ovarian tumors via regulation of PR expression; a functional ER signaling pathway promotes robust PR expression. While total PR levels are routinely measured in breast and endometrial cancers (but rarely in ovarian cancer) for clinical management and disease treatment, very few studies have examined the levels of PR isoforms in ovarian tumors. To our knowledge, only three studies (Akahira et al. 2000; Akahira et al. 2002; Lenhard et al. 2012) have reported differential expression of PR isoforms in ovarian tumors. These studies have reported a dominance of PR-B expression in ovarian tumors across all sub-types, with PR-B frequently expressed in the serous subtype. In contrast, PR-A expression was weakly expressed in mucinous and serous ovarian carcinomas and comparison of normal to malignant ovarian tissues revealed reduced to absent expression in malignant tumors relative to PR-B (Akahira et al. 2002).
Progesterone actions in ovarian cancer
The molecular mechanisms of progesterone's protective role in ovarian cancer are not well understood; both proliferative and inhibitory actions of progesterone have been reported in ovarian cancer cell line models. Several independent in vitro studies demonstrated anti-proliferative actions of progesterone at higher concentrations (≥1 μM) in ovarian cancer cells, primarily through the induction of apoptosis (Bu, et al. 1997; Keith Bechtel and Bonavida 2001; Syed and Ho 2003; Yu, et al. 2001), while fewer studies reported progesterone as proliferative in these cells at lower concentrations (Fauvet, et al. 2006; Syed, et al. 2001). The opposing cellular responses of ovarian cancer cells to progesterone may be attributed to cell context-dependent regulatory inputs to PR (discussed above), such as progesterone dosing, kinase activation state of the cells, cofactor availability, or PR-A and PR-B ratios. Ovarian cancer cells are susceptible to concentration-dependent and biphasic effects within the same cell model systems as mentioned previously for uterine and breast (in 2D culture systems) cancer cells. Similar to breast and uterus, crosstalk between PR and growth factor-mediated signaling pathways (i.e. protein kinases) presumably directs PR promoter selection and specific cell fates (e.g. apoptosis). The relative abundance of cofactors that associate with PR also varies in a tissue-specific manner (Giangrande et al. 2000; Han, et al. 2005). As in other tissues (discussed above), shifts in PR isoform ratios (PR-A and PR-B) and cofactor availability may contribute to variations in biological responses to progesterone.
PR-isoform specific actions are largely undefined in ovarian cancer. However, our recent study defined a mechanism for PR-B regulation of ovarian cancer cellular senescence in response to progesterone. Using ovarian cancer cell models, we demonstrated that ligand-activated PR-B acting through a FOXO1-dependent mechanism induced p21, a known mediator of cellular senescence. FOXO1, a transcriptional factor, has been shown to interact physically with other nuclear steroid hormone receptor proteins, such as AR (Fan, et al. 2007; Li, et al. 2003), ER-alpha (Schuur, et al. 2001), and both PR isoforms (Kim, et al. 2005; Rudd, et al. 2007). Our study demonstrated that PR-B and FOXO1 were co-recruited to a PRE-containing region in the upstream promoter of p21 upon progestin (R5020) treatment. Both proteins were required to cooperatively activate progestin-induced p21 expression and induce PR-dependent cellular senescence. PR-B appears to be a more potent driver of ovarian cancer cell senescence relative to PR-A; PR-B but not PR-A induces robust FOXO1 expression (Diep, Knutson, and Lange, unpublished results). As stated above for breast studies, we suspect that PR isoforms in ovarian cancer models are also exquisitely sensitive to kinase inputs that may alter this biological outcome. Both PR-B and FOXO1 are tightly regulated by phosphorylation events. Hormone-driven breast and gynecologic cancers frequently exhibit upregulated protein kinases, such as MAPK (Faivre, et al. 2005), CDK2 (Pierson-Mullany and Lange 2004a), and CK2 (Hagan et al. 2011b), which directly phosphorylate and modulate PR-B target gene selectivity (Figure 1). Notably, the same kinases that are recruited to PR-B in “rapid” signaling (i.e. extra-nuclear) complexes (i.e. CDK2 and MAPK) also inhibit FOXO1 via regulation of specific phosphorylation sites that favor nuclear export (Hedrick, et al. 2012). Deregulation of FOXO1 is associated with tumorigenesis and cancer progression. FOXO1 is downregulated in several carcinomas, including ovarian (Goto, et al. 2008), through alterations in upstream regulators, post-translational deregulation, or by genetic mutations (Myatt and Lam 2007). Specifically, AKT-mediated serine/threonine phospho-regulation of FOXO1 is well-defined and prevents FOXO1 nuclear accumulation, thus impairing target gene regulation (Myatt and Lam 2007). As mutations of PI3Ks or PTEN are common early events in cancer (particularly in breast, uterine, and ovarian cancers), activated AKT and other mitogenic protein kinases may prevent PR-induced senescence signaling by nuclear exclusion of FOXO1. Thus, the early loss or inactivation of FOXO1 may render PR “incompetent” at genes required for the induction of cellular senescence, leading to the loss of protective “sensing” by progesterone in ovarian tumors. Whether these events may redirect PR to “alternate” genes that instead favor tumor progression is unknown and a topic for further study.
Finally, mortality rates for ovarian cancer have remained largely unaffected despite clinical advances in detection methods, surgical techniques, and treatment regimens. Although extensive surgery followed by chemotherapy is often effective at inducing clinical remission, the treatment is toxic and rarely results in a cure. Other treatment regimens, such as hormonal therapy have been evaluated for ovarian cancer. The use of progestins alone (megestrol acetate and medroxyprogesterone acetate) as ovarian cancer therapies have been examined in several relatively small phase II clinical trials with variable inclusion criteria and modest response rates (Modugno et al. 2012). However, retrospective studies evaluating the association of total PR expression and progression-free disease survival (Akahira et al. 2000; Hempling et al. 1998; Hogdall et al. 2007; Lee et al. 2005; Lindgren et al. 2001; Munstedt et al. 2000; Sieh et al. 2013; Sinn et al. 2011; Tangjitgamol et al. 2009; Yang et al. 2009) support the concept that subsets of PR-positive ovarian tumors are highly sensitive to hormones and thus more likely to respond to endocrine therapy.
Overall, identifying the mechanisms governing PR-A versus PR-B specific gene regulation may provide insight for exploiting the protective actions of progesterone in PR-positive gynecological tumors to induce growth arrest and ultimately favor cell death. Namely, the development of PR isoform-specific ligands may allow for promotion of PR-B driven cellular senescence in ovarian cancer or induction of the protective actions of PR-A in uterine cancer. Growth arrested senescent cells cannot further divide, but depend upon specific kinase-mediated signal transduction pathways for prolonged survival, and thus may be more vulnerable to subsequent therapies that inhibit mitogenic protein kinases and thereby promote apoptosis. Thus, as part of novel combination therapies, PR-targeted strategies could provide a safe and useful means to improve treatment outcomes and increase overall patient survival.
Antiprogestins in preclinical and clinical development
Table 1 depicts antiprogestins currently under preclinical and clinical development in breast cancer, endometrial cancer, endometriosis, leiomyomas, and ovarian cancer. Mifepristone (RU486) has been studied in several Phase I and II clinical trials for breast and gynecological diseases and cancers as it blocks the transcriptional activity of PR by directly binding to and recruiting corepressors to PR (depending on cellular context) (Han, et al. 2007). Paradoxically, mifepristone was originally developed as a potent antiglucocorticoid compound, and was later discovered to have antiprogesterone activity when mifepristone caused termination of pregnancy in preclinical studies (Spitz and Bardin 1993). Likewise, mifepristone can also bind to the androgen receptor (Song, et al. 2004). While the structures between progesterone, glucocorticoid and androgen receptors are very similar, the varying affinity of mifepristone to these steroid receptors may account for the limited efficacy and substantial toxicity observed in several clinical trials for breast and ovarian cancer (Perrault, et al. 1996; Rocereto, et al. 2010). A new generation of PR antagonists attenuates malignant proliferation of tumors and is highly selective for PR with potent antiprogesterone activity but minimal antiglucocorticoid effects in in vitro and in vivo studies. These PR antagonists include APR19, CDB-2914 (ulipristal), CDB-4124 (telapristone), J867 (asoprisnil), ORG31710, WAY-255348, ZK230211 (lonaprisan), ZK98299 (onapristone), and a 17-fluorinated steroid branded as EC304 (Table 1) (reviewed in (Chabbert-Buffet, et al. 2005; Goyeneche and Telleria 2015; Knutson and Lange 2014; Spitz 2006)). Ultimately, the development of highly selective PR antagonists, the identification of patient cohorts that will benefit from antiprogestins, and their use in combination with other endocrine therapies may significantly advance hormone-modulation strategies for breast and gynecological cancers.
Table 1.
Current antiprogestins in preclinical and clinical development in breast and gynecological diseases.
| Antiprogestin | Phase | Disease | References |
|---|---|---|---|
| APR19 | Preclinical | Breast cancer | (Khan, et al. 2013) |
| EC304 | Preclinical | Breast cancer | (Nickisch, et al. 2013) |
| ORG31710 | Preclinical | Breast cancer | (Bakker, et al. 1990) |
| WAY-255348 | Preclinical | Breast cancer | (Yudt, et al. 2011) |
| Asoprisnil (J867) | II | Endometriosis | (Chwalisz, et al. 2007; DeManno, et al. 2003) |
| II | Leiomyoma | ||
| Lonaprisan (BAY86-5044, ZK230211) | II | Breast cancer | (Jonat, et al. 2013) |
| Mifepristone (RU486) | I - II | Breast cancer | (Klijn, et al. 1989; Perrault et al. 1996; Romieu, et al. 1987) |
| II | Endometrial cancer | (Ramondetta, et al. 2009) | |
| I - III | Leiomyoma | (Engman, et al. 2009; Yerushalmi, et al. 2014) | |
| II | Ovarian cancer | (Rocereto et al. 2010; Rocereto, et al. 2000) | |
| Onapristone (ZK98299) | II | Breast cancer | (Helle, et al. 1998; Robertson, et al. 1999) |
| I | PR+ tumors | ||
| Telapristone (CDB-4124, Proellex) | II | Breast cancer | (Gupta, et al. 2013) |
| II | Endometriosis | (Ioffe, et al. 2009) | |
| Ulipristal (CDB-2914) | II - III | Leiomyoma | (Levens, et al. 2008) |
Summary of Discussion
Herein, we have discussed the pivotal role of altered progesterone signaling in the development and progression of hormone-regulated tumors. In the breast, progesterone promotes a proliferative and pro-survival response (i.e. PR is a major downstream effector of estrogen signaling), but inhibits estrogen-induced growth in the reproductive tract. The paradoxical effects of progesterone observed in tumors arising from these tissues may be largely dependent on endogenous cell context and the tissue microenvironment. Namely, progesterone's opposing effects may be attributed to altered expression or activity of PR isoforms, the contextual interactions between the epithelial and stromal compartments observed in breast and endometrial tissues, changes in their relative regulation either by post-translational modifications and via differential crosstalk with cofactor binding partners that serve as major inputs to altered transcriptional activity and promoter selection in the various target tissues (see Table 2 for an overview).
Table 2.
Summary of PR isoform actions
| Tissue type | Isoforms | Isoform-specific actions of progesterone |
|---|---|---|
| Breast | PR-A | - Trans-represses PR, ER, AR, and GR activities - Weaker transcriptional activator relative to PR-B |
| PR-B | - Required for normal mammary gland development and expansion - Proliferative isoform in breast tumors |
|
| Uterus | PR-A | - Required for normal uterine development and function - Dominant isoform in normal stromal cells - Anti-proliferative actions |
| PR-B | - Dominant isoform in normal glandular epithelial cells - Proliferative isoform in endometrial cancer cells |
|
| Ovary | PR-A | - Essential for normal ovarian function - Reduced or absent expression in ovarian carcinomas |
| PR-B | - Dominant isoform in ovarian carcinomas - Anti-proliferative actions (e.g. senescence, apoptosis) |
Although elegant models have recently emerged (Karst, et al. 2011; Tanos et al. 2013), knowledge gaps still exist. What are the best methods and experimental models to elucidate progesterone-specific effects in hormone-responsive tumors? While breast, endometrial, and ovarian cancers are diagnosed in both pre- and post-menopausal populations, a majority of the current cell-based models were originally established from post-menopausal patients. To understand steroid receptor actions, cells are treated with varying concentrations of exogenous hormones that may or may not reflect true physiological levels experienced in a pre-menopausal (cyclical hormone exposure) or post-menopausal (constant/low hormone exposure) context. Are the hormone concentrations used in the laboratory relevant to these contexts and thus to the biology of the tumors that arise? In addition, decreased PR expression is associated with progression of disease in breast and gynecologic cancers (Balleine, et al. 1999; Gross, et al. 1984), whereas over 50% of acquired endocrine-resistant breast tumors retain PR expression (Encarnacion, et al. 1993; Johnston, et al. 1995). How do breast and other tumors lose PR expression and/or regain it during extended periods of endocrine (anti-estrogen therapy)? How should we model these changes? Concerning in vitro models, PR expression is often lost when primary isolates or immortalized cell lines are continuously cultured on 2D surfaces. The development of co-culture or 3D models may more accurately reflect in vivo cellular architecture relevant to paracrine signaling and tumor biology (Lo, et al. 2012) and will allow a more accurate characterization of the mechanisms and biological effects of hormone and antitumor treatments. Finally, routine detection and quantification of individual PR isoforms in clinical samples may provide valuable information as potentially distinct biomarkers of tumor behavior that could be used to further guide endocrine therapy.
Understanding how PRs function differentially in each normal and neoplastic tissue type will reveal how these highly modified receptors can be therapeutically targeted, perhaps as separate isoforms, to favor one biological outcome (growth inhibition, senescence, apoptosis) over another (proliferation, survival). Ultimately, in order to effectively manipulate PR action pharmacologically to treat tumors arising from different tissue types we must first appreciate their mechanistic complexity. Isoform-specific ligands as activators or inhibitors would be a valuable set of tools to accomplish this goal. In the current age of cancer genomics and personalized medicine, clinical readouts of PR-driven gene signatures may provide an additional means to discern context-dependent protective versus deleterious PR actions present in individual tissues and tumors.
Acknowledgments
None
Funding
This work was supported by NIH grant R01 CA159712 (to C.A.L.), a supplement to the parent NIH grant R01 CA15972-S1 (to C.A.L.), Cancer Biology Training Grant NIH T32 CA009138 (to C.H.D.) and National Center for Advancing Translational Sciences of the National Institutes of Health Award UL1TR000114 (to C.H.D).
Footnotes
Declaration of interest
We declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported and discussed.
References
- Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Hafiz H, Dudevoir ML, Horwitz KB. Mechanisms underlying the control of progesterone receptor transcriptional activity by SUMOylation. J Biol Chem. 2009;284:9099–9108. doi: 10.1074/jbc.M805226200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Hafiz H, Takimoto GS, Tung L, Horwitz KB. The inhibitory function in human progesterone receptor N termini binds SUMO-1 protein to regulate autoinhibition and transrepression. J Biol Chem. 2002;277:33950–33956. doi: 10.1074/jbc.M204573200. [DOI] [PubMed] [Google Scholar]
- Akahira J, Inoue T, Suzuki T, Ito K, Konno R, Sato S, Moriya T, Okamura K, Yajima A, Sasano H. Progesterone receptor isoforms A and B in human epithelial ovarian carcinoma: immunohistochemical and RT-PCR studies. Br J Cancer. 2000;83:1488–1494. doi: 10.1054/bjoc.2000.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akahira J, Suzuki T, Ito K, Kaneko C, Darnel AD, Moriya T, Okamura K, Yaegashi N, Sasano H. Differential expression of progesterone receptor isoforms A and B in the normal ovary, and in benign, borderline, and malignant ovarian tumors. Jpn J Cancer Res. 2002;93:807–815. doi: 10.1111/j.1349-7006.2002.tb01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Cancer Society . Cancer Facts and Figures 2014. GACS; Atlanta: [Google Scholar]
- Asselin-Labat ML, Shackleton M, Stingl J, Vaillant F, Forrest NC, Eaves CJ, Visvader JE, Lindeman GJ. Steroid hormone receptor status of mouse mammary stem cells. J Natl Cancer Inst. 2006;98:1011–1014. doi: 10.1093/jnci/djj267. [DOI] [PubMed] [Google Scholar]
- Balleine RL, Earl MJ, Greenberg ML, Clarke CL. Absence of progesterone receptor associated with secondary breast cancer in postmenopausal women. Br J Cancer. 1999;79:1564–1571. doi: 10.1038/sj.bjc.6690249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks E, Beral V, Reeves G. The epidemiology of epithelial ovarian cancer: a review. International Journal of Gynecological Cancer. 1997;7:425–438. [Google Scholar]
- Banno K, Kisu I, Yanokura M, Tsuji K, Masuda K, Ueki A, Kobayashi Y, Yamagami W, Nomura H, Susumu N, et al. Progestin therapy for endometrial cancer: the potential of fourth-generation progestin (review). Int J Oncol. 2012;40:1755–1762. doi: 10.3892/ijo.2012.1384. [DOI] [PubMed] [Google Scholar]
- Beleut M, Rajaram RD, Caikovski M, Ayyanan A, Germano D, Choi Y, Schneider P, Brisken C. Two distinct mechanisms underlie progesterone-induced proliferation in the mammary gland. Proc Natl Acad Sci U S A. 2010;107:2989–2994. doi: 10.1073/pnas.0915148107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–427. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- Boonyaratanakornkit V, McGowan E, Sherman L, Mancini MA, Cheskis BJ, Edwards DP. The role of extranuclear signaling actions of progesterone receptor in mediating progesterone regulation of gene expression and the cell cycle. Mol Endocrinol. 2007;21:359–375. doi: 10.1210/me.2006-0337. [DOI] [PubMed] [Google Scholar]
- Boonyaratanakornkit V, Scott MP, Ribon V, Sherman L, Anderson SM, Maller JL, Miller WT, Edwards DP. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol Cell. 2001;8:269–280. doi: 10.1016/s1097-2765(01)00304-5. [DOI] [PubMed] [Google Scholar]
- Brisken C. Progesterone signalling in breast cancer: a neglected hormone coming into the limelight. Nat Rev Cancer. 2013;13:385–396. doi: 10.1038/nrc3518. [DOI] [PubMed] [Google Scholar]
- Brisken C, O'Malley B. Hormone action in the mammary gland. Cold Spring Harb Perspect Biol. 2010;2:a003178. doi: 10.1101/cshperspect.a003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisken C, Park S, Vass T, Lydon JP, O'Malley BW, Weinberg RA. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc Natl Acad Sci U S A. 1998;95:5076–5081. doi: 10.1073/pnas.95.9.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu SZ, Yin DL, Ren XH, Jiang LZ, Wu ZJ, Gao QR, Pei G. Progesterone induces apoptosis and up-regulation of p53 expression in human ovarian carcinoma cell lines. Cancer. 1997;79:1944–1950. doi: 10.1002/(sici)1097-0142(19970515)79:10<1944::aid-cncr15>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Chabbert-Buffet N, Meduri G, Bouchard P, Spitz IM. Selective progesterone receptor modulators and progesterone antagonists: mechanisms of action and clinical applications. Hum Reprod Update. 2005;11:293–307. doi: 10.1093/humupd/dmi002. [DOI] [PubMed] [Google Scholar]
- Charles D. Iatrogenic Endometrial Patterns. J Clin Pathol. 1964;17:205–212. doi: 10.1136/jcp.17.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Pan H, Zhu L, Deng Y, Pollard JW. Progesterone inhibits the estrogen-induced phosphoinositide 3-kinase-->AKT-->GSK-3beta-->cyclin D1-->pRB pathway to block uterine epithelial cell proliferation. Mol Endocrinol. 2005;19:1978–1990. doi: 10.1210/me.2004-0274. [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Anderson GL. Changing concepts: Menopausal hormone therapy and breast cancer. J Natl Cancer Inst. 2012;104:517–527. doi: 10.1093/jnci/djs014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowski RT, Kuller LH, Prentice RL, Stefanick ML, Manson JE, Gass M, Aragaki AK, Ockene JK, Lane DS, Sarto GE, et al. Breast cancer after use of estrogen plus progestin in postmenopausal women. N Engl J Med. 2009;360:573–587. doi: 10.1056/NEJMoa0807684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodankar R, Kwang S, Sangiorgi F, Hong H, Yen HY, Deng C, Pike MC, Shuler CF, Maxson R, Dubeau L. Cell-nonautonomous induction of ovarian and uterine serous cystadenomas in mice lacking a functional Brca1 in ovarian granulosa cells. Curr Biol. 2005;15:561–565. doi: 10.1016/j.cub.2005.01.052. [DOI] [PubMed] [Google Scholar]
- Chung HH, Sze SK, Tay AS, Lin VC. Acetylation at lysine 183 of progesterone receptor by p300 accelerates DNA binding kinetics and transactivation of direct target genes. J Biol Chem. 2014;289:2180–2194. doi: 10.1074/jbc.M113.517896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicatiello L, Addeo R, Sasso A, Altucci L, Petrizzi VB, Borgo R, Cancemi M, Caporali S, Caristi S, Scafoglio C, et al. Estrogens and progesterone promote persistent CCND1 gene activation during G1 by inducing transcriptional derepression via c-Jun/c-Fos/estrogen receptor (progesterone receptor) complex assembly to a distal regulatory element and recruitment of cyclin D1 to its own gene promoter. Mol Cell Biol. 2004;24:7260–7274. doi: 10.1128/MCB.24.16.7260-7274.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cittelly DM, Finlay-Schultz J, Howe EN, Spoelstra NS, Axlund SD, Hendricks P, Jacobsen BM, Sartorius CA, Richer JK. Progestin suppression of miR-29 potentiates dedifferentiation of breast cancer cells via KLF4. Oncogene. 2013;32:2555–2564. doi: 10.1038/onc.2012.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy KB. Reproductive ecology and the endometrium: physiology, variation, and new directions. Am J Phys Anthropol. 2009;14049(Suppl):137–154. doi: 10.1002/ajpa.21188. [DOI] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Collaborative Group on Hormonal Factors in Breast C Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347:1713–1727. doi: 10.1016/s0140-6736(96)90806-5. [DOI] [PubMed] [Google Scholar]
- Condon JC, Hardy DB, Kovaric K, Mendelson CR. Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-kappaB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol. 2006;20:764–775. doi: 10.1210/me.2005-0242. [DOI] [PubMed] [Google Scholar]
- Conneely OM, Mulac-Jericevic B, Lydon JP. Progesterone-dependent regulation of female reproductive activity by two distinct progesterone receptor isoforms. Steroids. 2003;68:771–778. doi: 10.1016/s0039-128x(03)00126-0. [DOI] [PubMed] [Google Scholar]
- Conneely OM, Mulac-Jericevic B, Lydon JP, De Mayo FJ. Reproductive functions of the progesterone receptor isoforms: lessons from knock-out mice. Mol Cell Endocrinol. 2001;179:97–103. doi: 10.1016/s0303-7207(01)00465-8. [DOI] [PubMed] [Google Scholar]
- Cooke PS, Buchanan DL, Young P, Setiawan T, Brody J, Korach KS, Taylor J, Lubahn DB, Cunha GR. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci U S A. 1997;94:6535–6540. doi: 10.1073/pnas.94.12.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel AR, Faivre EJ, Lange CA. Phosphorylation-dependent antagonism of sumoylation derepresses progesterone receptor action in breast cancer cells. Mol Endocrinol. 2007;21:2890–2906. doi: 10.1210/me.2007-0248. [DOI] [PubMed] [Google Scholar]
- Daniel AR, Gaviglio AL, Czaplicki LM, Hillard CJ, Housa D, Lange CA. The Progesterone Receptor Hinge Region Regulates the Kinetics of Transcriptional Responses Through Acetylation, Phosphorylation, and Nuclear Retention. Mol Endocrinol. doi: 10.1210/me.2010-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel AR, Gaviglio AL, Czaplicki LM, Hillard CJ, Housa D, Lange CA. The progesterone receptor hinge region regulates the kinetics of transcriptional responses through acetylation, phosphorylation, and nuclear retention. Mol Endocrinol. 2010;24:2126–2138. doi: 10.1210/me.2010-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel AR, Gaviglio AL, Knutson TP, Ostrander JH, D'Assoro AB, Ravindranathan P, Peng Y, Raj GV, Yee D, Lange CA. Progesterone receptor-B enhances estrogen responsiveness of breast cancer cells via scaffolding PELP1- and estrogen receptor-containing transcription complexes. Oncogene. 2014 doi: 10.1038/onc.2013.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel AR, Hagan CR, Lange CA. Progesterone receptor action: defining a role in breast cancer. Expert Rev Endocrinol Metab. 2011;6:359–369. doi: 10.1586/eem.11.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel AR, Knutson TP, Lange CA. Signaling inputs to progesterone receptor gene regulation and promoter selectivity. Mol Cell Endocrinol. 2009;308:47–52. doi: 10.1016/j.mce.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel AR, Lange CA. Protein kinases mediate ligand-independent derepression of sumoylated progesterone receptors in breast cancer cells. Proc Natl Acad Sci U S A. 2009;106:14287–14292. doi: 10.1073/pnas.0905118106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel CW, Silberstein GB, Strickland P. Direct action of 17 beta-estradiol on mouse mammary ducts analyzed by sustained release implants and steroid autoradiography. Cancer Res. 1987;47:6052–6057. [PubMed] [Google Scholar]
- Diep CH, Charles NJ, Gilks CB, Kalloger SE, Argenta PA, Lange CA. Progesterone receptors induce FOXO1-dependent senescence in ovarian cancer cells. Cell Cycle. 2013;12:1433–1449. doi: 10.4161/cc.24550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressing GE, Hagan CR, Knutson TP, Daniel AR, Lange CA. Progesterone receptors act as sensors for mitogenic protein kinases in breast cancer models. Endocr Relat Cancer. 2009;16:351–361. doi: 10.1677/ERC-08-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressing GE, Knutson TP, Schiewer MJ, Daniel AR, Hagan CR, Diep CH, Knudsen KE, Lange CA. Progesterone receptor-cyclin d1 complexes induce cell cycle-dependent transcriptional programs in breast cancer cells. Mol Endocrinol. 2014;28:442–457. doi: 10.1210/me.2013-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson RJ, Monaghan JM. The epidemiology of ovarian cancer. Int J Gynecol Cancer. 2001;11:423–429. doi: 10.1046/j.1525-1438.2001.01053.x. [DOI] [PubMed] [Google Scholar]
- Encarnacion CA, Ciocca DR, McGuire WL, Clark GM, Fuqua SA, Osborne CK. Measurement of steroid hormone receptors in breast cancer patients on tamoxifen. Breast Cancer Res Treat. 1993;26:237–246. doi: 10.1007/BF00665801. [DOI] [PubMed] [Google Scholar]
- Faivre E, Skildum A, Pierson-Mullany L, Lange CA. Integration of progesterone receptor mediated rapid signaling and nuclear actions in breast cancer cell models: role of mitogen-activated protein kinases and cell cycle regulators. Steroids. 2005;70:418–426. doi: 10.1016/j.steroids.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Faivre EJ, Daniel AR, Hillard CJ, Lange CA. Progesterone receptor rapid signaling mediates serine 345 phosphorylation and tethering to specificity protein 1 transcription factors. Mol Endocrinol. 2008;22:823–837. doi: 10.1210/me.2007-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre EJ, Lange CA. Progesterone receptors upregulate Wnt-1 to induce epidermal growth factor receptor transactivation and c-Src-dependent sustained activation of Erk1/2 mitogen-activated protein kinase in breast cancer cells. Mol Cell Biol. 2007;27:466–480. doi: 10.1128/MCB.01539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Yanase T, Morinaga H, Okabe T, Nomura M, Daitoku H, Fukamizu A, Kato S, Takayanagi R, Nawata H. Insulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. J Biol Chem. 2007;282:7329–7338. doi: 10.1074/jbc.M610447200. [DOI] [PubMed] [Google Scholar]
- Fauvet R, Dufournet Etienne C, Poncelet C, Bringuier AF, Feldmann G, Darai E. Effects of progesterone and anti-progestin (mifepristone) treatment on proliferation and apoptosis of the human ovarian cancer cell line, OVCAR-3. Oncol Rep. 2006;15:743–748. [PubMed] [Google Scholar]
- Fleisch MC, Chou YC, Cardiff RD, Asaithambi A, Shyamala G. Overexpression of progesterone receptor A isoform in mice leads to endometrial hyperproliferation, hyperplasia and atypia. Mol Hum Reprod. 2009;15:241–249. doi: 10.1093/molehr/gap013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkins AK, Jarboe EA, Saleemuddin A, Lee Y, Callahan MJ, Drapkin R, Garber JE, Muto MG, Tworoger S, Crum CP. A candidate precursor to pelvic serous cancer (p53 signature) and its prevalence in ovaries and fallopian tubes from women with BRCA mutations. Gynecol Oncol. 2008;109:168–173. doi: 10.1016/j.ygyno.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107:103–111. doi: 10.1007/s10549-007-9523-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier A, Berrino F, Riboli E, Avenel V, Clavel-Chapelon F. Breast cancer risk in relation to different types of hormone replacement therapy in the E3N-EPIC cohort. Int J Cancer. 2005;114:448–454. doi: 10.1002/ijc.20710. [DOI] [PubMed] [Google Scholar]
- Franco HL, Rubel CA, Large MJ, Wetendorf M, Fernandez-Valdivia R, Jeong JW, Spencer TE, Behringer RR, Lydon JP, Demayo FJ. Epithelial progesterone receptor exhibits pleiotropic roles in uterine development and function. FASEB J. 2012;26:1218–1227. doi: 10.1096/fj.11-193334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabra H, Watson JE, Taylor KJ, Mackay J, Leonard RC, Steel CM, Porteous DJ, Smyth JF. Definition and refinement of a region of loss of heterozygosity at 11q23.3-q24.3 in epithelial ovarian cancer associated with poor prognosis. Cancer Res. 1996;56:950–954. [PubMed] [Google Scholar]
- Giangrande PH, Kimbrel EA, Edwards DP, McDonnell DP. The opposing transcriptional activities of the two isoforms of the human progesterone receptor are due to differential cofactor binding. Mol Cell Biol. 2000;20:3102–3115. doi: 10.1128/mcb.20.9.3102-3115.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulianelli S, Vaque JP, Soldati R, Wargon V, Vanzulli SI, Martins R, Zeitlin E, Molinolo AA, Helguero LA, Lamb CA, et al. Estrogen receptor alpha mediates progestin-induced mammary tumor growth by interacting with progesterone receptors at the cyclin D1/MYC promoters. Cancer Res. 2012;72:2416–2427. doi: 10.1158/0008-5472.CAN-11-3290. [DOI] [PubMed] [Google Scholar]
- Goto T, Takano M, Hirata J, Tsuda H. The involvement of FOXO1 in cytotoxic stress and drug-resistance induced by paclitaxel in ovarian cancers. Br J Cancer. 2008;98:1068–1075. doi: 10.1038/sj.bjc.6604279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyeneche AA, Telleria CM. Antiprogestins in gynecological diseases. Reproduction. 2015;149:R15–R33. doi: 10.1530/REP-14-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JD, Clarke CL. Physiological action of progesterone in target tissues. Endocr Rev. 1997;18:502–519. doi: 10.1210/edrv.18.4.0308. [DOI] [PubMed] [Google Scholar]
- Graham JD, Mote PA, Salagame U, van Dijk JH, Balleine RL, Huschtscha LI, Reddel RR, Clarke CL. DNA replication licensing and progenitor numbers are increased by progesterone in normal human breast. Endocrinology. 2009;150:3318–3326. doi: 10.1210/en.2008-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groshong SD, Owen GI, Grimison B, Schauer IE, Todd MC, Langan TA, Sclafani RA, Lange CA, Horwitz KB. Biphasic regulation of breast cancer cell growth by progesterone: role of the cyclin-dependent kinase inhibitors, p21 and p27(Kip1). Mol Endocrinol. 1997;11:1593–1607. doi: 10.1210/mend.11.11.0006. [DOI] [PubMed] [Google Scholar]
- Gross GE, Clark GM, Chamness GC, McGuire WL. Multiple progesterone receptor assays in human breast cancer. Cancer Res. 1984;44:836–840. [PubMed] [Google Scholar]
- Hagan CR, Daniel AR, Dressing GE, Lange CA. Role of phosphorylation in progesterone receptor signaling and specificity. Mol Cell Endocrinol. 2011a doi: 10.1016/j.mce.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan CR, Knutson TP, Lange CA. A Common Docking Domain in Progesterone Receptor-B links DUSP6 and CK2 signaling to proliferative transcriptional programs in breast cancer cells. Nucleic Acids Res. 2013;41:8926–8942. doi: 10.1093/nar/gkt706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan CR, Lange CA. Molecular determinants of context-dependent progesterone receptor action in breast cancer. BMC Med. 2014;12:32. doi: 10.1186/1741-7015-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan CR, Regan TM, Dressing GE, Lange CA. ck2-dependent phosphorylation of progesterone receptors (PR) on Ser81 regulates PR-B isoform-specific target gene expression in breast cancer cells. Mol Cell Biol. 2011b;31:2439–2452. doi: 10.1128/MCB.01246-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SJ, Jeong J, Demayo FJ, Xu J, Tsai SY, Tsai MJ, O'Malley BW. Dynamic cell type specificity of SRC-1 coactivator in modulating uterine progesterone receptor function in mice. Mol Cell Biol. 2005;25:8150–8165. doi: 10.1128/MCB.25.18.8150-8165.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SJ, Tsai SY, Tsai MJ, O'Malley BW. Distinct temporal and spatial activities of RU486 on progesterone receptor function in reproductive organs of ovariectomized mice. Endocrinology. 2007;148:2471–2486. doi: 10.1210/en.2006-1561. [DOI] [PubMed] [Google Scholar]
- Hankinson SE, Colditz GA, Hunter DJ, Spencer TL, Rosner B, Stampfer MJ. A quantitative assessment of oral contraceptive use and risk of ovarian cancer. Obstet Gynecol. 1992;80:708–714. [PubMed] [Google Scholar]
- Hecht JL, Mutter GL. Molecular and pathologic aspects of endometrial carcinogenesis. J Clin Oncol. 2006;24:4783–4791. doi: 10.1200/JCO.2006.06.7173. [DOI] [PubMed] [Google Scholar]
- Hedrick SM, Hess Michelini R, Doedens AL, Goldrath AW, Stone EL. FOXO transcription factors throughout T cell biology. Nat Rev Immunol. 2012;12:649–661. doi: 10.1038/nri3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempling RE, Piver MS, Eltabbakh GH, Recio FO. Progesterone receptor status is a significant prognostic variable of progression-free survival in advanced epithelial ovarian cancer. Am J Clin Oncol. 1998;21:447–451. doi: 10.1097/00000421-199810000-00005. [DOI] [PubMed] [Google Scholar]
- Hilton HN, Graham JD, Kantimm S, Santucci N, Cloosterman D, Huschtscha LI, Mote PA, Clarke CL. Progesterone and estrogen receptors segregate into different cell subpopulations in the normal human breast. Mol Cell Endocrinol. 2012;361:191–201. doi: 10.1016/j.mce.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Hogdall EV, Christensen L, Hogdall CK, Blaakaer J, Gayther S, Jacobs IJ, Christensen IJ, Kjaer SK. Prognostic value of estrogen receptor and progesterone receptor tumor expression in Danish ovarian cancer patients: from the ‘MALOVA’ ovarian cancer study. Oncol Rep. 2007;18:1051–1059. [PubMed] [Google Scholar]
- Hong H, Yen HY, Brockmeyer A, Liu Y, Chodankar R, Pike MC, Stanczyk FZ, Maxson R, Dubeau L. Changes in the mouse estrus cycle in response to BRCA1 inactivation suggest a potential link between risk factors for familial and sporadic ovarian cancer. Cancer Res. 2010;70:221–228. doi: 10.1158/0008-5472.CAN-09-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz KB, Dye WW, Harrell JC, Kabos P, Sartorius CA. Rare steroid receptor-negative basal-like tumorigenic cells in luminal subtype human breast cancer xenografts. Proc Natl Acad Sci U S A. 2008;105:5774–5779. doi: 10.1073/pnas.0706216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz KB, Sartorius CA. Progestins in hormone replacement therapies reactivate cancer stem cells in women with preexisting breast cancers: a hypothesis. J Clin Endocrinol Metab. 2008;93:3295–3298. doi: 10.1210/jc.2008-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X, Tan Y, Li M, Dey SK, Das SK. Canonical Wnt signaling is critical to estrogen-mediated uterine growth. Mol Endocrinol. 2004;18:3035–3049. doi: 10.1210/me.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovland AR, Powell RL, Takimoto GS, Tung L, Horwitz KB. An N-terminal inhibitory function, IF, suppresses transcription by the A-isoform but not the B-isoform of human progesterone receptors. J Biol Chem. 1998;273:5455–5460. doi: 10.1074/jbc.273.10.5455. [DOI] [PubMed] [Google Scholar]
- Hunter DJ, Colditz GA, Hankinson SE, Malspeis S, Spiegelman D, Chen W, Stampfer MJ, Willett WC. Oral contraceptive use and breast cancer: a prospective study of young women. Cancer Epidemiol Biomarkers Prev. 2010;19:2496–2502. doi: 10.1158/1055-9965.EPI-10-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen DM, Rosales MA, Paik DY, Lee DS, Smith DA, Witte ON, Iruela-Arispe ML, Memarzadeh S. Progesterone receptor signaling in the microenvironment of endometrial cancer influences its response to hormonal therapy. Cancer Res. 2013;73:4697–4710. doi: 10.1158/0008-5472.CAN-13-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Johnston SR, Saccani-Jotti G, Smith IE, Salter J, Newby J, Coppen M, Ebbs SR, Dowsett M. Changes in estrogen receptor, progesterone receptor, and pS2 expression in tamoxifen-resistant human breast cancer. Cancer Res. 1995;55:3331–3338. [PubMed] [Google Scholar]
- Jones MC, Fusi L, Higham JH, Abdel-Hafiz H, Horwitz KB, Lam EW, Brosens JJ. Regulation of the SUMO pathway sensitizes differentiating human endometrial stromal cells to progesterone. Proc Natl Acad Sci U S A. 2006;103:16272–16277. doi: 10.1073/pnas.0603002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongen V, Briet J, de Jong R, ten Hoor K, Boezen M, van der Zee A, Nijman H, Hollema H. Expression of estrogen receptor-alpha and -beta and progesterone receptor-A and -B in a large cohort of patients with endometrioid endometrial cancer. Gynecol Oncol. 2009;112:537–542. doi: 10.1016/j.ygyno.2008.10.032. [DOI] [PubMed] [Google Scholar]
- Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, Stingl J, Waterhouse PD, Khokha R. Progesterone induces adult mammary stem cell expansion. Nature. 465:803–807. doi: 10.1038/nature09091. [DOI] [PubMed] [Google Scholar]
- Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, Stingl J, Waterhouse PD, Khokha R. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465:803–807. doi: 10.1038/nature09091. [DOI] [PubMed] [Google Scholar]
- Kaku T, Yoshikawa H, Tsuda H, Sakamoto A, Fukunaga M, Kuwabara Y, Hataeg M, Kodama S, Kuzuya K, Sato S, et al. Conservative therapy for adenocarcinoma and atypical endometrial hyperplasia of the endometrium in young women: central pathologic review and treatment outcome. Cancer Lett. 2001;167:39–48. doi: 10.1016/s0304-3835(01)00462-1. [DOI] [PubMed] [Google Scholar]
- Kariagina A, Aupperlee MD, Haslam SZ. Progesterone receptor isoform functions in normal breast development and breast cancer. Crit Rev Eukaryot Gene Expr. 2008;18:11–33. doi: 10.1615/critreveukargeneexpr.v18.i1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst AM, Levanon K, Drapkin R. Modeling high-grade serous ovarian carcinogenesis from the fallopian tube. Proc Natl Acad Sci U S A. 2011;108:7547–7552. doi: 10.1073/pnas.1017300108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith Bechtel M, Bonavida B. Inhibitory effects of 17beta-estradiol and progesterone on ovarian carcinoma cell proliferation: a potential role for inducible nitric oxide synthase. Gynecol Oncol. 2001;82:127–138. doi: 10.1006/gyno.2001.6221. [DOI] [PubMed] [Google Scholar]
- Khunamornpong S, Suprasert P, Chiangmai WN, Siriaunkgul S. Metastatic tumors to the ovaries: a study of 170 cases in northern Thailand. Int J Gynecol Cancer. 2006;16(Suppl 1):132–138. doi: 10.1111/j.1525-1438.2006.00302.x. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Buzzio OL, Li S, Lu Z. Role of FOXO1A in the regulation of insulin-like growth factor-binding protein-1 in human endometrial cells: interaction with progesterone receptor. Biol Reprod. 2005;73:833–839. doi: 10.1095/biolreprod.105.043182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Chapman-Davis E. Role of progesterone in endometrial cancer. Semin Reprod Med. 2010;28:81–90. doi: 10.1055/s-0029-1242998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev. 2013;34:130–162. doi: 10.1210/er.2012-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson TP, Daniel AR, Fan D, Silverstein KA, Covington KR, Fuqua SA, Lange CA. Phosphorylated and small ubiquitin-like modifier protein-deficient progesterone receptors drive proliferative gene signatures during breast cancer progression. Breast Cancer Res. 2012a;14:R95. doi: 10.1186/bcr3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson TP, Daniel AR, Fan D, Silverstein KA, Covington KR, Fuqua SA, Lange CA. Phosphorylated and sumoylation-deficient progesterone receptors drive proliferative gene signatures during breast cancer progression. Breast Cancer Res. 2012b;14:R95. doi: 10.1186/bcr3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson TP, Lange CA. Tracking progesterone receptor-mediated actions in breast cancer. Pharmacol Ther. 2014;142:114–125. doi: 10.1016/j.pharmthera.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreizman-Shefer H, Pricop J, Goldman S, Elmalah I, Shalev E. Distribution of estrogen and progesterone receptors isoforms in endometrial cancer. Diagn Pathol. 2014;9:77. doi: 10.1186/1746-1596-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita T, Young P, Brody JR, Lydon JP, O'Malley BW, Cunha GR. Stromal progesterone receptors mediate the inhibitory effects of progesterone on estrogen-induced uterine epithelial cell deoxyribonucleic acid synthesis. Endocrinology. 1998;139:4708–4713. doi: 10.1210/endo.139.11.6317. [DOI] [PubMed] [Google Scholar]
- Kyo S, Sakaguchi J, Kiyono T, Shimizu Y, Maida Y, Mizumoto Y, Mori N, Nakamura M, Takakura M, Miyake K, et al. Forkhead transcription factor FOXO1 is a direct target of progestin to inhibit endometrial epithelial cell growth. Clin Cancer Res. 2011;17:525–537. doi: 10.1158/1078-0432.CCR-10-1287. [DOI] [PubMed] [Google Scholar]
- Lange CA, Shen T, Horwitz KB. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc Natl Acad Sci U S A. 2000;97:1032–1037. doi: 10.1073/pnas.97.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Rosen DG, Zhu C, Silva EG, Liu J. Expression of progesterone receptor is a favorable prognostic marker in ovarian cancer. Gynecol Oncol. 2005;96:671–677. doi: 10.1016/j.ygyno.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, Garber J, Birch C, Mou H, Gordon RW, et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211:26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- Lenhard M, Tereza L, Heublein S, Ditsch N, Himsl I, Mayr D, Friese K, Jeschke U. Steroid hormone receptor expression in ovarian cancer: Progesterone receptor B as prognostic marker for patient survival. BMC Cancer. 2012;12:553. doi: 10.1186/1471-2407-12-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie KK, Kumar NS, Richer J, Owen G, Takimoto G, Horwitz KB, Lange C. Differential expression of the A and B isoforms of progesterone receptor in human endometrial cancer cells. Only progesterone receptor B is induced by estrogen and associated with strong transcriptional activation. Ann N Y Acad Sci. 1997;828:17–26. doi: 10.1111/j.1749-6632.1997.tb48520.x. [DOI] [PubMed] [Google Scholar]
- Li CI, Beaber EF, Tang MT, Porter PL, Daling JR, Malone KE. Effect of depomedroxyprogesterone acetate on breast cancer risk among women 20 to 44 years of age. Cancer Res. 2012;72:2028–2035. doi: 10.1158/0008-5472.CAN-11-4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Lee H, Guo S, Unterman TG, Jenster G, Bai W. AKT-independent protection of prostate cancer cells from apoptosis mediated through complex formation between the androgen receptor and FKHR. Mol Cell Biol. 2003;23:104–118. doi: 10.1128/MCB.23.1.104-118.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Kannan A, DeMayo FJ, Lydon JP, Cooke PS, Yamagishi H, Srivastava D, Bagchi MK, Bagchi IC. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science. 2011;331:912–916. doi: 10.1126/science.1197454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren P, Backstrom T, Mahlck CG, Ridderheim M, Cajander S. Steroid receptors and hormones in relation to cell proliferation and apoptosis in poorly differentiated epithelial ovarian tumors. Int J Oncol. 2001;19:31–38. [PubMed] [Google Scholar]
- Lo AT, Mori H, Mott J, Bissell MJ. Constructing three-dimensional models to study mammary gland branching morphogenesis and functional differentiation. J Mammary Gland Biol Neoplasia. 2012;17:103–110. doi: 10.1007/s10911-012-9251-7. [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr., Shyamala G, Conneely OM, O'Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- Lyytinen HK, Dyba T, Ylikorkala O, Pukkala EI. A case-control study on hormone therapy as a risk factor for breast cancer in Finland: Intrauterine system carries a risk as well. Int J Cancer. 2010;126:483–489. doi: 10.1002/ijc.24738. [DOI] [PubMed] [Google Scholar]
- Maillot G, Lacroix-Triki M, Pierredon S, Gratadou L, Schmidt S, Benes V, Roche H, Dalenc F, Auboeuf D, Millevoi S, et al. Widespread estrogen-dependent repression of micrornas involved in breast tumor cell growth. Cancer Res. 2009;69:8332–8340. doi: 10.1158/0008-5472.CAN-09-2206. [DOI] [PubMed] [Google Scholar]
- McCampbell AS, Broaddus RR, Loose DS, Davies PJ. Overexpression of the insulin-like growth factor I receptor and activation of the AKT pathway in hyperplastic endometrium. Clin Cancer Res. 2006;12:6373–6378. doi: 10.1158/1078-0432.CCR-06-0912. [DOI] [PubMed] [Google Scholar]
- Mendelson CR. Minireview: fetal-maternal hormonal signaling in pregnancy and labor. Mol Endocrinol. 2009;23:947–954. doi: 10.1210/me.2009-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlino AA, Welsh TN, Tan H, Yi LJ, Cannon V, Mercer BM, Mesiano S. Nuclear progesterone receptors in the human pregnancy myometrium: evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A. J Clin Endocrinol Metab. 2007;92:1927–1933. doi: 10.1210/jc.2007-0077. [DOI] [PubMed] [Google Scholar]
- Mesiano S, Chan EC, Fitter JT, Kwek K, Yeo G, Smith R. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J Clin Endocrinol Metab. 2002;87:2924–2930. doi: 10.1210/jcem.87.6.8609. [DOI] [PubMed] [Google Scholar]
- Mesiano S, Wang Y, Norwitz ER. Progesterone receptors in the human pregnancy uterus: do they hold the key to birth timing? Reprod Sci. 2011;18:6–19. doi: 10.1177/1933719110382922. [DOI] [PubMed] [Google Scholar]
- Migliaccio A, Piccolo D, Castoria G, Di Domenico M, Bilancio A, Lombardi M, Gong W, Beato M, Auricchio F. Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. EMBO J. 1998;17:2008–2018. doi: 10.1093/emboj/17.7.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Watanabe J, Hata H, Jobo T, Kawaguchi M, Hattori M, Saito M, Kuramoto H. Significance of progesterone receptor-A and -B expressions in endometrial adenocarcinoma. J Steroid Biochem Mol Biol. 2004;92:111–118. doi: 10.1016/j.jsbmb.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Modugno F, Laskey R, Smith AL, Andersen CL, Haluska P, Oesterreich S. Hormone response in ovarian cancer: time to reconsider as a clinical target? Endocr Relat Cancer. 2012;19:R255–R279. doi: 10.1530/ERC-12-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mote PA, Balleine RL, McGowan EM, Clarke CL. Colocalization of progesterone receptors A and B by dual immunofluorescent histochemistry in human endometrium during the menstrual cycle. J Clin Endocrinol Metab. 1999;84:2963–2971. doi: 10.1210/jcem.84.8.5928. [DOI] [PubMed] [Google Scholar]
- Mote PA, Graham JD, Clarke CL. Progesterone receptor isoforms in normal and malignant breast. Ernst Schering Found Symp Proc. 2007:77–107. [PubMed] [Google Scholar]
- Mote PA, Leary JA, Avery KA, Sandelin K, Chenevix-Trench G, Kirk JA, Clarke CL, kConFab I. Germ-line mutations in BRCA1 or BRCA2 in the normal breast are associated with altered expression of estrogen-responsive proteins and the predominance of progesterone receptor A. Genes Chromosomes Cancer. 2004;39:236–248. doi: 10.1002/gcc.10321. [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289:1751–1754. doi: 10.1126/science.289.5485.1751. [DOI] [PubMed] [Google Scholar]
- Munstedt K, Steen J, Knauf AG, Buch T, von Georgi R, Franke FE. Steroid hormone receptors and long term survival in invasive ovarian cancer. Cancer. 2000;89:1783–1791. doi: 10.1002/1097-0142(20001015)89:8<1783::aid-cncr19>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Musgrove EA, Lee CS, Sutherland RL. Progestins both stimulate and inhibit breast cancer cell cycle progression while increasing expression of transforming growth factor alpha, epidermal growth factor receptor, c-fos, and c-myc genes. Mol Cell Biol. 1991;11:5032–5043. doi: 10.1128/mcb.11.10.5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7:847–859. doi: 10.1038/nrc2223. [DOI] [PubMed] [Google Scholar]
- Mylonas I, Jeschke U, Shabani N, Kuhn C, Kunze S, Dian D, Friedl C, Kupka MS, Friese K. Steroid receptors ERalpha, ERbeta, PR-A and PR-B are differentially expressed in normal and atrophic human endometrium. Histol Histopathol. 2007;22:169–176. doi: 10.14670/HH-22.169. [DOI] [PubMed] [Google Scholar]
- Need EF, Selth LA, Harris TJ, Birrell SN, Tilley WD, Buchanan G. Research resource: interplay between the genomic and transcriptional networks of androgen receptor and estrogen receptor alpha in luminal breast cancer cells. Mol Endocrinol. 2012;26:1941–1952. doi: 10.1210/me.2011-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norquist BM, Garcia RL, Allison KH, Jokinen CH, Kernochan LE, Pizzi CC, Barrow BJ, Goff BA, Swisher EM. The molecular pathogenesis of hereditary ovarian carcinoma: alterations in the tubal epithelium of women with BRCA1 and BRCA2 mutations. Cancer. 2010;116:5261–5271. doi: 10.1002/cncr.25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata K, Morland SJ, Watson RH, Hitchcock A, Chenevix-Trench G, Thomas EJ, Campbell IG. Frequent PTEN/MMAC mutations in endometrioid but not serous or mucinous epithelial ovarian tumors. Cancer Res. 1998;58:2095–2097. [PubMed] [Google Scholar]
- Owen GI, Richer JK, Tung L, Takimoto G, Horwitz KB. Progesterone regulates transcription of the p21(WAF1) cyclin- dependent kinase inhibitor gene through Sp1 and CBP/p300. J Biol Chem. 1998;273:10696–10701. doi: 10.1074/jbc.273.17.10696. [DOI] [PubMed] [Google Scholar]
- Pathiraja TN, Shetty PB, Jelinek J, He R, Hartmaier R, Margossian AL, Hilsenbeck SG, Issa JP, Oesterreich S. Progesterone receptor isoform-specific promoter methylation: association of PRA promoter methylation with worse outcome in breast cancer patients. Clin Cancer Res. 2011;17:4177–4186. doi: 10.1158/1078-0432.CCR-10-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault D, Eisenhauer EA, Pritchard KI, Panasci L, Norris B, Vandenberg T, Fisher B. Phase II study of the progesterone antagonist mifepristone in patients with untreated metastatic breast carcinoma: a National Cancer Institute of Canada Clinical Trials Group study. J Clin Oncol. 1996;14:2709–2712. doi: 10.1200/JCO.1996.14.10.2709. [DOI] [PubMed] [Google Scholar]
- Peters AA, Buchanan G, Ricciardelli C, Bianco-Miotto T, Centenera MM, Harris JM, Jindal S, Segara D, Jia L, Moore NL, et al. Androgen receptor inhibits estrogen receptor-alpha activity and is prognostic in breast cancer. Cancer Res. 2009;69:6131–6140. doi: 10.1158/0008-5472.CAN-09-0452. [DOI] [PubMed] [Google Scholar]
- Pieber D, Allport VC, Hills F, Johnson M, Bennett PR. Interactions between progesterone receptor isoforms in myometrial cells in human labour. Mol Hum Reprod. 2001;7:875–879. doi: 10.1093/molehr/7.9.875. [DOI] [PubMed] [Google Scholar]
- Pierson-Mullany LK, Lange CA. Phosphorylation of progesterone receptor serine 400 mediates ligand-independent transcriptional activity in response to activation of cyclin-dependent protein kinase 2. Mol Cell Biol. 2004a;24:10542–10557. doi: 10.1128/MCB.24.24.10542-10557.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson-Mullany LK, Lange CA. Phosphorylation of Progesterone Receptor Serine 400 Mediates Ligand-Independent Transcriptional Activity in Response to Activatoin of Cyclin-Dependent Protein Kinase2. Mol. Cell. Biol. 2004b;24 doi: 10.1128/MCB.24.24.10542-10557.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu M, Lange CA. MAP kinases couple multiple functions of human progesterone receptors: degradation, transcriptional synergy, and nuclear association. J Steroid Biochem Mol Biol. 2003;85:147–157. doi: 10.1016/s0960-0760(03)00221-8. [DOI] [PubMed] [Google Scholar]
- Ramsey EM, Houston ML, Harris JW. Interactions of the trophoblast and maternal tissues in three closely related primate species. Am J Obstet Gynecol. 1976;124:647–652. doi: 10.1016/0002-9378(76)90068-5. [DOI] [PubMed] [Google Scholar]
- Ren Y, Liu X, Ma D, Feng Y, Zhong N. Down-regulation of the progesterone receptor by the methylation of progesterone receptor gene in endometrial cancer cells. Cancer Genet Cytogenet. 2007;175:107–116. doi: 10.1016/j.cancergencyto.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem. 2002;277:5209–5218. doi: 10.1074/jbc.M110090200. [DOI] [PubMed] [Google Scholar]
- Richer JK, Lange CA, Manning NG, Owen G, Powell R, Horwitz KB. Convergence of progesterone with growth factor and cytokine signaling in breast cancer. Progesterone receptors regulate signal transducers and activators of transcription expression and activity. J Biol Chem. 1998;273:31317–31326. doi: 10.1074/jbc.273.47.31317. [DOI] [PubMed] [Google Scholar]
- Rocereto TF, Brady WE, Shahin MS, Hoffman JS, Small L, Rotmensch J, Mannel RS. A phase II evaluation of mifepristone in the treatment of recurrent or persistent epithelial ovarian, fallopian or primary peritoneal cancer: a gynecologic oncology group study. Gynecol Oncol. 2010;116:332–334. doi: 10.1016/j.ygyno.2009.10.071. [DOI] [PubMed] [Google Scholar]
- Rudd MD, Gonzalez-Robayna I, Hernandez-Gonzalez I, Weigel NL, Bingman WE, 3rd, Richards JS. Constitutively active FOXO1a and a DNA-binding domain mutant exhibit distinct co-regulatory functions to enhance progesterone receptor A activity. J Mol Endocrinol. 2007;38:673–690. doi: 10.1677/JME-07-0017. [DOI] [PubMed] [Google Scholar]
- Sakaguchi H, Fujimoto J, Hong BL, Nakagawa Y, Tamaya T. Drastic decrease of progesterone receptor form B but not A mRNA reflects poor patient prognosis in endometrial cancers. Gynecol Oncol. 2004;93:394–399. doi: 10.1016/j.ygyno.2004.01.042. [DOI] [PubMed] [Google Scholar]
- Salghetti SE, Caudy AA, Chenoweth JG, Tansey WP. Regulation of transcriptional activation domain function by ubiquitin. Science. 2001;293:1651–1653. doi: 10.1126/science.1062079. [DOI] [PubMed] [Google Scholar]
- Samarnthai N, Hall K, Yeh IT. Molecular profiling of endometrial malignancies. Obstet Gynecol Int. 2010;2010:162363. doi: 10.1155/2010/162363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Dharia A, Oh BR, Tanaka Y, Fujimoto S, Dahiya R. Progesterone receptor B gene inactivation and CpG hypermethylation in human uterine endometrial cancer. Cancer Res. 2001;61:97–102. [PubMed] [Google Scholar]
- Sato N, Tsunoda H, Nishida M, Morishita Y, Takimoto Y, Kubo T, Noguchi M. Loss of heterozygosity on 10q23.3 and mutation of the tumor suppressor gene PTEN in benign endometrial cyst of the ovary: possible sequence progression from benign endometrial cyst to endometrioid carcinoma and clear cell carcinoma of the ovary. Cancer Res. 2000;60:7052–7056. [PubMed] [Google Scholar]
- Schuur ER, Loktev AV, Sharma M, Sun Z, Roth RA, Weigel RJ. Ligand-dependent interaction of estrogen receptor-alpha with members of the forkhead transcription factor family. J Biol Chem. 2001;276:33554–33560. doi: 10.1074/jbc.M105555200. [DOI] [PubMed] [Google Scholar]
- Shabani N, Kuhn C, Kunze S, Schulze S, Mayr D, Dian D, Gingelmaier A, Schindlbeck C, Willgeroth F, Sommer H, et al. Prognostic significance of oestrogen receptor alpha (ERalpha) and beta (ERbeta), progesterone receptor A (PR-A) and B (PR-B) in endometrial carcinomas. Eur J Cancer. 2007;43:2434–2444. doi: 10.1016/j.ejca.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Shao R. Progesterone receptor isoforms A and B: new insights into the mechanism of progesterone resistance for the treatment of endometrial carcinoma. Ecancermedicalscience. 2013;7:381. doi: 10.3332/ecancer.2013.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieh W, Kobel M, Longacre TA, Bowtell DD, Defazio A, Goodman MT, Hogdall E, Deen S, Wentzensen N, Moysich KB, et al. Hormone-receptor expression and ovarian cancer survival: an Ovarian Tumor Tissue Analysis consortium study. Lancet Oncol. 2013 doi: 10.1016/S1470-2045(13)70253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinn BV, Darb-Esfahani S, Wirtz RM, Budczies J, Sehouli J, Chekerov R, Dietel M, Denkert C. Evaluation of a hormone receptor-positive ovarian carcinoma subtype with a favourable prognosis by determination of progesterone receptor and oestrogen receptor 1 mRNA expression in formalin-fixed paraffin-embedded tissue. Histopathology. 2011;59:918–927. doi: 10.1111/j.1365-2559.2011.04028.x. [DOI] [PubMed] [Google Scholar]
- Soini T, Hurskainen R, Grenman S, Maenpaa J, Paavonen J, Pukkala E. Cancer risk in women using the levonorgestrel-releasing intrauterine system in Finland. Obstet Gynecol. 2014;124:292–299. doi: 10.1097/AOG.0000000000000356. [DOI] [PubMed] [Google Scholar]
- Song LN, Coghlan M, Gelmann EP. Antiandrogen effects of mifepristone on coactivator and corepressor interactions with the androgen receptor. Mol Endocrinol. 2004;18:70–85. doi: 10.1210/me.2003-0189. [DOI] [PubMed] [Google Scholar]
- Spitz IM. Progesterone receptor antagonists. Curr Opin Investig Drugs. 2006;7:882–890. [PubMed] [Google Scholar]
- Spitz IM, Bardin CW. Mifepristone (RU 486)--a modulator of progestin and glucocorticoid action. N Engl J Med. 1993;329:404–412. doi: 10.1056/NEJM199308053290607. [DOI] [PubMed] [Google Scholar]
- Stoecklin E, Wissler M, Schaetzle D, Pfitzner E, Groner B. Interactions in the transcriptional regulation exerted by Stat5 and by members of the steroid hormone receptor family. J Steroid Biochem Mol Biol. 1999;69:195–204. doi: 10.1016/s0960-0760(99)00052-7. [DOI] [PubMed] [Google Scholar]
- Syed V, Ho SM. Progesterone-induced apoptosis in immortalized normal and malignant human ovarian surface epithelial cells involves enhanced expression of FasL. Oncogene. 2003;22:6883–6890. doi: 10.1038/sj.onc.1206828. [DOI] [PubMed] [Google Scholar]
- Syed V, Ulinski G, Mok SC, Yiu GK, Ho SM. Expression of gonadotropin receptor and growth responses to key reproductive hormones in normal and malignant human ovarian surface epithelial cells. Cancer Res. 2001;61:6768–6776. [PubMed] [Google Scholar]
- Takamoto N, Zhao B, Tsai SY, DeMayo FJ. Identification of Indian hedgehog as a progesterone-responsive gene in the murine uterus. Mol Endocrinol. 2002;16:2338–2348. doi: 10.1210/me.2001-0154. [DOI] [PubMed] [Google Scholar]
- Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA, et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147:1097–1121. doi: 10.1210/en.2005-1076. [DOI] [PubMed] [Google Scholar]
- Tang HY, Lin HY, Zhang S, Davis FB, Davis PJ. Thyroid hormone causes mitogen-activated protein kinase-dependent phosphorylation of the nuclear estrogen receptor. Endocrinology. 2004;145:3265–3272. doi: 10.1210/en.2004-0308. [DOI] [PubMed] [Google Scholar]
- Tangjitgamol S, Manusirivithaya S, Khunnarong J, Jesadapatarakul S, Tanwanich S. Expressions of estrogen and progesterone receptors in epithelial ovarian cancer: a clinicopathologic study. Int J Gynecol Cancer. 2009;19:620–627. doi: 10.1111/IGC.0b013e3181a44b62. [DOI] [PubMed] [Google Scholar]
- Tanos T, Sflomos G, Echeverria PC, Ayyanan A, Gutierrez M, Delaloye JF, Raffoul W, Fiche M, Dougall W, Schneider P, et al. Progesterone/RANKL is a major regulatory axis in the human breast. Sci Transl Med. 2013;5:182ra155. doi: 10.1126/scitranslmed.3005654. [DOI] [PubMed] [Google Scholar]
- Tong W, Pollard JW. Progesterone inhibits estrogen-induced cyclin D1 and cdk4 nuclear translocation, cyclin E- and cyclin A-cdk2 kinase activation, and cell proliferation in uterine epithelial cells in mice. Mol Cell Biol. 1999;19:2251–2264. doi: 10.1128/mcb.19.3.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tora L, Gronemeyer H, Turcotte B, Gaub MP, Chambon P. The N-terminal region of the chicken progesterone receptor specifies target gene activation. Nature. 1988;333:185–188. doi: 10.1038/333185a0. [DOI] [PubMed] [Google Scholar]
- Ushijima K, Yahata H, Yoshikawa H, Konishi I, Yasugi T, Saito T, Nakanishi T, Sasaki H, Saji F, Iwasaka T, et al. Multicenter phase II study of fertility-sparing treatment with medroxyprogesterone acetate for endometrial carcinoma and atypical hyperplasia in young women. J Clin Oncol. 2007;25:2798–2803. doi: 10.1200/JCO.2006.08.8344. [DOI] [PubMed] [Google Scholar]
- Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O'Malley BW, McDonnell DP. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol. 1993;7:1244–1255. doi: 10.1210/mend.7.10.8264658. [DOI] [PubMed] [Google Scholar]
- Venkitaraman AR. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell. 2002;108:171–182. doi: 10.1016/s0092-8674(02)00615-3. [DOI] [PubMed] [Google Scholar]
- Wang S, Counterman LJ, Haslam SZ. Progesterone action in normal mouse mammary gland. Endocrinology. 1990;127:2183–2189. doi: 10.1210/endo-127-5-2183. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hanifi-Moghaddam P, Hanekamp EE, Kloosterboer HJ, Franken P, Veldscholte J, van Doorn HC, Ewing PC, Kim JJ, Grootegoed JA, et al. Progesterone inhibition of Wnt/beta-catenin signaling in normal endometrium and endometrial cancer. Clin Cancer Res. 2009;15:5784–5793. doi: 10.1158/1078-0432.CCR-09-0814. [DOI] [PubMed] [Google Scholar]
- Wang Y, van der Zee M, Fodde R, Blok LJ. Wnt/Beta-catenin and sex hormone signaling in endometrial homeostasis and cancer. Oncotarget. 2010;1:674–684. doi: 10.18632/oncotarget.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward EC, Hoekstra AV, Blok LJ, Hanifi-Moghaddam P, Lurain JR, Singh DK, Buttin BM, Schink JC, Kim JJ. The regulation and function of the forkhead transcription factor, Forkhead box O1, is dependent on the progesterone receptor in endometrial carcinoma. Endocrinology. 2008;149:1942–1950. doi: 10.1210/en.2007-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetendorf M, DeMayo FJ. The progesterone receptor regulates implantation, decidualization, and glandular development via a complex paracrine signaling network. Mol Cell Endocrinol. 2012;357:108–118. doi: 10.1016/j.mce.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widschwendter M, Rosenthal AN, Philpott S, Rizzuto I, Fraser L, Hayward J, Intermaggio MP, Edlund CK, Ramus SJ, Gayther SA, et al. The sex hormone system in carriers of BRCA1/2 mutations: a case-control study. Lancet Oncol. 2013;14:1226–1232. doi: 10.1016/S1470-2045(13)70448-0. [DOI] [PubMed] [Google Scholar]
- Wu Q, Ishikawa T, Sirianni R, Tang H, McDonald JG, Yuhanna IS, Thompson B, Girard L, Mineo C, Brekken RA, et al. 27-Hydroxycholesterol promotes cell-autonomous, ER-positive breast cancer growth. Cell Rep. 2013;5:637–645. doi: 10.1016/j.celrep.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Dowdy SC, Gonzalez Bosquet J, Zhao Y, Eberhardt NL, Podratz KC, Jiang SW. Epigenetic-mediated upregulation of progesterone receptor B gene in endometrial cancer cell lines. Gynecol Oncol. 2005;99:135–141. doi: 10.1016/j.ygyno.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Yamaji D, Na R, Feuermann Y, Pechhold S, Chen W, Robinson GW, Hennighausen L. Development of mammary luminal progenitor cells is controlled by the transcription factor STAT5A. Genes Dev. 2009;23:2382–2387. doi: 10.1101/gad.1840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Thiel KW, Leslie KK. Progesterone: the ultimate endometrial tumor suppressor. Trends Endocrinol Metab. 2011;22:145–152. doi: 10.1016/j.tem.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Xiao X, Jia Y, Liu X, Zhang Y, Wang X, Winters CJ, Devor EJ, Meng X, Thiel KW, et al. Epigenetic modification restores functional PR expression in endometrial cancer cells. Curr Pharm Des. 2014;20:1874–1880. doi: 10.2174/13816128113199990532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XY, Xi MR, Yang KX, Yu H. Prognostic value of estrogen receptor and progesterone receptor status in young Chinese ovarian carcinoma patients. Gynecol Oncol. 2009;113:99–104. doi: 10.1016/j.ygyno.2008.12.018. [DOI] [PubMed] [Google Scholar]
- Yang-Hartwich Y, Gurrea-Soteras M, Sumi N, Joo WD, Holmberg JC, Craveiro V, Alvero AB, Mor G. Ovulation and extra-ovarian origin of ovarian cancer. Sci Rep. 2014;4:6116. doi: 10.1038/srep06116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen HY, Gabet Y, Liu Y, Martin A, Wu NL, Pike MC, Frenkel B, Maxson R, Dubeau L. Alterations in Brca1 expression in mouse ovarian granulosa cells have short-term and long-term consequences on estrogen-responsive organs. Lab Invest. 2012;92:802–811. doi: 10.1038/labinvest.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Lee M, Shin S, Park J. Apoptosis induced by progesterone in human ovarian cancer cell line SNU-840. J Cell Biochem. 2001;82:445–451. doi: 10.1002/jcb.1171. [DOI] [PubMed] [Google Scholar]
- Yudt MR, Berrodin TJ, Jelinsky SA, Hanna LA, Brown EL, Chippari S, Bhat RA, Winneker RC, Zhang Z. Selective and opposing actions of progesterone receptor isoforms in human endometrial stromal cells. Mol Cell Endocrinol. 2006;247:116–126. doi: 10.1016/j.mce.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Zhang PJ, Zhao J, Li HY, Man JH, He K, Zhou T, Pan X, Li AL, Gong WL, Jin BF, et al. CUE domain containing 2 regulates degradation of progesterone receptor by ubiquitin-proteasome. EMBO J. 2007;26:1831–1842. doi: 10.1038/sj.emboj.7601602. [DOI] [PMC free article] [PubMed] [Google Scholar]