Abstract
Heart disease remains a major contributor to morbidity and mortality in women in the United States and worldwide. This review highlights known and emerging risk factors for ischemic heart disease (IHD) in women. Traditional Framingham risk factors such as hypertension, hyperlipidemia, diabetes, smoking, as well as lifestyle habits such as unhealthy diet and sedentary lifestyle are all modifiable. Health care providers should be aware of emerging cardiac risk factors in women such as adverse pregnancy outcomes, systemic autoimmune disorders, obstructive sleep apnea, and radiation-induced heart disease; psychosocial factors such as mental stress, depression, anxiety, low socioeconomic status, and work and marital stress play an important role in IHD in women. Appropriate recognition and management of an array of risk factors is imperative given the growing burden of IHD and need to deliver cost-effective, quality care for women.
Keywords: women, ischemic heart disease, cardiac risk factors
Background
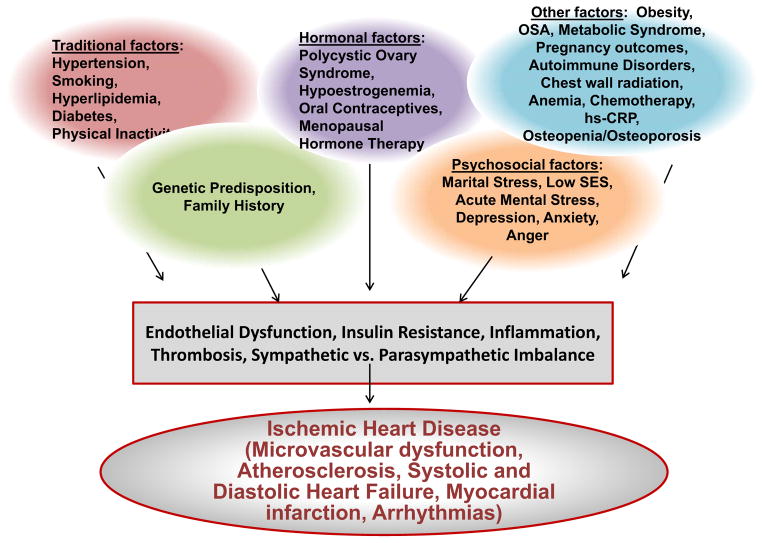
Despite the decline in (IHD) mortality in both women and men, women remain the majority who die from cardiovascular disease (CVD), with an estimated 515,000 women each year diagnosed with coronary heart disease (CHD) 1. Furthermore, data indicate that CVD mortality among young women between the ages of 35–54 is increasing 1. Traditional and non-traditional risk factors contribute to development of IHD in both women and men, but some risk factors are unique to women (e.g. pregnancy-related complications) or selectively disadvantage women (e.g. depression). This review highlights known and emerging risk factors for IHD in women (Figure). Many risk factors are implicated in IHD in women; while some risk factors are causal (i.e. hypertension, smoking, dyslipidemia), many risk factors are associative (i.e. depression) and it is often difficult to determine the exact contribution of non-traditional risk factors in isolation. The impact of non-traditional risk factors on IHD in women may be higher than in men due to the increased prevalence of certain risk factors in women; however, the pathophysiologic relationship between IHD and non-traditional risk factors such as mental stress-induced ischemia, depression, and anxiety in women is not clearly understood but is an active area of investigation 2, 3.
Figure. Ischemic Heart Disease Risk Factors in Women.
Many traditional and novel risk factors contribute to the development of IHD through various mechanistic pathways.
Risk Factors
Framingham Risk Factors and Risk Equivalents
While clinicians have relied on the Framingham Risk Score (FRS) to assess CVD risk, data show that FRS underestimates risk in women and classifies 90% of women as low risk 4. The 2013 ACC/AHA guidelines on the assessment of CVD risk recommend estimating a 10-year or lifetime atherosclerotic CVD risk derived from pooled cohort equations that take into account diverse ethnic populations and stroke risk in women 5.
Hypertension
Hypertension underdiagnosed and undertreated remains a major risk factor for IHD in women 1. The prevalence of hypertension in women and men continues to increase, and it is estimated that lifetime risk of developing hypertension is at least 90% 6. Hypertension significantly increases the risk of myocardial infarction, heart failure, atrial fibrillation, stroke, and renal failure. Identification and treatment of hypertension is imperative and despite the many anti-hypertensive medications available, only one of two hypertensive Americans are adequately treated 7. Pre-menopausal women are at a higher risk of hypertensive end-organ damage than age-matched men, including microalbuminuria and left ventricular hypertrophy 8. Blood pressure (BP) increases during the menopausal transition may be related to the decline in estrogen levels, which leads to upregulation of the renin-angiotensin system, production of vasoconstrictive factors such as endothelin, and increased salt sensitivity 9. Over age 65, hypertension prevalence is higher in women than men, but less than half receive adequate treatment 10, 11. The prevalence of hypertension is 25.3% among Hispanic women, and only 37.5% have controlled BP 12. Untreated white-coat hypertension is also associated with increased CVD risk and ambulatory BP monitoring has demonstrated that more women have “white coat” hypertension than men 13, 14. The 2014 hypertension guidelines are controversial and may selectively disadvantage women 60 years and older by raising the SBP threshold for initiation of treatment, given that recent NHANES data document that 62% of hypertensive Americans are women and over 40% of these are African American. Older women and African Americans are known to be at high risk for stroke, CKD, and heart failure complications due to hypertension 15.
Hyperlipidemia
Elevated serum lipid levels are the greatest contributor to development of IHD worldwide, and clinical trials have shown that low density lipoprotein cholesterol (LDL-C) reduction with statins leads to improved CVD outcomes 16, 17. The 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol advocates comparable initiation of statins in women and men with LDL-C > 190 mg/dL or an estimated 10-year ASCVD risk of ≥ 7.5% 17. Statin therapy has similar proportional benefits for women and men in CVD event reduction 18. Among the elderly, women are 20% less likely than men to use statins 19. This may reflect a potential higher vulnerability of elderly women to statin-induced myalgia or underprescription to this population, although data are lacking 20. High non-HDL-C and triglyceride levels are more important CVD risk factors in women than men, especially women with diabetes 21, 22.
Smoking
Tobacco use increases IHD risk, including progression of atherosclerosis, myocardial infarction (MI), and sudden cardiac death. Tobacco use is increasing among teenagers and young adults; and in particular, women use smoking as a weight management strategy. Smoking is a stronger risk factor for MI in middle-aged women compared to men, with a 6 fold increased risk vs. 3 fold increased risk in men 23. Smoking as few as 2–3 cigarettes daily increases risk of CVD; with cessation risk decreases substantially within 3 years 24. Tobacco is the most common preventable cause of heart disease; smoking status should be addressed at each visit and avoidance of environmental tobacco exposure recommended 25, 26.
Diabetes
A diabetic woman is at 4–6 times increased risk of developing IHD compared to 2–3 times increased risk in a diabetic man 27–29. The greater impact of diabetes on women may be partially due to a greater increase in adiposity and insulin resistance in diabetic women 30. Multiple studies indicate that diabetes is a stronger prognostic predictor of mortality in women than in men 31. Early diagnosis of diabetes is essential, particularly in ethnic groups at high risk for diabetes such as African Americans, Hispanics, American Indians, and Pacific Islander Americans. While glucose-lowering therapy reduces microvascular complications, data are inconclusive whether treatment of hyperglycemia reduces macrovascular complications 32.
Peripheral Arterial Disease (PAD)
PAD is a risk equivalent for IHD, and both share risk factors such as hypertension, diabetes and smoking. Ankle-brachial-index to screen for PAD is an inexpensive test for risk stratification in women and men, but unfortunately is not reimbursed for use for cardiac risk stratification by most payers. In women and men with stable IHD and PAD, depression was independently associated with PAD in women, and smoking and elevated fibrinogen levels with PAD in men 33. Women with PAD who undergo vascular intervention are more likely to be older, have more severe and complex disease, and are at risk of adverse outcomes with higher rates of transfusion and embolism, although they have similar procedural success as men 34. In the Jackson Heart Study of women and men (mean age of 55), lower ankle-brachial index was associated with coronary artery calcification 35.
Chronic Kidney Disease (CKD)
CKD is an IHD risk equivalent in both women and men 36. Risk factors such as hypertension, hyperlipidemia, and diabetes are higher in those with a reduced glomerular filtration rate, and CKD is associated with coronary artery calcification independent of traditional risk factors 37. In the Women’s Ischemia Syndrome Evaluation (WISE) Study, women with serum creatinine of 1.2 to 1.9 mg/dL were more likely to have significant angiographic stenosis (≥ 50% stenosis in ≥1 coronary artery) compared to women with a normal serum creatinine (61% vs. 37%; p<0.001) 38. Mortality from end stage renal disease is high, and approximately 50% of CKD deaths are related to CVD. In a large community based population, as estimated GFR decreased, rates of hospitalization, CVD events, and death increased 39.
Non-Framingham Risk Factors
Overweight and Obesity
While elevated BMI is associated with increased fatal and nonfatal IHD in both women and men, sex differences in fat distribution have been implicated in IHD. Females predominantly accumulate subcutaneous fat, whereas men accumulate significantly more visceral fat. Postmenopausal women have increased visceral fat accrual, which has implications for development of insulin resistance, inflammatory responses, and lipolysis 40. Excess visceral fat and pericardial fat are prominent risk factors for CVD morbidities independent of traditional measures of obesity 41. Several CVD risk factors are significantly associated with overweight and obesity. In particular, hypertension has a strong association with being overweight (OR=2.1) and obese (OR=5.2) in women, and diabetes has a strong association with abdominal obesity (OR=3.9) in women 42. Weight loss improves cholesterol profiles, lowers blood pressure, and reduces the risk of developing diabetes 43.
Current ACC/AHA/The Obesity Society (TOS) guidelines recommend that health care providers should measure waist circumference and calculate BMI at least annually, and overweight and obese individuals and women with waist circumference of >35 inches (88 cm) should be counseled regarding their elevated CVD risk 44. The guidelines recommend for weight loss in women, prescribing a 1,200–1,500 kcal/day diet with a 500–750 kcal/day energy deficit, and referral for nutrition counseling. Providers should strongly recommend a long-term weight loss maintenance program that involves regular contact with a weight loss/nutrition professional. Bariatric surgery can be considered in women with a BMI ≥ 40 or in those with BMI ≥ 35 with comorbid condition related to obesity 44.
Family History
A family history of IHD is present in the majority of patients with premature IHD, but family history is not incorporated in the recent ACC/AHA Atherosclerotic CVD Risk Estimator 5, 45. Family history of MI is an independent prognostic indicator of increased IHD risk in both women and men 46. Gender differences in the impact of family history on IHD risk have been controversial. In a multivariate analysis of traditional Framingham risk factors, family history was predictive of major adverse CVD events in women but there was no significant increased risk in men 47. A larger study showed gender differences as well. Examination of the Physician’s Health Study and Women’s Health Study revealed that a history of maternal MI up to 79 years was associated with increased CVD risk for both sons and daughters, while paternal MI conferred increased CVD risk for sons, but only premature paternal MI (<50 years) conferred increased risk for daughters 48.
Metabolic Syndrome
The metabolic syndrome is a clustering of risk factors that increases risk of IHD 49. Recent research has focused on the individual risk factors of the metabolic syndrome, and the risk of diabetes increases as the number of components of the syndrome increase50,51. Dysregulation of metabolic factors such as tumor necrosis factor-3, leptin, and adiponectin contributes to the development of CVD, and serum adiponectin levels predict CVD risk and mortality in men and women 52, 53. Adiponectin may be a novel therapeutic target for metabolic syndrome and CVD 53.
Polycystic Ovary Syndrome (PCOS)
Cardiac risk factors such as diabetes, obesity, hypertension, and metabolic syndrome are prevalent in women with PCOS, and education of such women regarding weight management and exercise can improve their cumulative cardiac risk. Although no long-term outcome studies evaluate increased CVD risk in PCOS, women with PCOS have elevated coronary artery calcium compared to healthy matched controls with normal ovulation 54.
Adverse Pregnancy Outcomes
Pregnancy is a metabolic stress test, and serves to predict future CV health. The 2011 guidelines for CVD prevention in women incorporated adverse pregnancy outcomes (APOs) as cardiac risk factors 1. These include gestational diabetes, pre-eclampsia, eclampsia, and pre-term delivery, all associated with increased future heart disease risk 55, 56. A diagnosis of pre-eclampsia doubles the risk of future diabetes and stroke57, and quadruples the risk of hypertension later in life. If woman has a history of preeclampsia, she should be screened within 6 months to 1 year post-partum for hypertension and then screened annually 58, 59. Women with gestational diabetes should be screened within 6 weeks post-partum for diabetes; if they have impaired fasting glucose at 6 weeks post-partum, they should be screened annually 60–62. When assessing a woman’s cardiac risk, it is important to obtain a thorough pregnancy history. No outcome data reflect the impact of early treatment of women with APOs, but risk factors should be assessed and current ASCVD prevention guidelines followed.
Systemic Autoimmune Disorders
Systemic autoimmune disorders, such as systemic lupus erythematosis (SLE) and rheumatoid arthritis are highly prevalent in women, and have substantial evidence linking them with increased CVD risk 1. IHD is a leading cause of morbidity and mortality in SLE patients, and rheumatoid arthritis is associated with a two-to-threefold higher risk of MI and increased CVD mortality 63. Traditional CVD risk factors do not fully explain the substantially increased rate of IHD in patients with SLE, suggesting that underlying chronic inflammation may contribute to increased IHD risk. An increased risk is attributed to systemic inflammation and rupture of vulnerable plaque 64. To complicate the matters, treatment for autoimmune disorders involves corticosteroids, which are associated with metabolic syndrome and premature atherosclerosis 65. Coronary artery calcium may be more predictive of CVD risk than Framingham risk scores in women with SLE and rheumatoid arthritis 66. Coronary microvascular dysfunction (CMD) has also been suggested to contribute to ischemia and angina in SLE patients without obstructive CAD 67. The CVD risk of SLE and other autoimmune disorders in men is not well described due to the low prevalence of these conditions in men compared to women.
Radiation Therapy
Chest wall or mediastinal radiation for treatment of malignancies such as Hodgkin’s lymphoma and breast cancer is associated with coronary atherosclerosis and IHD, as well as pericardial and valvular disease. The risk of radiation-induced IHD increases in the presence of risk factors, mandating increased risk factor control in such women. Heart disease risk starts within the first 5 years after radiation exposure, and continues to at least 20 years, and rate of events increases by 7.4% per gray of radiation 68. Left-breast radiation doses to the heart are higher than right-sided breast radiation; left breast and chest wall radiation is associated with mid-left anterior descending, distal diagonal, and proximal right coronary artery atherosclerosis 69. In women and men, complications of radiation therapy for head and neck cancer include subclavian/carotid artery atherosclerosis and thrombosis; a review demonstrated a dose response relationship between frequency of carotid stenosis and radiation dose exposure 70. It is critically important to recommend continued screening for secondary and tertiary cancers in these women, while addressing CVD prevention.
Obstructive Sleep Apnea (OSA)
OSA contributes to CVD risk by increased sympathetic drive, oxidative stress, and inflammation 71. Apneic episodes and sleep disordered breathing contribute to systemic and pulmonary hypertension, as well as significant electrical disturbances and a reduction in heart rate variability 71. There are gender differences in presentation of OSA, with women more likely to present with insomnia, have a history of depression and thyroid dysfunction 72. OSA is more frequently reported in men and may be underdiagnosed in women 73. Clinicians should be astute in suspecting and screening for OSA in women as they may present atypical complaints of morning headaches, insomnia, and depression.
Osteopenia/Osteoporosis
Severity of osteoporosis is associated with cardiac events 74. Women have lower vitamin D levels compared to men, and a low vitamin D level is associated with hypertension, diabetes, and IHD. In Framingham Offspring Study participants, those with low vitamin D levels had a 1.62 times increased events compared to those with higher levels 75. However, data from NHANES III showed that a low vitamin D level was not associated with increased mortality 76. No randomized controlled trials have shown any benefit of Vitamin D or calcium supplementation on CVD risk, and at present the use of Vitamin D and calcium is only recommended for bone health and not for primary prevention of CVD 77–79.
Anemia
In the Atherosclerosis Risk in Communities (ARIC) study, 13 % of women were anemic (vs. 4.8% men); during a follow-up of 6.1 years, a low hemoglobin level was an independent risk factor for CVD events 80. In the WISE study, 21% of women were anemic (Hg < 12 g/dL); anemia was not associated with severity of CAD or ejection fraction, but associated with higher risk of adverse outcomes; women with anemia were more likely to have renal dysfunction, diabetes, hypertension, and be non-white 81. While anemia is a risk factor, one of the hypotheses as to why premenopausal women are protected from CAD implicates the monthly iron loss through menstruation as beneficial 82, 83.
C-reactive protein
Elevated levels of C-reactive protein (hs-CRP), a marker of inflammation, are associated with increased risk of cardiovascular events. The Reynolds Risk Score 84 incorporates hs-CRP and family history in an algorithm that improves on the FRS calculation. In the Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) 85, rosuvastatin decreased CVD outcomes at 1.9 years in those with LDL <130 mg/dL and hs-CRP > 2 mg/L. Studies have reported a gender difference in hs-CRP prediction of CVD mortality; elevated hs-CRP levels are associated with higher mortality in men compared to women86,87. In a subgroup analysis of JUPITER, the incidence of venous thromboembolism was also significantly reduced in women with the addition of rosuvastatin.
Lifestyle Factors
Alcohol
Alcohol intake has a J-shaped relationship with IHD risk in generally healthy populations, and healthy women are recommended to have no more than one drink per day on average. Excessive alcohol consumption is associated with hyperlipidemia, hypertension, vasoconstriction, hypercoagulability, and a lower ventricular fibrillation threshold 88. Moderate alcohol consumption is associated with reduced IHD risk, even in higher risk women with type 2 diabetes mellitus 89. The cardioprotective benefit of moderate alcohol intake may be due to increased HDL-C, increased endothelial vasorelaxation, decrease in platelet aggregation/function, and increased fibrinolysis 88.
Physical Activity and Cardiac Rehabilitation
Physical inactivity is higher among women than men, especially in non-Hispanic black and Hispanic adults 11. In one study of women the population risk of heart disease attributable to physical inactivity outweighed that of other traditional CVD risk factors 90. By enhancing coronary endothelial function, improving ventricular systolic and diastolic function, and decreasing CVD risk factors, exercise is important for the management of angina and prevention of MI 91.
Cardiac rehabilitation is a cost-effective modality in the treatment of IHD 92–94; for improving CVD risk factors, functional capacity, and heart rate recovery 95, 96, and for rebuilding patient confidence and overall well-being. Participation in cardiac rehabilitation provides a mortality benefit above usual care alone 97. It is a Class I indication per ACC/AHA guidelines for stable IHD, MI, post percutaneous coronary intervention and coronary artery bypass grafting, heart failure, as well as valvular heart disease 98–105. A comprehensive cardiac rehabilitation program includes a personalized exercise prescription, smoking cessation counseling, nutrition and weight management education, medication management strategies, and psychological support. Despite its known benefits, women and the elderly are less likely to be referred to cardiac rehabilitation and it is underutilized, particularly among low socioeconomic groups and in rural areas 106,92, 107, 108.
Nutrition
Nutrition is a modifiable risk factor that influences atherogenesis and inflammation. The Mediterranean diet supplemented with extra-virgin olive oil or nuts reduced the incidence of major CV events by ~30% in people at high CV risk 109. On the other hand, a traditional low-fat dietary approach did not result in CV benefit 110. Trans fatty acid intake has been associated with sudden cardiac death in women with clinical atherosclerosis, as well as overall all-cause mortality111, 112. Adherence to the DASH diet appears to lower blood pressure and risk of heart failure, but does not reduce the risk of sudden cardiac death in postmenopausal women 113. Recent investigation of the relationship between the intestinal microbiome and IHD has led to improved understanding of the CVD risk of red meat consumption 114. Dietary L-carnitine and phosphatidylcholine are converted to trimethylamine-N-oxide (TMAO) by gut microflora, and increased TMAO levels are associated with CVD events. Atherosclerosis is suppressed in mice when TMAO levels are reduced by administration of antibiotics targeted at intestinal microbes 115. These findings introduce the intestinal microbiome as an exciting new potential therapeutic target for IHD.
Psychosocial Variables
Acute Mental Stress
Acute mental stress-related ischemia, infarction, and arrhythmias occur in both women and men as seen during disasters such as earthquakes and wars 116. A mental stress-associated acute syndrome, stress cardiomyopathy or Takotsubo-cardiomyopathy, is highly prevalent in post-menopausal women (80% of cases) 117–119. It appears related to an abnormal response to a catecholamine surge in the setting of an acute emotional (i.e. death of a spouse) or a physical stressor (i.e. acute respiratory illness) 120. Coronary endothelial and non-endothelial microvascular dysfunction (CMD), is a significant contributor to IHD in women, and has been associated with Takotsubo-cardiomyopathy 121. An important sex-based difference in IHD that has emerged in the past decade is that women and men differ in the degree of epicardial obstructive CAD noted on conventional angiography; coronary endothelial/non-endothelial microvascular dysfunction (CMD) has been proposed as playing a greater role in IHD in women with signs and symptoms of myocardial ischemia and no obstructive CAD. One recent study in women with CMD found that dyslipidemia and elevated BMI was related to CMD, and that the traditional risk factors modestly contribute to CMD (Agrawal M et al. Cardiovascular Diagnostics and Therapeutics 2013;3(3):146–52). It has been hypothesized that factors such as depression may play an important role in CMD and mechanistic links are under investigation. Ischemia from mental stress is more common in younger women (age ≤ 50) post-MI compared to age-matched men 122.
Depression
Depression is associated with increased IHD risk and IHD mortality, but is currently not listed as an official risk factor in the guidelines, although recently depression has been proposed to be elevated to status of a CVD risk factor 123. Particularly in young women (age ≤ 55), depressive symptoms were associated with increased risk of death compared to men ≤ 55 years 124. Depression was associated with increased risk of CVD and was an independent predictor of CVD death in the Women’s Health Initiative (WHI) 125. In the Nurses Health Study (NHS), depression was associated with fatal IHD events; use of anti-depressant medication was associated with an increased risk of sudden cardiac death 126. The mechanism by which depression impacts IHD risk is under investigation; while inflammatory biomarkers (CRP and Il-6) are elevated in women with depression, inflammation is felt to explain only a small portion of association between depression and CVD 3. Depression is associated with decreased control of modifiable risk factors and lower medication adherence 127, 128.
No studies have shown that treatment of depression impacts cardiac mortality. In the ENRICHD trial, treatment of depression with cognitive behavioral therapy and selective serotonin reuptake inhibitors did not reduce death or MI 129. Treatment with sertraline did not improve mortality in patients with heart disease in the Sertraline Antidepressant Heart Attack Randomized Trial (SADHART) 130. In a more recent trial on pharmacological and non-pharmacological treatment of depression, treatment did not reduce cardiac morbidity or mortality 131. In the Stockholm Women’s Intervention Trial for Coronary Heart Disease (SWITCHD), a randomized trial, group-based psychosocial intervention had a protective effect and prolonged life for women hospitalized for CHD 132. Given that depression is a CVD risk factor, women should be screened for depression using the Patient Health Questionnaire (PHQ-2 or PHQ-9) during routine CVD preventive visits. Depression should be addressed and treated with a mental health provider, as recommended by the AHA and endorsed by the American Psychiatric Association 133.
Anxiety
Anxious patients post-MI are at increased risk of new cardiac events, cardiac mortality, and all-cause mortality. A meta-analysis reported that post-MI anxiety is associated with a 36% increased risk of adverse outcomes 134. In another meta-analysis, anxiety was an independent risk factor for IHD and cardiac mortality 135.
Anger
Anger is associated with adverse cardiac outcomes in both women and men. The pathogenesis of anger-related cardiac events implicates acute mental stress induced ischemia, coronary vasospasm, endothelial dysfunction in the setting of increased catecholamines, plaque instability, enhanced thrombogenicity, and arrhythmias. In the Atherosclerosis Risk in Communities (ARIC) study, those with normal BP prone to anger were at higher risk of acute MI, revascularization, and cardiac mortality compared to those with a low anger trait, although this association was not present in hypertensive patients136. In a case-crossover analysis in the Stockholm Heart Epidemiology Program (SHEEP) of women and men, anger was associated with an increased risk of MI up to one hour after an outburst of anger 137. A more recent meta-analysis of the association of anger with MI, stroke, ruptured aneurysm, and ventricular arrhythmias, reported increased CV events within 2 hours of anger outburst 138.
Marital Status
In the Stockholm Female Coronary Risk (FemCorRisk) study cohort of community-based women with IHD, marital stress (but not work stress) predicted poor prognosis at a median follow up of 4.8 years. Women with severe marital stress had a 3x increased risk of coronary events despite controlling for standard risk factors and left ventricular dysfunction 139.
Socioeconomic Status (SES)
Lower SES is associated with a slightly higher risk of IHD in women compared to men. Higher burden of modifiable risk factors is found in lower socio-economic status women; and a low SES is inversely related to body mass index 140. In the Women’s Health Study, educated women were less likely to smoke, have hypertension, diabetes, or obesity; a decrease in incident CVD events was observed with increasing levels of education and income 141. Low socioeconomic status and work stress also increased IHD risk in the FemCorRisk study 142.
Hormones and CVD Risk
Oral Contraceptives (OCPs)
OCPs in young (age < 30 years) healthy women with no risk factors do not represent a major risk factor for IHD. Smoking combined with OCPs increases risk 7-fold 143 OCP use is associated with a significant increase in diastolic BP and poor hypertension control in hypertensive women, independent of age and weight 144. The fourth generation OCPs (containing drospirenone) can have a BP lowering effect as they have same mechanism of action as spironolactone; they are also associated with a slightly increased risk of VTE 145. The 2010 Centers for Disease Control guidelines recommend progestin-only OCP (level 1) over combined OCP (level 3) in women with controlled hypertension. The risk of MI is increased among second generation OCP (e.g. levonorgestrel) users aged 18–49, and this risk is similar among OCP users regardless of prothrombotic mutation 146.
Menopause and menopausal hormone therapy (MHT)
The incidence of IHD increases after menopause. Declining estrogen levels are implicated, but an altered unfavorable ratio of sex hormones (testosterone/estrogen) may contribute to the risk 147. IHD increases with age in both women and men, and menopause itself may not be an independent risk factor, as other risk factors such as diabetes, hypertension, and metabolic syndrome increase after menopause. Although observational data suggested that hormone therapy may reduce IHD risk, results from the randomized Women’s Health Initiative (WHI) indicated that estrogen combined with progestin vs. placebo was associated with adverse outcomes in women. Menopausal hormone therapy is no longer recommended for primary or secondary prevention of IHD 148. Prior to WHI, The Heart and Estrogen/Progestin Replacement Study (HERS) did not report benefit of hormone therapy in women with IHD 149. In a younger middle-aged cohort of post-menopausal women 50–59 years, there was decreased coronary artery calcification in the estrogen arm vs. placebo 150; in the Kronos Early Estrogen Prevention (KEEPS) Study, although use of hormone therapy reduced vasomotor symptoms, there was no difference in coronary artery calcification or carotid intima-medial thickness after 4 years of follow up 151, 152. In women with a history of IHD who have significant vasomotor menopausal symptoms, non-hormonal options are available for management of these symptoms which include selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors (SNRI), life style and nutrition adjustments, sleep hygiene, and acupuncture 153. If an SNRI is initiated, blood pressure should be monitored as these agents can cause hypertension. There are no long-term follow up data on the recently approved pharmacologic agent for menopause, bazedoxifene (an estrogen agonist and antagonist), to determine its impact on CVD risk.
Knowledge Gaps
While awareness of heart disease as a major health threat to women has increased among women over the past 15 years, awareness appears to have plateaued based on the AHA survey conducted in 2012. Awareness particularly lags behind in African American and Hispanic women. A better understanding of socioeconomic and cultural factors specific to women that pose as barriers for guideline-based preventive strategies is needed. There also remain extensive knowledge gaps in the pathogenesis of how psychosocial risk factors impact IHD in women, and regarding optimal treatment of those risk factors for risk reduction. Several clinically relevant questions regarding primary prevention also remain: Does pre-treatment with statin prevent radiation-induced accelerated atherosclerosis? Does early treatment of cardiac risk factors in women with adverse pregnancy outcomes lead to a significant impact on IHD mortality? Should preventive strategies such as aspirin be recommended in all patients with SLE and other autoimmune disorders if there are no contraindications?
Conclusion
IHD is a significant contributor to morbidity and mortality in women. Traditional Framingham risk factors such as hypertension, hyperlipidemia, diabetes, tobacco use, as well as lifestyle habits such as unhealthy diet and sedentary lifestyle are all modifiable. Health care providers should be aware of emerging risk factors in women such as metabolic syndrome, pregnancy related disorders, autoimmune disorders, sleep apnea, and chronic kidney disease; psychosocial factors such as depression, anxiety, low socioeconomic status, and work and marital stress play an important role in IHD in women. Education of women both in the medical office setting and in the community is important because timely treatment of risk factors can reduce the burden of IHD in women. Appropriate recognition, discussion, education, and treatment of risk factors is imperative given the expected growing burden of IHD in a healthcare system that focuses on delivering cost-effective, quality care for all patients.
Acknowledgments
This work was supported by the National Heart, Lung and Blood Institute grants K23HL105787 and T32HL116273, the Barbra Streisand Women’s Cardiovascular Research and Education Program, and the Linda Joy Pollin Women’s Heart Health Program, Cedars-Sinai Medical Center, Los Angeles, CA.
Footnotes
Conflicts of Interest:
Dr. Mehta reports grants from Gilead Sciences and General Electric
Dr. Wei reports no conflicts of interest
Dr. Wenger reports Research Grants/Contracts/Trial Steering Commttee/Trial Data Safety and Monitoring Board from Gilead Sciences, Merck, NHLBI, Pfizer; Consultantship from AstraZeneca. Gilead Sciences, Merck, and Pfizer
References
- 1.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: A guideline from the american heart association. Circulation. 2011;123:1243–1262. doi: 10.1161/CIR.0b013e31820faaf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mulle JG, Vaccarino V. Cardiovascular disease, psychosocial factors, and genetics: The case of depression. Progress in cardiovascular diseases. 2013;55:557–562. doi: 10.1016/j.pcad.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaccarino V, Johnson BD, Sheps DS, Reis SE, Kelsey SF, Bittner V, et al. Depression, inflammation, and incident cardiovascular disease in women with suspected coronary ischemia: The national heart, lung, and blood institute-sponsored wise study. Journal of the American College of Cardiology. 2007;50:2044–2050. doi: 10.1016/j.jacc.2007.07.069. [DOI] [PubMed] [Google Scholar]
- 4.Pasternak RC, Abrams J, Greenland P, Smaha LA, Wilson PW, Houston-Miller N. 34th bethesda conference: Task force #1--identification of coronary heart disease risk: Is there a detection gap? Journal of the American College of Cardiology. 2003;41:1863–1874. doi: 10.1016/s0735-1097(03)00358-9. [DOI] [PubMed] [Google Scholar]
- 5.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Sr, Gibbons R, et al. 2013 acc/aha guideline on the assessment of cardiovascular risk: A report of the american college of cardiology/american heart association task force on practice guidelines. Circulation. 2013 doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Messerli FH, Williams B, Ritz E. Essential hypertension. Lancet. 2007;370:591–603. doi: 10.1016/S0140-6736(07)61299-9. [DOI] [PubMed] [Google Scholar]
- 7.Daugherty SL, Masoudi FA, Ellis JL, Ho PM, Schmittdiel JA, Tavel HM, et al. Age-dependent gender differences in hypertension management. Journal of hypertension. 2011;29:1005–1011. doi: 10.1097/HJH.0b013e3283449512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palatini P, Mos L, Santonastaso M, Saladini F, Benetti E, Mormino P, et al. Premenopausal women have increased risk of hypertensive target organ damage compared with men of similar age. Journal of women’s health. 2011;20:1175–1181. doi: 10.1089/jwh.2011.2771. [DOI] [PubMed] [Google Scholar]
- 9.Maas AH, Franke HR. Women’s health in menopause with a focus on hypertension. Neth Heart J. 2009;17:68–72. doi: 10.1007/BF03086220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu Q, Burt VL, Paulose-Ram R, Dillon CF. Gender differences in hypertension treatment, drug utilization patterns, and blood pressure control among us adults with hypertension: Data from the national health and nutrition examination survey 1999–2004. Am J Hypertens. 2008;21:789–798. doi: 10.1038/ajh.2008.185. [DOI] [PubMed] [Google Scholar]
- 11.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics--2013 update: A report from the american heart association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorlie PD, Allison MA, Aviles-Santa ML, Cai J, Daviglus ML, Howard AG, et al. Prevalence of hypertension, awareness, treatment, and control in the hispanic community health study/study of latinos. Am J Hypertens. 2014;27:793–800. doi: 10.1093/ajh/hpu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de la Sierra A, Segura J, Banegas JR, Gorostidi M, de la Cruz JJ, Armario P, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57:898–902. doi: 10.1161/HYPERTENSIONAHA.110.168948. [DOI] [PubMed] [Google Scholar]
- 14.Stergiou GS, Asayama K, Thijs L, Kollias A, Niiranen TJ, Hozawa A, et al. Prognosis of white-coat and masked hypertension: International database of home blood pressure in relation to cardiovascular outcome. Hypertension. 2014;63:675–682. doi: 10.1161/HYPERTENSIONAHA.113.02741. [DOI] [PubMed] [Google Scholar]
- 15.Krakoff LR, Gillespie RL, Ferdinand KC, Fergus IV, Akinboboye O, Williams KA, et al. 2014 hypertension recommendations from the eighth joint national committee panel members raise concerns for elderly black and female populations. Journal of the American College of Cardiology. 2014;64:394–402. doi: 10.1016/j.jacc.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the interheart study): Case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 17.Stone NJ, Robinson J, Lichtenstein AH, Merz CN, Blum CB, Eckel RH, et al. 2013 acc/aha guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the american college of cardiology/american heart association task force on practice guidelines. Circulation. 2013 [Google Scholar]
- 18.Kostis WJ, Cheng JQ, Dobrzynski JM, Cabrera J, Kostis JB. Meta-analysis of statin effects in women versus men. Journal of the American College of Cardiology. 2012;59:572–582. doi: 10.1016/j.jacc.2011.09.067. [DOI] [PubMed] [Google Scholar]
- 19.Bhattacharjee S, Findley PA, Sambamoorthi U. Understanding gender differences in statin use among elderly medicare beneficiaries: An application of decomposition technique. Drugs Aging. 2012;29:971–980. doi: 10.1007/s40266-012-0032-1. [DOI] [PubMed] [Google Scholar]
- 20.Bhardwaj S, Selvarajah S, Schneider EB. Muscular effects of statins in the elderly female: A review. Clin Interv Aging. 2013;8:47–59. doi: 10.2147/CIA.S29686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu W, Resnick HE, Jablonski KA, Jones KL, Jain AK, Howard WJ, et al. Non-hdl cholesterol as a predictor of cardiovascular disease in type 2 diabetes: The strong heart study. Diabetes Care. 2003;26:16–23. doi: 10.2337/diacare.26.1.16. [DOI] [PubMed] [Google Scholar]
- 22.Shai I, Rimm EB, Hankinson SE, Curhan G, Manson JE, Rifai N, et al. Multivariate assessment of lipid parameters as predictors of coronary heart disease among postmenopausal women: Potential implications for clinical guidelines. Circulation. 2004;110:2824–2830. doi: 10.1161/01.CIR.0000146339.57154.9B. [DOI] [PubMed] [Google Scholar]
- 23.Njolstad I, Arnesen E, Lund-Larsen PG. Smoking, serum lipids, blood pressure, and sex differences in myocardial infarction. A 12-year follow-up of the finnmark study. Circulation. 1996;93:450–456. doi: 10.1161/01.cir.93.3.450. [DOI] [PubMed] [Google Scholar]
- 24.Teo KK, Ounpuu S, Hawken S, Pandey MR, Valentin V, Hunt D, et al. Tobacco use and risk of myocardial infarction in 52 countries in the interheart study: A case-control study. Lancet. 2006;368:647–658. doi: 10.1016/S0140-6736(06)69249-0. [DOI] [PubMed] [Google Scholar]
- 25.Huxley RR, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: A systematic review and meta-analysis of prospective cohort studies. Lancet. 2011;378:1297–1305. doi: 10.1016/S0140-6736(11)60781-2. [DOI] [PubMed] [Google Scholar]
- 26.Smith SC, Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, et al. Aha/accf secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: A guideline from the american heart association and american college of cardiology foundation endorsed by the world heart federation and the preventive cardiovascular nurses association. Journal of the American College of Cardiology. 2011;58:2432–2446. doi: 10.1016/j.jacc.2011.10.824. [DOI] [PubMed] [Google Scholar]
- 27.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: Meta-analysis of 37 prospective cohort studies. Bmj. 2006;332:73–78. doi: 10.1136/bmj.38678.389583.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gregg EW, Gu Q, Cheng YJ, Narayan KM, Cowie CC. Mortality trends in men and women with diabetes, 1971 to 2000. Ann Intern Med. 2007;147:149–155. doi: 10.7326/0003-4819-147-3-200708070-00167. [DOI] [PubMed] [Google Scholar]
- 29.Kalyani RR, Lazo M, Ouyang P, Turkbey E, Chevalier K, Brancati F, et al. Sex differences in diabetes and risk of incident coronary artery disease in healthy young and middle-aged adults. Diabetes Care. 2014;37:830–838. doi: 10.2337/dc13-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wannamethee SG, Papacosta O, Lawlor DA, Whincup PH, Lowe GD, Ebrahim S, et al. Do women exhibit greater differences in established and novel risk factors between diabetes and non-diabetes than men? The british regional heart study and british women’s heart health study. Diabetologia. 2012;55:80–87. doi: 10.1007/s00125-011-2284-4. [DOI] [PubMed] [Google Scholar]
- 31.Golden SH, Brown A, Cauley JA, Chin MH, Gary-Webb TL, Kim C, et al. Health disparities in endocrine disorders: Biological, clinical, and nonclinical factors--an endocrine society scientific statement. J Clin Endocrinol Metab. 2012;97:E1579–1639. doi: 10.1210/jc.2012-2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mannucci E, Dicembrini I, Lauria A, Pozzilli P. Is glucose control important for prevention of cardiovascular disease in diabetes? Diabetes Care. 2013;36 (Suppl 2):S259–263. doi: 10.2337/dcS13-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grenon SM, Cohen BE, Smolderen K, Vittinghoff E, Whooley MA, Hiramoto J. Peripheral arterial disease, gender, and depression in the heart and soul study. Journal of vascular surgery. 2014 doi: 10.1016/j.jvs.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jackson EA, Munir K, Schreiber T, Rubin JR, Cuff R, Gallagher KA, et al. Impact of gender on morbidity and mortality rates following lower extremity interventions for peripheral arterial disease: Observations from the blue cross blue shield of michigan cardiovascular consortium. Journal of the American College of Cardiology. 2014 doi: 10.1016/j.jacc.2014.03.036. [DOI] [PubMed] [Google Scholar]
- 35.Tullos BW, Sung JH, Lee JE, Criqui MH, Mitchell ME, Taylor HA. Ankle-brachial index (abi), abdominal aortic calcification (aac), and coronary artery calcification (cac): The jackson heart study. The international journal of cardiovascular imaging. 2013;29:891–897. doi: 10.1007/s10554-012-0145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the american heart association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Hypertension. 2003;42:1050–1065. doi: 10.1161/01.HYP.0000102971.85504.7c. [DOI] [PubMed] [Google Scholar]
- 37.Budoff MJ, Rader DJ, Reilly MP, Mohler ER, 3rd, Lash J, Yang W, et al. Relationship of estimated gfr and coronary artery calcification in the cric (chronic renal insufficiency cohort) study. Am J Kidney Dis. 2011;58:519–526. doi: 10.1053/j.ajkd.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reis SE, Olson MB, Fried L, Reeser V, Mankad S, Pepine CJ, et al. Mild renal insufficiency is associated with angiographic coronary artery disease in women. Circulation. 2002;105:2826–2829. doi: 10.1161/01.cir.0000021597.63026.65. [DOI] [PubMed] [Google Scholar]
- 39.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 40.Fuente-Martin E, Argente-Arizon P, Ros P, Argente J, Chowen JA. Sex differences in adipose tissue: It is not only a question of quantity and distribution. Adipocyte. 2013;2:128–134. doi: 10.4161/adip.24075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahabadi AA, Massaro JM, Rosito GA, Levy D, Murabito JM, Wolf PA, et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: The framingham heart study. Eur Heart J. 2009;30:850–856. doi: 10.1093/eurheartj/ehn573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Felix-Redondo FJ, Grau M, Baena-Diez JM, Degano IR, de Leon AC, Guembe MJ, et al. Prevalence of obesity and associated cardiovascular risk: The darios study. BMC Public Health. 2013;13:542. doi: 10.1186/1471-2458-13-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 aha/acc/tos guideline for the management of overweight and obesity in adults: A report of the american college of cardiology/american heart association task force on practice guidelines and the obesity society. Circulation. 2013 doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 aha/acc/tos guideline for the management of overweight and obesity in adults: A report of the american college of cardiology/american heart association task force on practice guidelines and the obesity society. Journal of the American College of Cardiology. 2014;63:2985–3023. doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 45.O’Donnell CJ. Family history, subclinical atherosclerosis, and coronary heart disease risk: Barriers and opportunities for the use of family history information in risk prediction and prevention. Circulation. 2004;110:2074–2076. doi: 10.1161/01.CIR.0000145539.77021.AC. [DOI] [PubMed] [Google Scholar]
- 46.Paixao AR, Berry JD, Neeland IJ, Ayers CR, Rohatgi A, de Lemos JA, et al. Coronary artery calcification and family history of myocardial infarction in the dallas heart study. JACC Cardiovasc Imaging. 2014 doi: 10.1016/j.jcmg.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 47.Kim C, Chang HJ, Cho I, Sung JM, Choi D, Jeong MH, et al. Impact of family history on the presentation and clinical outcomes of coronary heart disease: Data from the korea acute myocardial infarction registry. The Korean journal of internal medicine. 2013;28:547–556. doi: 10.3904/kjim.2013.28.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sesso HD, Lee IM, Gaziano JM, Rexrode KM, Glynn RJ, Buring JE. Maternal and paternal history of myocardial infarction and risk of cardiovascular disease in men and women. Circulation. 2001;104:393–398. doi: 10.1161/hc2901.093115. [DOI] [PubMed] [Google Scholar]
- 49.Ninomiya JK, L’Italien G, Criqui MH, Whyte JL, Gamst A, Chen RS. Association of the metabolic syndrome with history of myocardial infarction and stroke in the third national health and nutrition examination survey. Circulation. 2004;109:42–46. doi: 10.1161/01.CIR.0000108926.04022.0C. [DOI] [PubMed] [Google Scholar]
- 50.Klein BE, Klein R, Lee KE. Components of the metabolic syndrome and risk of cardiovascular disease and diabetes in beaver dam. Diabetes Care. 2002;25:1790–1794. doi: 10.2337/diacare.25.10.1790. [DOI] [PubMed] [Google Scholar]
- 51.Reilly MP, Rader DJ. The metabolic syndrome: More than the sum of its parts? Circulation. 2003;108:1546–1551. doi: 10.1161/01.CIR.0000088846.10655.E0. [DOI] [PubMed] [Google Scholar]
- 52.Wu ZJ, Cheng YJ, Gu WJ, Aung LH. Adiponectin is associated with increased mortality in patients with already established cardiovascular disease: A systematic review and meta-analysis. Metabolism. 2014 doi: 10.1016/j.metabol.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 53.Lee S, Kwak HB. Role of adiponectin in metabolic and cardiovascular disease. J Exerc Rehabil. 2014;10:54–59. doi: 10.12965/jer.140100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Christian RC, Dumesic DA, Behrenbeck T, Oberg AL, Sheedy PF, 2nd, Fitzpatrick LA. Prevalence and predictors of coronary artery calcification in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:2562–2568. doi: 10.1210/jc.2003-030334. [DOI] [PubMed] [Google Scholar]
- 55.Wenger NK. Recognizing pregnancy-associated cardiovascular risk factors. The American journal of cardiology. 2014;113:406–409. doi: 10.1016/j.amjcard.2013.08.054. [DOI] [PubMed] [Google Scholar]
- 56.Ahmed R, Dunford J, Mehran R, Robson S, Kunadian V. Pre-eclampsia and future cardiovascular risk among women: A review. Journal of the American College of Cardiology. 2014;63:1815–1822. doi: 10.1016/j.jacc.2014.02.529. [DOI] [PubMed] [Google Scholar]
- 57.Carr DB, Newton KM, Utzschneider KM, Tong J, Gerchman F, Kahn SE, et al. Preeclampsia and risk of developing subsequent diabetes. Hypertension in pregnancy : official journal of the International Society for the Study of Hypertension in Pregnancy. 2009;28:435–447. doi: 10.3109/10641950802629675. [DOI] [PubMed] [Google Scholar]
- 58.Bushnell C, McCullough LD, Awad IA, Chireau MV, Fedder WN, Furie KL, et al. Guidelines for the prevention of stroke in women: A statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2014;45:1545–1588. doi: 10.1161/01.str.0000442009.06663.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.American College of O, Gynecologists, Task Force on Hypertension in P. Hypertension in pregnancy. Report of the american college of obstetricians and gynecologists’ task force on hypertension in pregnancy. Obstetrics and gynecology. 2013;122:1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 60.Expert Committee on the D, Classification of Diabetes M. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26 (Suppl 1):S5–20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 61.Committee on Practice B-O. Practice bulletin no. 137: Gestational diabetes mellitus. Obstetrics and gynecology. 2013;122:406–416. doi: 10.1097/01.AOG.0000433006.09219.f1. [DOI] [PubMed] [Google Scholar]
- 62.National institutes of health consensus development conference statement: Diagnosing gestational diabetes mellitus, march 4–6, 2013. Obstetrics and gynecology. 2013;122:358–369. doi: 10.1097/AOG.0b013e31829c3e64. [DOI] [PubMed] [Google Scholar]
- 63.Zhang J, Chen L, Delzell E, Muntner P, Hillegass WB, Safford MM, et al. The association between inflammatory markers, serum lipids and the risk of cardiovascular events in patients with rheumatoid arthritis. Ann Rheum Dis. 2014;73:1301–1308. doi: 10.1136/annrheumdis-2013-204715. [DOI] [PubMed] [Google Scholar]
- 64.Chung CP, Giles JT, Kronmal RA, Post WS, Gelber AC, Petri M, et al. Progression of coronary artery atherosclerosis in rheumatoid arthritis: Comparison with participants from the multi-ethnic study of atherosclerosis. Arthritis Res Ther. 2013;15:R134. doi: 10.1186/ar4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zeller CB, Appenzeller S. Cardiovascular disease in systemic lupus erythematosus: The role of traditional and lupus related risk factors. Current cardiology reviews. 2008;4:116–122. doi: 10.2174/157340308784245775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chung CP, Oeser A, Avalos I, Raggi P, Stein CM. Cardiovascular risk scores and the presence of subclinical coronary artery atherosclerosis in women with systemic lupus erythematosus. Lupus. 2006;15:562–569. doi: 10.1177/0961203306071870. [DOI] [PubMed] [Google Scholar]
- 67.Ishimori ML, Martin R, Berman DS, Goykhman P, Shaw LJ, Shufelt C, et al. Myocardial ischemia in the absence of obstructive coronary artery disease in systemic lupus erythematosus. JACC Cardiovasc Imaging. 2011;4:27–33. doi: 10.1016/j.jcmg.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 68.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Bronnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 69.Nilsson G, Holmberg L, Garmo H, Duvernoy O, Sjogren I, Lagerqvist B, et al. Distribution of coronary artery stenosis after radiation for breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:380–386. doi: 10.1200/JCO.2011.34.5900. [DOI] [PubMed] [Google Scholar]
- 70.Schultz-Hector S, Kallfass E, Sund M. radiation sequelae in the large arteries. A review of clinical and experimental data. Strahlentherapie und Onkologie : Organ der Deutschen Rontgengesellschaft ... [et al] 1995;171:427–436. [PubMed] [Google Scholar]
- 71.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, et al. Sleep apnea and cardiovascular disease: An american heart association/american college of cardiology foundation scientific statement from the american heart association council for high blood pressure research professional education committee, council on clinical cardiology, stroke council, and council on cardiovascular nursing. In collaboration with the national heart, lung, and blood institute national center on sleep disorders research (national institutes of health) Circulation. 2008;118:1080–1111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 72.Shepertycky MR, Banno K, Kryger MH. Differences between men and women in the clinical presentation of patients diagnosed with obstructive sleep apnea syndrome. Sleep. 2005;28:309–314. [PubMed] [Google Scholar]
- 73.Quintana-Gallego E, Carmona-Bernal C, Capote F, Sanchez-Armengol A, Botebol-Benhamou G, Polo-Padillo J, et al. Gender differences in obstructive sleep apnea syndrome: A clinical study of 1166 patients. Respiratory medicine. 2004;98:984–989. doi: 10.1016/j.rmed.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 74.Tanko LB, Christiansen C, Cox DA, Geiger MJ, McNabb MA, Cummings SR. Relationship between osteoporosis and cardiovascular disease in postmenopausal women. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2005;20:1912–1920. doi: 10.1359/JBMR.050711. [DOI] [PubMed] [Google Scholar]
- 75.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin d deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin d levels and the risk of mortality in the general population. Archives of internal medicine. 2008;168:1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fortmann SP, Burda BU, Senger CA, Lin JS, Whitlock EP. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: An updated systematic evidence review for the u.S. Preventive services task force. Ann Intern Med. 2013;159:824–834. doi: 10.7326/0003-4819-159-12-201312170-00729. [DOI] [PubMed] [Google Scholar]
- 78.Gunta SS, Thadhani RI, Mak RH. The effect of vitamin d status on risk factors for cardiovascular disease. Nature reviews. Nephrology. 2013;9:337–347. doi: 10.1038/nrneph.2013.74. [DOI] [PubMed] [Google Scholar]
- 79.Pilz S, Gaksch M, O’Hartaigh B, Tomaschitz A, Marz W. The role of vitamin d deficiency in cardiovascular disease: Where do we stand in 2013? Archives of toxicology. 2013;87:2083–2103. doi: 10.1007/s00204-013-1152-z. [DOI] [PubMed] [Google Scholar]
- 80.Sarnak MJ, Tighiouart H, Manjunath G, MacLeod B, Griffith J, Salem D, et al. Anemia as a risk factor for cardiovascular disease in the atherosclerosis risk in communities (aric) study. Journal of the American College of Cardiology. 2002;40:27–33. doi: 10.1016/s0735-1097(02)01938-1. [DOI] [PubMed] [Google Scholar]
- 81.Arant CB, Wessel TR, Olson MB, Bairey Merz CN, Sopko G, Rogers WJ, et al. Hemoglobin level is an independent predictor for adverse cardiovascular outcomes in women undergoing evaluation for chest pain: Results from the national heart, lung, and blood institute women’s ischemia syndrome evaluation study. Journal of the American College of Cardiology. 2004;43:2009–2014. doi: 10.1016/j.jacc.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 82.Mascitelli L, Goldstein MR, Pezzetta F. Explaining sex difference in coronary heart disease: Is it time to shift from the oestrogen hypothesis to the iron hypothesis? Journal of cardiovascular medicine. 2011;12:64–65. doi: 10.2459/JCM.0b013e3283403942. [DOI] [PubMed] [Google Scholar]
- 83.Sullivan JL. Iron and the sex difference in heart disease risk. Lancet. 1981;1:1293–1294. doi: 10.1016/s0140-6736(81)92463-6. [DOI] [PubMed] [Google Scholar]
- 84.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: The reynolds risk score. JAMA : the journal of the American Medical Association. 2007;297:611–619. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 85.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated c-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 86.Doran B, Zhu W, Muennig P. Gender differences in cardiovascular mortality by c-reactive protein level in the united states: Evidence from the national health and nutrition examination survey iii. Am Heart J. 2013;166:45–51. doi: 10.1016/j.ahj.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 87.Emerging Risk Factors C. Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367:1310–1320. doi: 10.1056/NEJMoa1107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Costanzo S, Di Castelnuovo A, Donati MB, Iacoviello L, de Gaetano G. Cardiovascular and overall mortality risk in relation to alcohol consumption in patients with cardiovascular disease. Circulation. 2010;121:1951–1959. doi: 10.1161/CIRCULATIONAHA.109.865840. [DOI] [PubMed] [Google Scholar]
- 89.Solomon CG, Hu FB, Stampfer MJ, Colditz GA, Speizer FE, Rimm EB, et al. Moderate alcohol consumption and risk of coronary heart disease among women with type 2 diabetes mellitus. Circulation. 2000;102:494–499. doi: 10.1161/01.cir.102.5.494. [DOI] [PubMed] [Google Scholar]
- 90.Brown WJ, Pavey T, Bauman AE. Comparing population attributable risks for heart disease across the adult lifespan in women. Br J Sports Med. 2014 doi: 10.1136/bjsports-2013-093090. [DOI] [PubMed] [Google Scholar]
- 91.Wienbergen H, Hambrecht R. Physical exercise and its effects on coronary artery disease. Curr Opin Pharmacol. 2013;13:218–225. doi: 10.1016/j.coph.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 92.Wenger NK. Current status of cardiac rehabilitation. Journal of the American College of Cardiology. 2008;51:1619–1631. doi: 10.1016/j.jacc.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 93.Ades PA, Grunvald MH. Cardiopulmonary exercise testing before and after conditioning in older coronary patients. Am Heart J. 1990;120:585–589. doi: 10.1016/0002-8703(90)90015-p. [DOI] [PubMed] [Google Scholar]
- 94.Williams MA, Ades PA, Hamm LF, Keteyian SJ, LaFontaine TP, Roitman JL, et al. Clinical evidence for a health benefit from cardiac rehabilitation: An update. Am Heart J. 2006;152:835–841. doi: 10.1016/j.ahj.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 95.Sanderson BK, Shewchuk RM, Bittner V. Cardiac rehabilitation and women: What keeps them away? J Cardiopulm Rehabil Prev. 2010;30:12–21. doi: 10.1097/HCR.0b013e3181c85859. [DOI] [PubMed] [Google Scholar]
- 96.Anjo D, Santos M, Rodrigues P, Brochado B, Sousa MJ, Barreira A, et al. The benefits of cardiac rehabilitation in coronary heart disease: A gender issue? Rev Port Cardiol. 2014;33:79–87. doi: 10.1016/j.repc.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 97.Goel K, Lennon RJ, Tilbury RT, Squires RW, Thomas RJ. Impact of cardiac rehabilitation on mortality and cardiovascular events after percutaneous coronary intervention in the community. Circulation. 2011;123:2344–2352. doi: 10.1161/CIRCULATIONAHA.110.983536. [DOI] [PubMed] [Google Scholar]
- 98.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, et al. 2012 accf/aha/acp/aats/pcna/scai/sts guideline for the diagnosis and management of patients with stable ischemic heart disease: A report of the american college of cardiology foundation/american heart association task force on practice guidelines, and the american college of physicians, american association for thoracic surgery, preventive cardiovascular nurses association, society for cardiovascular angiography and interventions, and society of thoracic surgeons. Circulation. 2012;126:e354–471. doi: 10.1161/CIR.0b013e318277d6a0. [DOI] [PubMed] [Google Scholar]
- 99.Jneid H, Anderson JL, Wright RS, Adams CD, Bridges CR, Casey DE, Jr, et al. 2012 accf/aha focused update of the guideline for the management of patients with unstable angina/non-st-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2012;126:875–910. doi: 10.1161/CIR.0b013e318256f1e0. [DOI] [PubMed] [Google Scholar]
- 100.O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr, Chung MK, de Lemos JA, et al. 2013 accf/aha guideline for the management of st-elevation myocardial infarction: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2013;127:e362–425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 101.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 accf/aha/scai guideline for percutaneous coronary intervention: A report of the american college of cardiology foundation/american heart association task force on practice guidelines and the society for cardiovascular angiography and interventions. Circulation. 2011;124:e574–651. doi: 10.1161/CIR.0b013e31823ba622. [DOI] [PubMed] [Google Scholar]
- 102.Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, et al. 2011 accf/aha guideline for coronary artery bypass graft surgery: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2011;124:e652–735. doi: 10.1161/CIR.0b013e31823c074e. [DOI] [PubMed] [Google Scholar]
- 103.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, 3rd, Guyton RA, et al. 2014 aha/acc guideline for the management of patients with valvular heart disease: A report of the american college of cardiology/american heart association task force on practice guidelines. Circulation. 2014;129:e521–643. doi: 10.1161/CIR.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 104.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, et al. 2013 accf/aha guideline for the management of heart failure: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2013;128:e240–327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 105.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. 2009 focused update incorporated into the acc/aha 2005 guidelines for the diagnosis and management of heart failure in adults: A report of the american college of cardiology foundation/american heart association task force on practice guidelines: Developed in collaboration with the international society for heart and lung transplantation. Circulation. 2009;119:e391–479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 106.Witt BJ, Jacobsen SJ, Weston SA, Killian JM, Meverden RA, Allison TG, et al. Cardiac rehabilitation after myocardial infarction in the community. Journal of the American College of Cardiology. 2004;44:988–996. doi: 10.1016/j.jacc.2004.05.062. [DOI] [PubMed] [Google Scholar]
- 107.Suaya JA, Shepard DS, Normand SL, Ades PA, Prottas J, Stason WB. Use of cardiac rehabilitation by medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation. 2007;116:1653–1662. doi: 10.1161/CIRCULATIONAHA.107.701466. [DOI] [PubMed] [Google Scholar]
- 108.Arena R, Williams M, Forman DE, Cahalin LP, Coke L, Myers J, et al. Increasing referral and participation rates to outpatient cardiac rehabilitation: The valuable role of healthcare professionals in the inpatient and home health settings: A science advisory from the american heart association. Circulation. 2012;125:1321–1329. doi: 10.1161/CIR.0b013e318246b1e5. [DOI] [PubMed] [Google Scholar]
- 109.Estruch R, Ros E, Martinez-Gonzalez MA. Mediterranean diet for primary prevention of cardiovascular disease. The New England journal of medicine. 2013;369:676–677. doi: 10.1056/NEJMc1306659. [DOI] [PubMed] [Google Scholar]
- 110.Howard BV, Van Horn L, Hsia J, Manson JE, Stefanick ML, Wassertheil-Smoller S, et al. Low-fat dietary pattern and risk of cardiovascular disease: The women’s health initiative randomized controlled dietary modification trial. JAMA : the journal of the American Medical Association. 2006;295:655–666. doi: 10.1001/jama.295.6.655. [DOI] [PubMed] [Google Scholar]
- 111.Chiuve SE, Rimm EB, Manson JE, Whang W, Mozaffarian D, Stampfer MJ, et al. Intake of total trans, trans-18:1, and trans-18:2 fatty acids and risk of sudden cardiac death in women. Am Heart J. 2009;158:761–767. doi: 10.1016/j.ahj.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kiage JN, Merrill PD, Robinson CJ, Cao Y, Malik TA, Hundley BC, et al. Intake of trans fat and all-cause mortality in the reasons for geographical and racial differences in stroke (regards) cohort. Am J Clin Nutr. 2013;97:1121–1128. doi: 10.3945/ajcn.112.049064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bertoia ML, Triche EW, Michaud DS, Baylin A, Hogan JW, Neuhouser ML, et al. Mediterranean and dietary approaches to stop hypertension dietary patterns and risk of sudden cardiac death in postmenopausal women. Am J Clin Nutr. 2014;99:344–351. doi: 10.3945/ajcn.112.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nature medicine. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (tako-tsubo or stress cardiomyopathy): A mimic of acute myocardial infarction. Am Heart J. 2008;155:408–417. doi: 10.1016/j.ahj.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 117.Kohan AA, Levy Yeyati E, De Stefano L, Dragonetti L, Pietrani M, Perez de Arenaza D, et al. Usefulness of mri in takotsubo cardiomyopathy: A review of the literature. Cardiovascular diagnosis and therapy. 2014;4:138–146. doi: 10.3978/j.issn.2223-3652.2013.10.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pelliccia F, Greco C, Vitale C, Rosano G, Gaudio C, Kaski JC. Takotsubo syndrome (stress cardiomyopathy): An intriguing clinical condition in search of its identity. Am J Med. 2014 doi: 10.1016/j.amjmed.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 119.Summers MR, Prasad A. Takotsubo cardiomyopathy: Definition and clinical profile. Heart failure clinics. 2013;9:111–122. vii. doi: 10.1016/j.hfc.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 120.Sharkey SW, Windenburg DC, Lesser JR, Maron MS, Hauser RG, Lesser JN, et al. Natural history and expansive clinical profile of stress (tako-tsubo) cardiomyopathy. Journal of the American College of Cardiology. 2010;55:333–341. doi: 10.1016/j.jacc.2009.08.057. [DOI] [PubMed] [Google Scholar]
- 121.Patel SM, Lerman A, Lennon RJ, Prasad A. Impaired coronary microvascular reactivity in women with apical ballooning syndrome (takotsubo/stress cardiomyopathy) European heart journal. Acute cardiovascular care. 2013;2:147–152. doi: 10.1177/2048872613475891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vaccarino V, Shah AJ, Rooks C, Ibeanu I, Nye JA, Pimple P, et al. Sex differences in mental stress- induced myocardial ischemia in young survivors of an acute myocardial infarction. Psychosomatic medicine. 2014;76:171–180. doi: 10.1097/PSY.0000000000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lichtman JH, Froelicher ES, Blumenthal JA, Carney RM, Doering LV, Frasure-Smith N, et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: Systematic review and recommendations: A scientific statement from the american heart association. Circulation. 2014;129:1350–1369. doi: 10.1161/CIR.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 124.Shah AJ, Ghasemzadeh N, Zaragoza-Macias E, Patel R, Eapen DJ, Neeland IJ, et al. Sex and age differences in the association of depression with obstructive coronary artery disease and adverse cardiovascular events. J Am Heart Assoc. 2014;3:e000741. doi: 10.1161/JAHA.113.000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wassertheil-Smoller S, Shumaker S, Ockene J, Talavera GA, Greenland P, Cochrane B, et al. Depression and cardiovascular sequelae in postmenopausal women. The women’s health initiative (whi) Archives of internal medicine. 2004;164:289–298. doi: 10.1001/archinte.164.3.289. [DOI] [PubMed] [Google Scholar]
- 126.Whang W, Kubzansky LD, Kawachi I, Rexrode KM, Kroenke CH, Glynn RJ, et al. Depression and risk of sudden cardiac death and coronary heart disease in women: Results from the nurses’ health study. Journal of the American College of Cardiology. 2009;53:950–958. doi: 10.1016/j.jacc.2008.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: A meta-analysis. Psychosomatic medicine. 2001;63:619–630. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 128.Rutledge T, Linke SE, Johnson BD, Bittner V, Krantz DS, Cornell CE, et al. Relationships between cardiovascular disease risk factors and depressive symptoms as predictors of cardiovascular disease events in women. Journal of women’s health. 2012;21:133–139. doi: 10.1089/jwh.2011.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: The enhancing recovery in coronary heart disease patients (enrichd) randomized trial. JAMA : the journal of the American Medical Association. 2003;289:3106–3116. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 130.Glassman AH, Bigger JT, Jr, Gaffney M. Psychiatric characteristics associated with long-term mortality among 361 patients having an acute coronary syndrome and major depression: Seven-year follow-up of sadhart participants. Archives of general psychiatry. 2009;66:1022–1029. doi: 10.1001/archgenpsychiatry.2009.121. [DOI] [PubMed] [Google Scholar]
- 131.Zuidersma M, Conradi HJ, van Melle JP, Ormel J, de Jonge P. Depression treatment after myocardial infarction and long-term risk of subsequent cardiovascular events and mortality: A randomized controlled trial. Journal of psychosomatic research. 2013;74:25–30. doi: 10.1016/j.jpsychores.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 132.Orth-Gomer K, Schneiderman N, Wang HX, Walldin C, Blom M, Jernberg T. Stress reduction prolongs life in women with coronary disease: The stockholm women’s intervention trial for coronary heart disease (switchd) Circulation. Cardiovascular quality and outcomes. 2009;2:25–32. doi: 10.1161/CIRCOUTCOMES.108.812859. [DOI] [PubMed] [Google Scholar]
- 133.Lichtman JH, Bigger JT, Jr, Blumenthal JA, Frasure-Smith N, Kaufmann PG, Lesperance F, et al. Depression and coronary heart disease: Recommendations for screening, referral, and treatment: A science advisory from the american heart association prevention committee of the council on cardiovascular nursing, council on clinical cardiology, council on epidemiology and prevention, and interdisciplinary council on quality of care and outcomes research: Endorsed by the american psychiatric association. Circulation. 2008;118:1768–1775. doi: 10.1161/CIRCULATIONAHA.108.190769. [DOI] [PubMed] [Google Scholar]
- 134.Roest AM, Martens EJ, Denollet J, de Jonge P. Prognostic association of anxiety post myocardial infarction with mortality and new cardiac events: A meta-analysis. Psychosomatic medicine. 2010;72:563–569. doi: 10.1097/PSY.0b013e3181dbff97. [DOI] [PubMed] [Google Scholar]
- 135.Roest AM, Martens EJ, de Jonge P, Denollet J. Anxiety and risk of incident coronary heart disease: A meta-analysis. Journal of the American College of Cardiology. 2010;56:38–46. doi: 10.1016/j.jacc.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 136.Williams JE, Paton CC, Siegler IC, Eigenbrodt ML, Nieto FJ, Tyroler HA. Anger proneness predicts coronary heart disease risk: Prospective analysis from the atherosclerosis risk in communities (aric) study. Circulation. 2000;101:2034–2039. doi: 10.1161/01.cir.101.17.2034. [DOI] [PubMed] [Google Scholar]
- 137.Moller J, Hallqvist J, Diderichsen F, Theorell T, Reuterwall C, Ahlbom A. Do episodes of anger trigger myocardial infarction? A case-crossover analysis in the stockholm heart epidemiology program (sheep) Psychosomatic medicine. 1999;61:842–849. doi: 10.1097/00006842-199911000-00019. [DOI] [PubMed] [Google Scholar]
- 138.Mostofsky E, Penner EA, Mittleman MA. Outbursts of anger as a trigger of acute cardiovascular events: A systematic review and meta-analysisdagger. Eur Heart J. 2014;35:1404–1410. doi: 10.1093/eurheartj/ehu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Orth-Gomer K, Wamala SP, Horsten M, Schenck-Gustafsson K, Schneiderman N, Mittleman MA. Marital stress worsens prognosis in women with coronary heart disease: The stockholm female coronary risk study. JAMA : the journal of the American Medical Association. 2000;284:3008–3014. doi: 10.1001/jama.284.23.3008. [DOI] [PubMed] [Google Scholar]
- 140.Lee JR, Paultre F, Mosca L. The association between educational level and risk of cardiovascular disease fatality among women with cardiovascular disease. Womens Health Issues. 2005;15:80–88. doi: 10.1016/j.whi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 141.Albert MA, Glynn RJ, Buring J, Ridker PM. Impact of traditional and novel risk factors on the relationship between socioeconomic status and incident cardiovascular events. Circulation. 2006;114:2619–2626. doi: 10.1161/CIRCULATIONAHA.106.660043. [DOI] [PubMed] [Google Scholar]
- 142.Wamala SP, Mittleman MA, Horsten M, Schenck-Gustafsson K, Orth-Gomer K. Job stress and the occupational gradient in coronary heart disease risk in women. The stockholm female coronary risk study. Social science & medicine. 2000;51:481–489. doi: 10.1016/s0277-9536(00)00006-x. [DOI] [PubMed] [Google Scholar]
- 143.WHO. Ischaemic stroke and combined oral contraceptives: Results of an international, multicentre, case-control study. Who collaborative study of cardiovascular disease and steroid hormone contraception. Lancet. 1996;348:498–505. [PubMed] [Google Scholar]
- 144.Lubianca JN, Faccin CS, Fuchs FD. Oral contraceptives: A risk factor for uncontrolled blood pressure among hypertensive women. Contraception. 2003;67:19–24. doi: 10.1016/s0010-7824(02)00429-8. [DOI] [PubMed] [Google Scholar]
- 145.Shufelt CL, Bairey Merz CN. Contraceptive hormone use and cardiovascular disease. Journal of the American College of Cardiology. 2009;53:221–231. doi: 10.1016/j.jacc.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tanis BC, van den Bosch MA, Kemmeren JM, Cats VM, Helmerhorst FM, Algra A, et al. Oral contraceptives and the risk of myocardial infarction. N Engl J Med. 2001;345:1787–1793. doi: 10.1056/NEJMoa003216. [DOI] [PubMed] [Google Scholar]
- 147.Rexrode KM, Manson JE, Lee IM, Ridker PM, Sluss PM, Cook NR, et al. Sex hormone levels and risk of cardiovascular events in postmenopausal women. Circulation. 2003;108:1688–1693. doi: 10.1161/01.CIR.0000091114.36254.F3. [DOI] [PubMed] [Google Scholar]
- 148.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the women’s health initiative randomized controlled trial. JAMA : the journal of the American Medical Association. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 149.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and estrogen/progestin replacement study (hers) research group. JAMA : the journal of the American Medical Association. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 150.Manson JE, Allison MA, Rossouw JE, Carr JJ, Langer RD, Hsia J, et al. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356:2591–2602. doi: 10.1056/NEJMoa071513. [DOI] [PubMed] [Google Scholar]
- 151.Wolff EF, He Y, Black DM, Brinton EA, Budoff MJ, Cedars MI, et al. Self-reported menopausal symptoms, coronary artery calcification, and carotid intima-media thickness in recently menopausal women screened for the kronos early estrogen prevention study (keeps) Fertility and sterility. 2013;99:1385–1391. doi: 10.1016/j.fertnstert.2012.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Brinton EA, NN, PNH, Santoro N, et al. Oral estrogen vs. Transdermal estrogen effects on lipids and lipoproteins in recently menopausal women: The kronos early estrogen prevention study (keeps) Menopause. 2013;19:1365–1404. [Google Scholar]
- 153.Painovich JM, Shufelt CL, Azziz R, Yang Y, Goodarzi MO, Braunstein GD, et al. A pilot randomized, single-blind, placebo-controlled trial of traditional acupuncture for vasomotor symptoms and mechanistic pathways of menopause. Menopause. 2012;19:54–61. doi: 10.1097/gme.0b013e31821f9171. [DOI] [PMC free article] [PubMed] [Google Scholar]