Abstract
A novel, promising potential cancer vaccine strategy was proposed to use a two-injection procedure for solid tumors to prompt the immune system to identify and systemically eliminate the primary and metastatic cancers. The two-injection procedure consists of local photothermal application on a selected tumor intended to liberate whole cell tumor antigens, followed by a local injection of an immunoadjuvant that consists of a semi-synthetic functionalized glucosamine polymer, N-dihydro-galacto-chitosan (GC), which is intended to activate antigen presenting cells and facilitate an increased uptake of tumor antigens. This strategy is thus proposed as an in situ autologous cancer vaccine (inCVAX) that may activate antigen presenting cells and expose them to tumor antigens in situ, with the intention of inducing a systemic tumor specific T-cell response. Here, the development of inCVAX for the treatment of metastatic cancers in the past decades are systematically reviewed. The antitumor immune responses of local photothermal treatment and immunological stimulation with GC are also discussed. This treatment approach is also commonly referred to as laser immunotherapy (LIT).
Keywords: inCVAX, Laser immunotherapy (LIT), N-dihydro-galacto-chitosan GC, metastatic cancers, antitumor immune responses
1. Introduction
Metastases are the major cause of treatment failure and cancer-related deaths. Unfortunately, tumor metastases to multiple sites often occur so early that by the time primary tumors are detected, a large portion of the patients with solid tumors already have either clinically apparent metastases or clinically occult micro-metastases [1–3]. Many approaches such as chemotherapy, radiation therapy, hormonal therapy, and other targeted therapies have been applied to treat metastatic cancers. Although some types of metastatic cancers can be treated effectively with current treatments, many cannot.
The ultimate control of cancer has been suggested to lie in the host immune surveillance and defense system [4–6]. Immunotherapy has been considered a promising treatment approach [7], and various strategies have been proposed, including cytokine therapy [8–9], dendritic cell-based vaccines [10–11], and check-point inhibitors and immune-activating antibodies, many of which have begun to be used in clinical studies, either alone or in various combinations with other therapies [12–14]. However, immunotherapy is only accepted as a treatment option for a few indications, and most cancers still avoid or escape immune control [15–17]. Death from metastatic cancer remains the most likely prognosis.
It is theorized that a metastasis originates from a primary cancer. Moreover, metastatic cancer cells and cells of the original cancer usually have some molecular features in common, such as the expression of certain proteins or the presence of specific chromosome changes [1]. The ideal cancer therapy should not only destroy the primary tumors, but also at the same time trigger the immune system to recognize, track down, and destroy any remaining tumor cells, be they at or near the site of the primary tumor or at distant sites. In view of these desirable properties, some local therapy modalities that are combined with immunotherapy have been developed for metastatic cancers [12, 18–19]. One such strategy is to combine photothermal therapy with an injection of an immunoadjuvant that consists of a semi-synthetic functionalized glucosamine polymer, N-dihydro-galacto-chitosan (GC), which in turn induces an in situ autologous cancer vaccine (inCVAX). This treatment approach is also commonly referred to as laser immunotherapy (LIT) [18, 20–23].
It is well known that one of the major reasons that tumors escape the host immune system is their insufficient expression of molecules necessary for antigen processing and presentation [17]. Laser-induced tumor cell death, on the other hand, can release tumor antigens into the surrounding milieu [24]. Concomitantly, immunoadjuvants for cancer immunotherapy can promote antigen uptake and presentation by antigen-presenting cells (APCs), thus triggering specific antitumor immunity [20,22]. The pre-clinical studies of inCVAX showed that the combination of the selective photothermal interaction and GC, both applied locally, could not only destroy the treated primary tumors but could also eradicate untreated metastases at distant sites [20–21, 25–27]. First-in-human clinical studies on late-stage, metastatic melanoma and breast cancer patients were also conducted, and the preliminary data indicates that inCVAX shows promise [28–29].
It is noticeable that the photothermally induced tumor cell death by inCVAX provided a source of tumor antigens released from the host’s own tumor, which contrasts other current vaccine strategies (peptide or cDNA), most of which concentrate on a single and identified tumor-specific antigen (TSA) [30]. Since TSA vary from tumor to tumor and from patient to patient, inCVAX, in lieu of current vaccine strategies, may have the potential to vaccinate patients against multiple TSA that originate from the individual patient’s own tumors.
2. Components of inCVAX
inCVAX utilizes two major interactions: (1) a selective local tumor cell death and tumor antigens release by direct delivery of laser energy into tumor tissue; (2) the local administration of GC as an immunoadjuvant, enhancing antigen uptake and presentation, eliciting a specific antitumor immunity [21,31]. The two components of inCVAX play a unique role on the induced antitumor immune response.
2.1 Photothermal therapy (PTT)
PTT is a therapeutic strategy for local treatment of cancer that uses heat generated from absorbed light energy to destroy tumor cells, which could be highly specific, much less invasive, and rather effective due to the intensive light directed to the target tumor [32–33]. Laser ablation can generate a thermal gradient inside the target tissue, which may induce different biological responses. It should be noted that at temperatures above 105°C, carbonization and evaporation of tissue are induced, which is generally considered undesirable in laser ablation, as it changes the optical properties of the tissue and reduces the light penetration into the deeper target tissue [34]. To increase its efficacy, the parameters of laser should be controlled according to the interactions with tumor tissue.
To study the thermal effect on tumor tissue, selective photothermal interaction using laser, and in situ light-absorbing dye, was initially developed. Specifically, an 805-nm laser was used in conjunction with indocyanine green (ICG), since biological tissues exhibit a deep penetrability with low absorption of NIR photons in the wavelength of 805 nm, and ICG solution has a high absorption peak around 800 nm, which made it an ideal candidate for selective photothermal therapy [32,35–36].
In the past decade, PTT using various types of NIR-absorbing agents has also been explored to enhance the local thermal effects, including gold nanoparticles, carbon nanotubes and other nanomaterials [37–42]. Moreover, direct application of PPT thorugh interstitial fibers, without the use of NIR-abosrbing agents, has been explored, yeiding positive results. However, although PTT has been demonstrated to be a powerful approach to destroy or interrup tumor cells, the control of metastatic tumors using PPT alone has not been achieved thus far.
2.2 Immunoadjuvant
2.2.1 Clinical application of adjuvants
Adjuvants are immunological agents that modify or augment an immune response, usually to a vaccine, without having any specific antigenic effect on their own [43–44]. Although hundreds of adjuvant candidates have been developed by preclinical and clinical testing, most of them failed to be approved for routine application [45]. Much progress has been made during the past decades. Several novel adjuvants are licensed for human use in different countries, including aluminum salts, squalene-oil-water emulsion (MF-59), monophosphory lipid A (MPL) and virosomes [46–47]. The most common adjuvants for human use today are still aluminum hydroxide and aluminum phosphate. However, the application of aluminum hydroxide has not been widely used because of aluminum-related macrophage myofascitis. Novel adjuvants still need to be developed for clinical practice. An “ideal” adjuvant would elicit a persistent, high affinity immune response to an antigen while being non-toxic, biodegradable, non-immunogenic and chemically defined for reproducible manufacture [43, 48–51].
Immunoadjuvants are often used to modify or augment the effects of cancer vaccine by stimulating the immune system to respond to the vaccine more vigorously, and thus providing increased immunity against cancer [52–53]. Adjuvants exert their effects through different mechanisms [54–55]: (1) elevate the presence of antigens in the blood; (2) help absorb the antigen by antigen presenting cells; (3) activate macrophages, lymphocytes, and other immune cells; (4) support the production of cytokines.
2.2.2 N-dihydro-galacto-chitosan (GC)
Chitin, sometimes referred to as Poly-N-acetylglucosamine (PNAG), is a naturally occuring biopolymer that is an important component in, for example, many biofilms, exoskeletons of crustaceans and insects, and cell walls in fungi. While only soluble in acidic solutions [56–57], it has been found that chitin and its N-deacetylated form (dPNAG), referred to as chitosan if the degree of deacetylation is >50%, have certain immune stimulating properties, and they have thus been proposed as potential adjuvants for clinical practice [58–59]. The immune response induced by PNAG and dPNAG is influenced by the presence of antigens [60], and it is believed that chitosan in particular could enhance both humoral and cell-mediated immune responses when vaccinated with antigens [61–64].
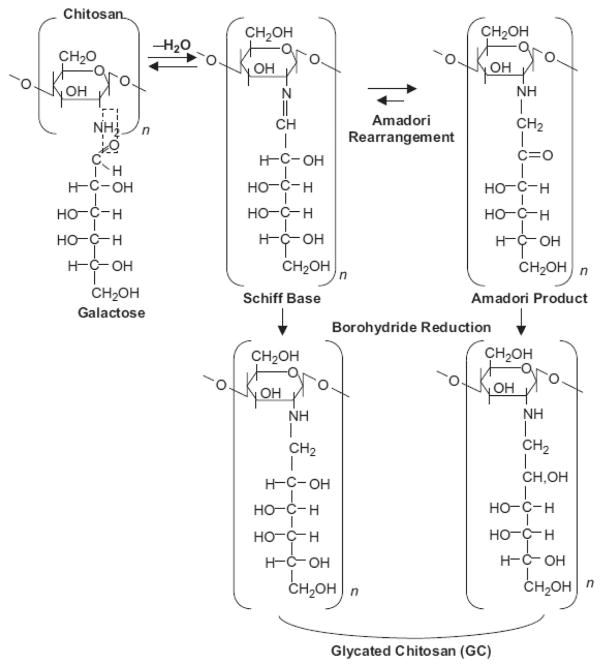
To enhance the immunological functions of chitosan, and to improve its bioavailability and usability in biomedical applications by increasing its water solubility, N-dihydro-galacto-chitosan (GC) was synthesized by attaching galactose molecules to the free amino groups on chitosan [31, 65], as shown in Figure 1. As a functionalized, soluble new chemical entity, GC apparently possesses improved properties for immunological stimulation in non-clinical and clinical studies, indicating its effect as immunoadjuvant in the treatment of metastatic cancers with the inCVAX approach [18, 20–21, 25–27, 31].
Figure 1.
Reaction of primary amino group of N-deacetylated poly-N-acetylglucosamine (dPNAG), or chitosan, with the aldehyde group of galactose yields a Schiff base which is in equilibrium with its Amadori product. Subsequent treatment with sodium borohydride leads to the reduction of the double bonds and the formation of stable functionalized chitosan, or glycated chitosan (GC). Reproduced with permission from [31]. Copyright 2003, Humana Press Inc.
3. Effects of inCVAX
As a novel technique, inCVAX was performed with a consistent demonstration of a long-term response of the immunological defense system against residual primary and metastatic tumor cells. Through local intervention, inCVAX could destroy treated primary tumors while also eradicating untreated metastases at distant sites [18,20,26,31].
3.1 Effects of inCVAX on animal tumor model
The initially proposed inCVAX approach combined three major components: a near-infrared laser (805 nm), a light-absorbing agent (ICG), and an immunoadjuvant (GC). For the inCVAX implementation, ICG was injected intratumorally, followed by topical laser irradiation to enhance light absorption in the tumor tissue [18,27]. The procedure has since then been improved by removing the ICG component, and the optical fiber is now injected intratumorally for direct photothermal application. GC, as an immunological stimulant, is injected into the same location (in the tumor) where the laser irradiation was performed to further enhance immune response after the photothermal treatment [21, 66]. Figure 2 shows the non-invasive laser irradiation of rat tumors.
Figure 2.

Non-invasive laser irradiation of rat tumors after the injection of inCVAX solution.
With a poorly immunogenic rat mammary DMBA-4 tumor model [27], inCVAX, referred to as LIT in early literatures, was performed; the experimental designs and the outcomes are shown in Table 1. In our previous studies, inCVAX treatment resulted in a 38% survival rate until the end of the study at 120 days, with metastases completely destroyed in 40 days. However, in the untreated group, all rats died by 40 days with multiple metastases in the inguinal and axillary areas. Only rats administered the effective photothermal application and GC achieved long-term survival [18,21]. In addition, the lack of tumor resistance observed in rats following freeze-thaw lysate immunization and surgical resection revealed a low level of immunogenicity in the DMBA-4 tumor model [27]. This implies that there may be strongly augmented antitumor immune responses induced in this tumor model.
TABLE I.
Permutations and treatment parameters of different components of inCVAX for treatment of DMBA-4 metastatic breast tumors in female rats.
| Group | Parameters | # of Rats | |
|---|---|---|---|
| Laser | Dye/Adjuvant | ||
|
| |||
| Control | 35* | ||
|
| |||
| ICG Injection Only | 0.25% ICG** | 12 | |
|
| |||
| GC Injection Only | 1% GC** | 12 | |
|
| |||
| Laser Only | 2 watts; 10 min. | 12 | |
|
| |||
| Laser + ICG | 2 watts; 10 min. | 0.25% ICG | 12 |
|
| |||
| Laser + GC | 2 watts; 10 min. | 1% GC | 12 |
|
| |||
| ICG + GC Injection | 0.25% ICG / 1% GC** | 12 | |
|
| |||
| Laser + ICG +GC | 2 watts; 10 min. | 0.25% ICG / 1% GC | 31* |
The data were collected from two separate experiments.
The ICG, GC, or ICG-GC solution (200 μl) was injected directly to the center of the primary tumor.
Reproduced with permission from [21]. Copyright 2002 American Association for Cancer Research.
The function of GC is to enhance the host immune response after direct cancer cell destruction by a selective laser photothermal interaction. To further test its effects, Chen et al. evaluated several different adjuvants for immunological stimulation, in combination with a selective photothermal interaction, including complete Freund (CF) adjuvant, incomplete Freund (IF) adjuvant and corynebacterium parvum (CP). In the treatment of DMBA-4 rat tumors, CF, IF and CP raised the cure rates from 0% to 18%, 7% and 9%, respectively. In comparison, GC resulted in an average of 29% long-term survival [67–68]. The results indicate that GC was more effective than other common adjuvants in inducing antitumor immunity in combination with laser treatment, leading to long-term survival of tumor-bearing rats.
3.2 Long-term antitumor effect of inCVAX
Tumor rechallenge was performed as shown in Figure 3, to confirm the long-term antitumor immunity induced by inCVAX [20]. The protective ability of induced immunity was evaluated in several groups that were challenged repeatedly with increased inoculation doses of viable tumor cells. In tumor rechallenge experiments, fifteen rats that had been successfully treated with inCVAX were rechallenged with 106 viable tumor cells 120 days after initial inoculation (105 viable tumor cells); these rats showed total resistance to the challenge, with neither primary tumors nor metastases observed. Eighteen naive age-matched rats were inoculated with same number of tumor cells for comparative purposes; then developed primary and metastatic tumors and all died within 30 days after inoculation. After the first rechallenge, the rats from several experimental groups were followed by two subsequent challenges in a time interval from 1 to 5 months, with 106 viable tumor cells. The rats successfully treated by inCVAX were totally refractory to all three tumor challenges [20].
Figure 3.
Schematic of anti-tumor immunity induced by inCVAX in tumor rechallenge.
Following the rechallenge study, an adoptive immunity transfer experiment was performed as demonstrated in Figure 4, to further evaluate the induced long-term antitumor immunity in the successfully treated rats [20]. The spleen cells harvested from successfully treated tumor-bearing rats provided 100% immunity in the naive recipients. The passively protected first cohort rats were immune to tumor challenge with an increased tumor dose; their splenocytes also prevented the establishment of tumors in the second cohort of naive recipient rats.
Figure 4.
Schematic of anti-tumor immunity induced by inCVAX demonstrated by adoptive immunity transfer after treatment.
After tumor rechallenge, sera was obtained and analysed for tumor selective antibodies production with histochemical assays [25, 27]. Compared to sera from untreated tumor bearing rats, the sera from cured tumor-bearing rats by inCVAX has contained antibodies that strongly bound to the plasma membrane of both living and preserved tumor cells. In the western blot assay, two distinct bands were observed at approximately 45 and 35 kDa using sera from successfully treated tumor-bearing rats; however, the bands intensity was much weaker using serum from the control tumor bearing rat [25, 27]. These results indicate that inCVAX has produced certain antibodies in rats that bind or intensify the binding of specific tumor proteins.
3.3 Combination of GC with other cancer treatment modalities
As the immunoadjuvant in inCVAX, the application of GC was tested with other local tumor destruction modalities. In the treatment of EMT6 mammary tumor in mice, GC of 0.5% and 1.5% concentrations increased the cure rates of Photofrin-based PDT treatment from 38% to 63% and 75%, respectively [67], in this highly immunogenic tumor model. GC of 0.5% concentrations increased the cure rates of carbon nanotubes-based PTT treatment of EMT6-bearing mice from 43.75% to 100% and all the cured mice showed resistance to tumor rechallenge [22]. In the treatment of line 1 lung adenocarcinoma in mice, a poorly immunogenic tumor model, a 1.67% GC solution enabled a noncurative mesosubstituted tetra (meta-hydroxy-phenyl) chlorin–based PDT (mTHPC-PDT) to cure 37% of the tumor-bearing mice [67]. In the treatment of 4T1 mammary sarcoma in mice, another poorly immunogenic tumor model, 1% GC solution decreased the sizes of tumor metastases when combined with high-intensity focused ultrasound (HIFU) treatment (instead of photothermal application), and the serum from treated mice showed significant cytotoxic effects on 4T1 cells [69]. These results confirmed our previous studies, showing that GC is a critical component in the application of inCVAX for the treatment of metastatic cancer.
4. Mechanism of inCVAX
4.1 Selective photothermal effect induced by inCVAX
4.1.1 Thermal effect on tumor tissue
Effects of tissue temperature elevation have been well established in terms of blood flow increases in tumor and normal tissues (T>40°C) [70], cellular cytotoxicity (T>41.5°C) [71] and vascular destruction within tumor tissue (T>42.5°C) [72]. The rate of “cell kill” doubles for every 1°C increase beyond 43°C [73–74]. In addition, tumor tissue is more sensitive to temperature increase than normal tissue [75].
Raising temperature in tumor tissue can cause cell death or cell stress, leading to the release of tumor antigens and the development of anti-tumor immunity [24,76]. These antigens include tumor-associated antigens, thermally induced heat shock proteins (HSPs), and a large amount of self-antigens. Antigen presenting cells (APCs), particularly dendritic cells (DCs), can capture these antigens, migrate to lymph nodes, and present the antigens to T cells to induce anti-tumor immune responses [77–78]. Specifically, thermal treatment of primary tumors can induce the release of unique tumor antigenic peptides that are bound to HSPs [79–80], which in turn can enhance immune responses by facilitating peptide presentation to CD8+ T cells [81].
4.1.2 Photothermal interaction with tumor tissue
An important aspect of photothermal interaction through laser ablation is that a thermal gradient is generated inside the tissue. Temperature-dependent biological reactions are crucial in thermal therapy for cancer treatment. When the temperature rises from 43 to 100°C, tumor cells undergo coagulative necrosis, but reversible changes in tissue occurs only with temperature below 60°C [82]. In addition, tumor cells swell and break into fragments allowing antigen release, which can induce tumor-specific immune response in the host.
The selective photothermal effect of inCVAX can thus induce a high temperature increase in the target tissue [35–36]. Chen et al. applied magnetic resonance thermometry (MRT) to monitor laser-induced high thermal gradient temperature distribution of target tissue with high spatial resolution. Figure 5 shows temperature gradients in liver tissue acquired by MRT. The temperature mapping results showed that the selective laser photothermal effect could increase tumor temperatures in a range of 10 to 25°C [83]. In a study by Le et al, different power settings (1.0, 1.25, and 1.5 W) were applied for 10 min in a rat tumor, demonstrating an increase in temperature that ranged from 8°C to 15°C based on different laser power and distance from the injected optical fiber [84].
Figure 5.

Two-dimensional temperature distributions in liver tissue immediately after interstitial laser irradiation (1.5 W for 10 min) at different locations from the center of the tumor (left panel) to the outer edge of the tumor (right panel). Reproduced with permission from [84]. Copyright 2011 Society of Photo Optical Instrumentation Engineers.
Different tissue temperatures can induce various immune responses in the host. Within the temperature range of 40 to 60°C, photothermal treatment may induce high level of cell apoptosis in the tumor tissue, which can create a large antigen load for the generation of antitumor immunity [22,85]. In therapeutic terms, the immunogenicity of apoptosis is preferable for immunological stimulation compared to necrosis [86]. The released antigens include tumor associated antigens and a large amount of self-antigens, which can be recognized and captured by APCs. Carrying the antigens, matured APCs migrate to lymph nodes, where they present these antigens to T cells, thus activating cytotoxic T-lymphocytes (CTLs). This process is thus intended to induce a specific cell-mediated antitumor immune response that is effective against tumor cell antigens [22].
As a chaperone, HSPs worked as an endogenous danger signal in the immune surveillance system, extracellular HSP-peptide complexes released from damaged cells can promote the cross-presentation of HSP-bound peptide antigens to major histocompatibility complex (MHC) class I molecules in DCs, leading to efficient induction of antigen-specific CTL [87–89]. Treatment by inCVAX could enhance HSPs expression in the tumor cells, and externalization of the apoptotic cells, which could recognized by APCs through toll like receptor (TLRs), followed by cytokine release via Myd88 and NF-kB signaling pathways [85–86,90]. HSPs on the surface of apoptotic cells could connect the APCs and the tumor cells as well as enhance the antigen acquisition and activation of APCs, which could initiate a specific antitumor immunity. The roles of HSPs in stimulating both innate immunity and adaptive immunity can explain at least in part the molecular mechanism by which thermal stress bolsters the host immune system [91–92].
4.2 Properties of GC
GC works as an immunological stimulant in inCVAX that is inteneded to further enhance the antitumor immune response [18,21]. With the direct immunostimulation properties, GC could stimulate macrophage activation, as measured by TNF-α secretion and NO production [85], and enhance CD80 expression that indicates the DCs maturation [22]. Studies on the direct interaction with tumor cells showed that GC has no cytotoxicity on tumor cells, but could decrease the expression of Twist-1 and Slug, proto-oncogenes commonly implicated in metastasis [69]. When GC alone is used as a treatment agent on tumor bearing mice, the metastases can be decreased, and survival time can be extended; however, the survival rate of animals has not been improved [21–22,69], demonstrating the adjuvant nature of GC.
Although photothermal application could release a large amount of different antigens for the generation of antitumor immunity, the response of the host immune system is limited [22,85]. When combined with GC, TNF-α secretion of macrophage and CD80 expression on DCs could be significantly enhanced [22,85], so could be the antigen acquisition and antigen presentation by APCs [22], thus activating CTLs [22,85]. These results showed that tumor antigen release by laser thermal therapy and the GC enhancement for the generation of antitumor immunity were both crucial.
It is not fully understood how GC functions as an immunoadjuvant, but it is possible that the carbohydrate structure [31] plays an important role. It has been reported that certain receptors on the immune cells can preferentially bind to carbohydrate structure, such as TLRs and C-type lectin family [93]. These TLRs and C-type lectins have been associated with antigen uptake and may be important for the migration of DCs and their interactions with lymphocytes [94].
4.3 Synergistic effect of inCVAX
Thermal interaction may induce a local immune-stimulating effect and released tumor antigen could lead to the development of theraml-based autologous vaccines [95–96]. However, as mentioned above, tumor debris is aparently not sufficient in inducing a potent anti-tumor response [97]. Therefore, additional immunologic intervention is required to invoke the immune system to achieve an effective and protective immune response against residual tumor cells.
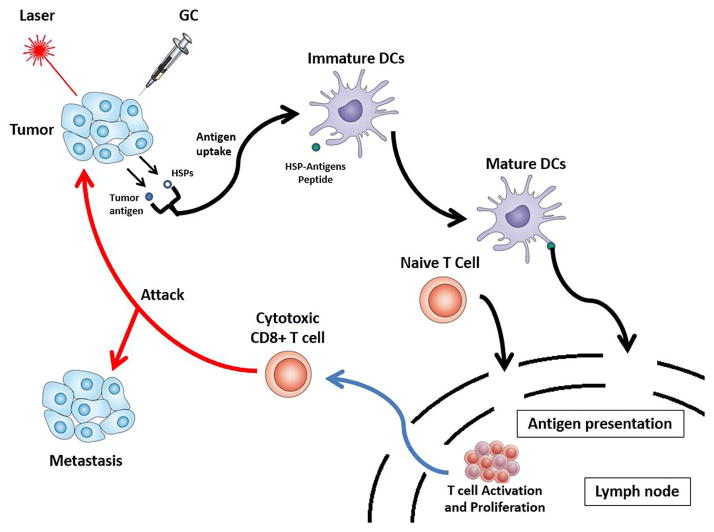
The principal idea behind inCVAX is an induced systemic, tumor-specific immune response through 1) laser irradiation to selectively interrupt tumor cells, releasing tumor antigens and 2) local administration of GC to activate immune cells, targeting specific tumor cells. In fact, its mechanism, using the treated tumor cells as sources of tumor antigens, can be considered as in situ autologous whole-cell cancer vaccination. The schematic of antitumor immune response induced by inCVAX is shown in Figure 6. Inducing systemic anti-tumor immune responses through local, selective photothermal interaction and active immunological stimulation with the unique properties of GC, inCVAX may be a promising approach against metastatic cancers [28–29].
Figure 6.
Schematic of the systemic antitumor immune response induced by inCVAX.
5. Novel features of inCVAX
5.1. Individualized therapy
As an autologous vaccine-like approach using patients’ own whole tumor cells as the source of antigens, the vaccine has access to the whole spectrum of unique and shared antigens expressed by the targeted tumor in each individual patient. Furhtermore, inCVAX requires no ex vivo selection and processing of patient antigens, and, with the unique properties of GC, may overcome other obstacles, including poor immunogenicity or different antigen expressions.
5.2. Minimally invasive
inCVAX is a local, minimally invasive modality that, based on preliminary studies [29], mainly appears to induce local discomfort; the most common adverse effects have been shown to be local pain, mild swelling, and blisters at the treatment sites. While additional clinical trials are necessary, to date, no grade 3 or 4 adverse events were reported in the initial clinical trials [29], which suggests that inCVAX may have the potential to improve the quality of patients’ lives.
The local injection of GC is a simple procedure. The technique of laser irradiation is intended to be straightforward for use by physicians. The equipment for inCVAX is small and can be stored and transported in a briefcase, making it suitable for professional clinical centers as well as small clinics in rural areas and in developing countries.
6. Prospect
The effect of inCVAX has been investigated through cellular and animal studies. Furthermore, inCVAX has been administered in initial clinical trials for the treatment of patients with metastatic breast cancer, many of whom have failed other available modalities, with promising outcomes [28–29]. Specifically, the data indicates that inCVAX may be capable of reducing or eliminating treated primary breast tumors and untreated metastases, as well as prolonging patient survival [29]. We intend to further develop inCVAX so that it can be administered to other solid tumors endoscopically, such as cervical cancer and colon cancers. As a new technology under development, much remains to be understood, but with further studies of the fundamental mechanism of inCVAX, and with continued clinical trials, it is believed that inCVAX may benefit cancer patients in the future, particularly those who have metastatic tumors and have failed conventional therapies.
The concept of inCVAX, using the synergistic effect of acute, local tumor cell destruction and prolonged, systemic immunological stimulation, may potentially be used with many cancer treatment modalities. For example, radiation therapy, a well-established local treatment method, may be an excellent candidate for concomitant use with inCVAX or GC. Radiation, through its direct, precise, and effective interaction, interrupts DNAs in tumor cells, leading to tumor cell death, mainly through apoptotic pathways. These dead or dying cells could be ideal sources of tumor antigens. Therefore, ionizing radiation, in combination with the in situ application of GC, holds promise for the treatment of metastatic tumors. Further investigation on the effects of the combination of radiation and GC is ongoing.
Highlights.
Laser immunotherapy (LIT) is a novel and promising cancer treatment strategy that uses local photothermal application on selected tumor as the source of antigens, and a local injection of an immunoadjuvant, glycated chitosan (GC), which working in tandem with tumor antigens liberated by local laser irradiation, creates an in situ autologous cancer vaccine (inCVAX). Furthermore, inCVAX has been administered in initial clinical trials for the treatment of patients with metastatic breast cancer, many of whom have failed other available modalities, with promising outcomes.
Acknowledgments
This study was supported in part by a grant from the US National Institutes of Health (R21 EB0155091-01), as well as by grants from National Natural Science Foundation of China (no. 81472796; 81000994), Beijing Natural Science Foundation (4153064), and Beijing Nova Program (Z131107000413104).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liotta LA, Stetler-Stevenson WG. Principles of molecular cell biology of cancer: Cancer metastasis. In: DeVita VT, Hellman S, Rosenberg SA, editors. Cancer: Principles & Practice of Oncology. J.B. Lippincott Co; Philadelphia, PA: 1993. pp. 134–149. [Google Scholar]
- 2.Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127(4):679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Kraljevic Pavelic S, Sedic M, Bosnjak H, Spaventi S, Pavelic K. Metastasis: new perspectives on an old problem. Mol Cancer. 2011;10:22. doi: 10.1186/1476-4598-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blattman JN, Greenberg PD. Cancer immunotherapy: a treatment for the masses. Science. 2004;305(5681):200–205. doi: 10.1126/science.1100369. [DOI] [PubMed] [Google Scholar]
- 5.Finn OJ. Cancer immunology. N Engl J Med. 2008;358(25):2704–2715. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411(6835):380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 7.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carpi A, Nicolini A, Antonelli A, Ferrari P, Rossi G. Cytokines in the management of high risk or advanced breast cancer: an update and expectation. Curr Cancer Drug Targets. 2009;9(8):888–903. doi: 10.2174/156800909790192392. [DOI] [PubMed] [Google Scholar]
- 9.Kim-Schulze S, Taback B, Kaufman HL. Cytokine therapy for cancer. Surg Oncol Clin N Am. 2007;16(4):793–818. doi: 10.1016/j.soc.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Eksioglu EA, Eisen S, Reddy V. Dendritic cells as therapeutic agents against cancer. Front Biosci. 2010;15:321–347. doi: 10.2741/3623. [DOI] [PubMed] [Google Scholar]
- 11.Kalinski P, Urban J, Narang R, Berk E, Wieckowski E, Muthuswamy R. Dendritic cell-based therapeutic cancer vaccines: what we have and what we need. Future Oncol. 2009;5(3):379–390. doi: 10.2217/FON.09.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer. 2006;6(7):535–545. doi: 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang HG, Mehta K, Cohen P, Guha C. Hyperthermia on immune regulation: a temperature’s story. Cancer Lett. 2008;271(2):191–204. doi: 10.1016/j.canlet.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 14.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12(4):237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmad M, Rees RC, Ali SA. Escape from immunotherapy: possible mechanisms that influence tumor regression/progression. Cancer Immunol Immunother. 2004;53(10):844–854. doi: 10.1007/s00262-004-0540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5(4):263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 17.Zindl CL, Chaplin DD. Immunology: tumor immune evasion. Science. 2010;328(5979):697–698. doi: 10.1126/science.1190310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen WR, Adams RL, Carubelli R, Nordquist RE. Laser-photosensitizer assisted immunotherapy: A novel modality in cancer treatment. Cancer Lett. 1997;115(1):25–30. doi: 10.1016/s0304-3835(97)04707-1. [DOI] [PubMed] [Google Scholar]
- 19.Mitsunaga M, Ogawa M, Kosaka N, Rosenblum LT, Choyke PL, Kobayashi H. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat Med. 2012;17(12):1685–1691. doi: 10.1038/nm.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen WR, Singhal AK, Liu H, Nordquist RE. Antitumor Immunity Induced by Laser Immunotherapy and Its Adoptive Transfer. Cancer Res. 2001;61(2):459–461. [PubMed] [Google Scholar]
- 21.Chen WR, Liu H, Ritchey JW, Bartels KE, Lucroy MD, Nordquist RE. Effect of different components of laser immunotherapy in treatment of metastatic tumors in rats. Cancer Res. 2002;62(15):4295–4299. [PubMed] [Google Scholar]
- 22.Zhou F, Wu S, Song S, Chen WR, Resasco DE, Xing D. Antitumor immunologically modified carbon nanotubes for photothermal therapy. Biomaterals. 2012;33(11):3235–3242. doi: 10.1016/j.biomaterials.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hode T, Li X, Naylor MF, Hode L, Jenkins P, Ferrel G, Nordquist RE, Adalsteinsson O, Lunn J, Hamblin MR, Alleruzzo L, Chen WR. Laser Immunotherapy. In: Hamblin MR, Sharma SK, editors. Handbook of Photomedicine. Taylor & Francis; USA: 2013. [Google Scholar]
- 24.den Brok MH, Sutmuller RP, van der Voort R, Bennink EJ, Figdor CG, Ruers TJ, Adema GJ. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res. 2004;64(11):4024–4029. doi: 10.1158/0008-5472.CAN-03-3949. [DOI] [PubMed] [Google Scholar]
- 25.Chen WR, Zhu WG, Dynlacht JR, Liu H, Nordquist RE. Long-term tumor resistance induced by laser photo-immunotherapy. Int J Cancer. 1999;81(5):808–812. doi: 10.1002/(sici)1097-0215(19990531)81:5<808::aid-ijc23>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 26.Chen WR, Liu H, Nordquist JA, Nordquist RE. Tumor cell damage and leukocyte infiltration after laser immunotherapy treatment. Lasers Med Sci. 2000;15(1):43–48. doi: 10.1007/s101030050046. [DOI] [PubMed] [Google Scholar]
- 27.Chen WR, Jeong SW, Lucroy MD, Wolf RF, Howard EW, Liu H, Nordquist RE. Induced anti-tumor immunity against DMBA-4 metastatic mammary tumors in rats using a novel approach. Int J Cancer. 2003;107(6):1053–1057. doi: 10.1002/ijc.11501. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Naylor MF, Nordquist RE, Kent TT, Anthony HC, Cynthia M, Chen WR. In situ photoimmunotherapy for late-stage melanoma patients: a preliminary study. Cancer Biol Ther. 2010;10(11):1077–1214. doi: 10.4161/cbt.10.11.13434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Ferrel GL, Guerra MC, Hode T, Lunn JA, Adalsteinsson O, Nordquist RE, Liu H, Chen WR. Preliminary safety and efficacy results of laser immunotherapy for the treatment of metastatic breast cancer patients. Photochem Photobiol Sci. 2011;10(5):817–821. doi: 10.1039/c0pp00306a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Copier J, Dalgleish A. Overview of tumor cell-based vaccines. Int Rev Immunol. 2006;25(5–6):297–319. doi: 10.1080/08830180600992472. [DOI] [PubMed] [Google Scholar]
- 31.Chen WR, Carubelli R, Liu H, Nordquist RE. Laser immunotherapy: a novel treatment modality for metastatic tumors. Mol Biotechnol. 2003;25(1):37–43. doi: 10.1385/MB:25:1:37. [DOI] [PubMed] [Google Scholar]
- 32.Chen WR, Adams RL, Heaton S, Dickey DT, Bartels KE, Nordquist RE. Chromophore-enhanced laser-tumor tissue photothermal interaction using 808 nm diode laser. Cancer Lett. 1995;88(1):15–19. doi: 10.1016/0304-3835(94)03609-m. [DOI] [PubMed] [Google Scholar]
- 33.Liang C, Diao S, Wang C, Gong H, Liu T, Hong G, Shi X, Dai H, Liu Z. Tumor metastasis inhibition by imaging-guided photothermal therapy with single-walled carbon nanotubes. Adv Mater. 2014;26(32):5646–5652. doi: 10.1002/adma.201401825. [DOI] [PubMed] [Google Scholar]
- 34.Iizuka MN, Vitkin IA, Kolios MC, Sherar MD. The effects of dynamic optical properties during interstitial laser photocoagulation. Phys Med Biol. 2000;45(5):1335–1357. doi: 10.1088/0031-9155/45/5/317. [DOI] [PubMed] [Google Scholar]
- 35.Chen WR, Adams RL, Bartels KE, Nordquist RE. Chromophore-enhanced in vivo tumor cell destruction using an 808-nm diode laser. Cancer Lett. 1995;94(2):125–131. doi: 10.1016/0304-3835(95)03837-m. [DOI] [PubMed] [Google Scholar]
- 36.Chen WR, Adams RL, Higgins AK, Bartels KE, Nordquist RE. Photothermal effects on murine mammary tumors using indocyanine green and an 808-nm diode laser: An in vivo efficacy study. Cancer Lett. 1996;98(2):169–173. [PubMed] [Google Scholar]
- 37.Huang XH, El-Sayed IH, Qian W, El-Sayed MA. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc. 2006;128(6):2115–2120. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- 38.Zhou F, Xing D, Ou Z, Wu B, Resasco DE, Chen WR. Cancer photothermal therapy in the near-infrared region by using single-walled carbon nanotubes. J Biomed Opt. 2009;14:021009. doi: 10.1117/1.3078803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang K, Zhang S, Zhang G, Sun X, Lee ST, Liu Z. Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010;10(9):3318–3323. doi: 10.1021/nl100996u. [DOI] [PubMed] [Google Scholar]
- 40.Cheng L, Yang K, Li Y, Chen J, Wang C, Shao M, Lee ST, Liu Z. Facile preparation of multifunctional upconversion nanoprobes for multimodal imaging and dual-targeted photothermal therapy. Angew Chem Int Edit. 2011;50(32):7385–7390. doi: 10.1002/anie.201101447. [DOI] [PubMed] [Google Scholar]
- 41.Zhou F, Wu S, Wu B, Chen WR, Xing D. Mitochondria-targeting single-walled carbon nanotubes for cancer photothermal therapy. Small. 2011;7(19):2727–2735. doi: 10.1002/smll.201100669. [DOI] [PubMed] [Google Scholar]
- 42.Jaque D, Martínez Maestro L, del Rosal B, Haro-Gonzalez P, Benayas A, Plaza JL, Martín Rodríguez E, García Solé J. Nanoparticles for photothermal therapies. Nanoscale. 2014;6:9494–9530. doi: 10.1039/c4nr00708e. [DOI] [PubMed] [Google Scholar]
- 43.Li X, Min M, Du N, Gu Y, Hode T, Naylor M, Chen D, Nordquist RE, Chen WR. Chitin, chitosan, and glycated chitosan regulate immune responses: the novel adjuvants for cancer vaccine. Clin Dev Immunol. 2013;2013:387023. doi: 10.1155/2013/387023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montomoli E, Piccirella S, Khadang B, Mennitto E, Camerini R, De Rosa A. Current adjuvants and new perspectives in vaccine formulation. Expert Rev Vaccines. 2011;10(7):1053–1061. doi: 10.1586/erv.11.48. [DOI] [PubMed] [Google Scholar]
- 45.Vogel FR, Powell MF. A compendium of vaccine adjuvants and excipients. Pharm Biotechnol. 1995;6:141–228. doi: 10.1007/978-1-4615-1823-5_7. [DOI] [PubMed] [Google Scholar]
- 46.Yang M, Yan Y, Fang M, Wan M, Wu X, Zhang X, Zhao T, Wei H, Song D, Wang L, Yu Y. MF59 formulated with CpG ODN as a potent adjuvant of recombinant HSP65-MUC1 for inducing anti-MUC1(+) tumor immunity in mice. Int Immunopharmacol. 2012;13(4):408–416. doi: 10.1016/j.intimp.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cluff CW. Monophosphoryl lipid A (MPL) as an adjuvant for anti-cancer vaccines: clinical results. Adv Exp Med Biol. 2010;667:111–123. doi: 10.1007/978-1-4419-1603-7_10. [DOI] [PubMed] [Google Scholar]
- 48.Gupta RK, Siber GR. Adjuvants for human vaccines-current status, problems and future prospects. Vaccine. 1995;13(14):1263–1276. doi: 10.1016/0264-410x(95)00011-o. [DOI] [PubMed] [Google Scholar]
- 49.Hoft DF, Brusic V, Sakala IG. Optimizing vaccine development. Cell Microbiol. 2011;13(7):934–942. doi: 10.1111/j.1462-5822.2011.01609.x. [DOI] [PubMed] [Google Scholar]
- 50.Cebon J. Cancer vaccines: Where are we going? Asia Pac J Clin Oncol. 2010;S1:S9–15. doi: 10.1111/j.1743-7563.2010.01270.x. [DOI] [PubMed] [Google Scholar]
- 51.Schlom J. Therapeutic cancer vaccines: current status and moving forward. J Natl Cancer Inst. 2012;104(8):599–613. doi: 10.1093/jnci/djs033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leroux-Roels G. Unmet needs in modern vaccinology: adjuvants to improve the immune response. Vaccine. 2010;28(S3):C25–C36. doi: 10.1016/j.vaccine.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 53.Mesa C, Fernández LE. Challenges facing adjuvants for cancer immunotherapy. Immunol Cell Biol. 2004;82:644–650. doi: 10.1111/j.0818-9641.2004.01279.x. [DOI] [PubMed] [Google Scholar]
- 54.Schijns VE. Mechanisms of vaccine adjuvant activity: initiation and regulation of immune responses by vaccine adjuvants. Vaccine. 2003;21(9–10):829–831. doi: 10.1016/s0264-410x(02)00527-3. [DOI] [PubMed] [Google Scholar]
- 55.McKee AS, MacLeod MKL, Kappler JW, Marrack P. Immune mechanisms of protection: can adjuvants rise to the challenge? BMC Biology. 2010;8:37. doi: 10.1186/1741-7007-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sugano M, Watanabe S, Kishi A, Izume M, Ohtakara A. Hypocholesterolemic action of chitosans with different viscosity in rats. Lipids. 1988;23(3):187–191. doi: 10.1007/BF02535456. [DOI] [PubMed] [Google Scholar]
- 57.Chung YC, Yeh JY, Tsai CF. Antibacterial characteristics and activity of water-soluble chitosan derivatives prepared by the Maillard reaction. Molecules. 2011;16(10):8504–8514. doi: 10.3390/molecules16108504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suzuki K, Okawa Y, Hashimoto K, Suzuki S, Suzuki M. Protecting effect of chitin and chitosan on experimentally induced murine candidiasis. Microbiol Immunol. 1984;28(8):903–912. doi: 10.1111/j.1348-0421.1984.tb00746.x. [DOI] [PubMed] [Google Scholar]
- 59.Jarmila V, Vavríková E. Chitosan derivatives with antimicrobial, antitumour and antioxidant activities—a review. Curr Pharm Des. 2011;17(32):3596–3607. doi: 10.2174/138161211798194468. [DOI] [PubMed] [Google Scholar]
- 60.Nishimura K, Nishimura S, Nishi N, Saiki I, Tokura S, Azuma I. Immunological activity of chitin and its derivatives. Vaccine. 1984;2(1):93–99. doi: 10.1016/s0264-410x(98)90039-1. [DOI] [PubMed] [Google Scholar]
- 61.van der Lubben IM, Verhoef JC, Borchard G, Junginger HE. Chitosan for mucosal vaccination. Adv Drug Deliv Rev. 2001;52(2):139–144. doi: 10.1016/s0169-409x(01)00197-1. [DOI] [PubMed] [Google Scholar]
- 62.Read RC, Naylor SC, Potter CW, Bond J, Jabbal-Gill I, Fisher A, Illum L, Jennings R. Effective nasal influenza vaccine delivery using chitosan. Vaccine. 2005;23(35):4367–4374. doi: 10.1016/j.vaccine.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 63.McNeela EA, Jabbal-Gill I, Illum L, Pizza M, Rappuoli R, Podda A, Lewis DJ, Mills KH. Intranasal immunization with genetically detoxified diphtheria toxin induces T cell responses in humans: enhancement of Th2 responses and toxin-neutralizing antibodies by formulation with chitosan. Vaccine. 2004;22(8):909–914. doi: 10.1016/j.vaccine.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 64.Zaharoff DA, Rogers CJ, Hance KW, Schlom J, Greiner JW. Chitosan solution enhances both humoral and cell-mediated immune responses to subcutaneous vaccination. Vaccine. 2007;25(11):2085–2094. doi: 10.1016/j.vaccine.2006.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu F, Liu H, Wu X, Jiang H, Nordquist RE, Chen WR. Measurement of x-ray attenuation coefficients of aqueous solutions of indocyanine green and glycated chitosan. Med Phys. 1999;26(7):1371–1374. doi: 10.1118/1.598633. [DOI] [PubMed] [Google Scholar]
- 66.Chen WR, Liu H, Yue W, Wang J, Nordquist RE. Dynamically observing intratumor injection of laser-absorbing dye and immunoadjuvant using digital x-ray imaging technique. Opt Eng. 2001;40(7):1249–1254. [Google Scholar]
- 67.Chen WR, Korbelik M, Bartels KE, Liu H, Sun J, Nordquist RE. Enhancement of laser cancer treatment by a chitosan-derived immunoadjuvant. Photochem Photobiol. 2005;81(1):190–195. doi: 10.1562/2004-07-20-RA-236. [DOI] [PubMed] [Google Scholar]
- 68.Chen WR, Huang Z, Korbelik M, Nordquist RE, Liu H. Photoimmunotherapy for cancer treatment. J Environ Pathol Toxicol Oncol. 2006;25(1–2):281–291. doi: 10.1615/jenvironpatholtoxicoloncol.v25.i1-2.180. [DOI] [PubMed] [Google Scholar]
- 69.Chen YL, Wang CY, Yang FY, Wang BS, Chen JY, Lin LT, Leu JD, Chiu SJ, Chen FD, Lee YJ, Chen WR. Synergistic effects of glycated chitosan with high-intensity focused ultrasound on suppression of metastases in a syngeneic breast tumor model. Cell Death Dis. 2014;5:e1178. doi: 10.1038/cddis.2014.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reinhold HS, Endrich B. Tumor microcirculation as a target for hyperthermia. Int J Hyperthermia. 1986;2(2):111–137. doi: 10.3109/02656738609012389. [DOI] [PubMed] [Google Scholar]
- 71.Thistlethwaite AJ, Leeper DB, Moylan DJ, Nerlinger RE. pH distribution in human tumors. Int J Radiat Oncol Biol Phys. 1985;11(9):1647–1652. doi: 10.1016/0360-3016(85)90217-2. [DOI] [PubMed] [Google Scholar]
- 72.Dewey WC, Hopwood LE, Sapareto SA, Gerweck LE. Cellular responses to combinations of hyperthermia and radiation. Radiology. 1977;123(2):463–474. doi: 10.1148/123.2.463. [DOI] [PubMed] [Google Scholar]
- 73.Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys. 1984;10(6):787–800. doi: 10.1016/0360-3016(84)90379-1. [DOI] [PubMed] [Google Scholar]
- 74.Field SB, Morris CC. The relationship between heating time and temperature: Its relevance to clinical hyperthermia. Radiother Oncol. 1983;1(2):179–186. doi: 10.1016/s0167-8140(83)80020-6. [DOI] [PubMed] [Google Scholar]
- 75.Anghileri LJ, Robert J. Hyperthermia in Cancer Treatment. CRC Press; Boca Raton, FL: 1986. [Google Scholar]
- 76.Yoon TJ, Kim JY, Kim H, Hong C, Lee H, Lee CK, Lee KH, Hong S, Park SH. Anti-tumor immunostimulatory effect of heat-killed tumor cells. Exp Mol Med. 2008;40(1):130–144. doi: 10.3858/emm.2008.40.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jager E, Jager D, Knuth A. Antigen-specific immunotherapy and cancer vaccines. Int J Cancer. 2003;106(6):817–820. doi: 10.1002/ijc.11292. [DOI] [PubMed] [Google Scholar]
- 78.Mukhopadhaya A, Mendecki J, Dong X, Liu L, Kalnicki S, Garg M, Alfieri A, Guha C. Localized hyperthermia combined with intratumoral dendritic cells induces systemic antitumor immunity. Cancer Res. 2007;67(16):7798–7806. doi: 10.1158/0008-5472.CAN-07-0203. [DOI] [PubMed] [Google Scholar]
- 79.Chen T, Guo J, Han C, Yang M, Cao X. Heat shock protein 70, released from heat-stressed tumor cells, initiates antitumor immunity by inducing tumor cell chemokine production and activating dendritic cells via TLR4 pathway. J Immunol. 2009;182(3):1449–1459. doi: 10.4049/jimmunol.182.3.1449. [DOI] [PubMed] [Google Scholar]
- 80.Rylander MN, Feng Y, Bass J, Diller KR. Heat shock protein expression and injury optimization for laser therapy design. Lasers Surg Med. 2007;39(9):731–746. doi: 10.1002/lsm.20546. [DOI] [PubMed] [Google Scholar]
- 81.Prohaszka Z. Chaperones as part of immune networks. Adv Exp Med Biol. 2007;594:159–166. doi: 10.1007/978-0-387-39975-1_14. [DOI] [PubMed] [Google Scholar]
- 82.Hamblin MR, Huang YY. Handbook of Photomedicine. CRC press; 2013. [Google Scholar]
- 83.Chen Y, Gnyawali SC, Wu F, Liu H, Tesiram YA, Abbott A, Towner RA, Chen WR. Magnetic resonance imaging guidance for laser photothermal therapy. J Biomed Opt. 2008;13(4):044033. doi: 10.1117/1.2960020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Le K, Li X, Figueroa D, Towner RA, Garteiser P, Saunders D, Smith N, Liu H, Hode T, Nordquist RE, Chen WR. Assessment of thermal effects of interstitial laser phototherapy on mammary tumors using proton resonance frequency method. J Biomed Opt. 2011;16(12):128001. doi: 10.1117/1.3659200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou F, Song S, Chen WR, Xing D. Immunostimulatory properties of glycated chitosan. J Xray Sci Technol. 2011;19(2):285–292. doi: 10.3233/XST-2011-0293. [DOI] [PubMed] [Google Scholar]
- 86.Zhou F, Xing D, Chen WR. Regulation of HSP70 on activating macrophages using PDT-induced apoptotic cells. Int J Cancer. 2009;125(6):1380–1389. doi: 10.1002/ijc.24520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gong J, Zhang Y, Durfee J, Weng D, Liu C, Koido S, Song B, Apostolopoulos V, Calderwood SK. A heat shock protein 70-based vaccine with enhanced immunogenicity for clinical use. J Immunol. 2010;184(1):488–496. doi: 10.4049/jimmunol.0902255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Segal BH, Wang XY, Dennis CG, Youn R, Repasky EA, Manjili MH, Subjeck JR. Heat shock proteins as vaccine adjuvants in infections and cancer. Drug Discov Today. 2006;11(11–12):534–540. doi: 10.1016/j.drudis.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 89.Nishikawa M, Takemoto S, Takakura Y. Heat shock protein derivatives for delivery of antigens to antigen presenting cells. Int J Pharm. 2008;354(1–2):23–27. doi: 10.1016/j.ijpharm.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 90.Song S, Zhou F, Chen WR, Xing D. PDT-induced HSP70 externalization up-regulates NO production via TLR2 signal pathway in macrophages. FEBS Lett. 2013;587(2):128–135. doi: 10.1016/j.febslet.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 91.Torigoe T, Tamura Y, Sato N. Heat shock proteins and immunity: application of hyperthermia for immunomodulation. Int J Hyperthermia. 2009;25(8):610–616. doi: 10.3109/02656730903315831. [DOI] [PubMed] [Google Scholar]
- 92.Yang WL, Nair DG, Makizumi R, Gallos G, Ye X, Sharma RR, Ravikumar TS. Heat shock protein 70 is induced in mouse human colon tumor xenografts after sublethal radiofrequency ablation. Ann Surg Oncol. 2004;11(4):399–406. doi: 10.1245/ASO.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 93.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182(2):389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Figdor CG, van Kooyk Y, Adema GJ. C-type lectin receptors on dendritic cells and langerhan cells. Nat Rev Immunol. 2002;2(2):77–84. doi: 10.1038/nri723. [DOI] [PubMed] [Google Scholar]
- 95.Möller PH, Ivarsson K, Stenram U, Radnell M, Tranberg K-G. Comparison between interstitial laser thermotherapy and excision of an adenocarcinoma transplanted into rat liver. Br J Cancer. 1998;77(11):1884–1892. doi: 10.1038/bjc.1998.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sanchez-Ortiz RF, Tannir N, Ahrar K, Wood CG. Spontaneous regression of pulmonary metastases from renal cell carcinoma after radio frequency ablation of primary tumor: an in situ tumor vaccine? J Urol. 2003;170(1):178–179. doi: 10.1097/01.ju.0000070823.38336.7b. [DOI] [PubMed] [Google Scholar]
- 97.Jager E, Jager D, Knuth A. Antigen-specific immunotherapy and cancer vaccines. Int J Cancer. 2003;106(6):820–817. doi: 10.1002/ijc.11292. [DOI] [PubMed] [Google Scholar]