Abstract
Among alcohols, methanol intoxication is the most frequently associated with cerebral toxicity, causing retinal damage and putaminal necrosis. This consequence is believed to be due to the transformation of methanol into formic acid. We describe the case of a patient who presented with acute impairment of consciousness and tetraparesis after she had been drinking several bottles of a topical antiseptic solution (Lysoform Medical) containing 2-bromo-2-nitro-1,3-propandiol (bronopol) among excipients, in order to lose weight during previous months. Moreover, she had been on a strict slimming diet. Soon after admission, a severe respiratory and metabolic impairment became rapidly evident, requiring an intensive care unit admission. Cerebral MRI showed the presence of bilateral putaminal necrosis. She recovered in 10 days, surprisingly, without any evident clinical neurological signs. Methanol, also bronopol, when diluted in aqueous solution, at warm temperature and/or higher pH, may release formaldehyde, which is converted into formic acid, a basal ganglia toxic compound.
Background
The 2-bromo-2-nitro-1,3-propandiol, or bronopol, is an alcohol produced by the bromination of di(hydroxymethyl)nitromethane. It is used as a microbiocide in air washer systems, air conditioning/humidifying systems, cooling water systems, topic antiseptic solutions, papermills, absorbent clays, metal working fluids, printing ink and paint.1 It is stable when the pH of the system is borderline between acid and neutral. Conversely, bronopol decomposes in alkaline solutions and very low levels of formaldehyde are produced. Elevated temperatures speed up the decomposition process.1 2 Liberated formaldehyde is not responsible for the biological activity associated with bronopol.3 However, formaldehyde is metabolised in the liver into formic acid, a compound that has toxic effects on neurones.3 To the best of our knowledge, no cases of bronopol intoxication due to chronic ingestion have been reported in the literature, while an allergic contact dermatitis related to the topic use of cosmetics or antiseptic solutions containing bronopol is reported.4 We describe a case of chronic oral bronopol ingestion resulting in severe neuropathological damage with putaminal bilateral necrosis in a patient who had been on a strict diet for 4 months for aesthetic reasons.
Case presentation
A middle-aged patient presented to our neurology department because of a persistent impairment of consciousness for 3 h. She was found on the grounds of her house with difficulty in speaking. Her relatives reported she had fever and nausea, and had been vomiting for 48 h.
On clinical examination, she had a Glasgow Coma Scale score of 7/15. Her pupils were in reactive mydriasis. She had a tetraparesis with weak deep tendon reflexes but no evidence of sensitivity impairment or extrapyramidal signs. Significant cardiovascular system impairment with blood pressure at 80/50 mm Hg, tachycardia and cold limb extremities were found. Blood oxygen saturation was equal to 83%, with periodic breath and inspiratory accessory muscle recruitment.
Investigations
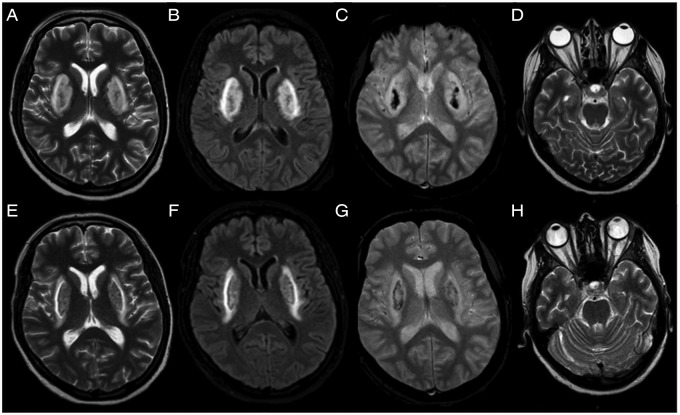
The laboratory tests showed a metabolic acidosis (pH 7.03; pCO2 11 mm Hg; pO2 122 mm Hg; Na++ 131 mmol/L; K+ 6.8 mmol/L; HCO3− 2.9 mmol/L; BE −25 mmol/L; glucose 163 mg/dL, lactate 0.8 mmol/L) with elevated anion gap (37.8); increased liver, pancreatic and muscular enzymes (γ-glutamyl transpeptidase (GT) 341 U/L (normal value (nv) 7–38), alanine transaminase 285 U/L (nv 2–40), aspartate transaminase 61 U/L (nv 2–40), lipase 109 U/L (nv 7–60); α-amylase 696 U/L (nv 30–130) and troponin T 379 ng/L (nv 0–50); lactate dehydrogenase 944 U/L (nv 150–500); creatine kinase isoenzyme 13.3 ng/mL (nv 0.6–6.3)). A moderate impairment of renal function was found (creatinine 2.38 mg/dL (nv 0.20–1.10), azotaemia 49 mg/dL (nv 10–50), urea 1.9 mg/dL (nv 2.30–6.10)). Urine examination was normal except for the presence of ketones (3+). Toxicological screening for cannabinoids, opiates, barbiturates, phencyclidine, ethanol, cocaine, amphetamines and benzodiazepine was negative. Laboratory tests for research of infectious diseases were negative. ECG and echocardiogram excluded any cardiac abnormalities. A cerebral CT scan showed a bilateral putaminal hypodensity. Based on the suggestive latter finding, serum methanol and ethylene glycol dosages were performed without their detection. A cerebral MRI, performed the day after the admission, confirmed the presence of a bilateral putaminal necrosis (figure 1A–D). Neuroradiological features have been already described in detail by Grasso et al.5
Figure 1.
(A–D) Brain MRI performed on the second day after admission. Axial T2-weighted images showing a symmetrical bilateral hyperintensity of the putamen, extending also to external capsule and corona radiata (A and B). The gradient echo T2-weighted image showing the presence of haemosiderin inside the lesions as haemorrhagic necrosis (C). Optic nerves have a normal appearance (D). (E–H) Brain MRI performed on the 20th day after admission. T2-weighted images showing a slight reduction in size of bilateral hyperintensity of the putamen (E and F). The amount of haemosiderin inside the lesions is reduced (G). No abnormalities of the optical nerves are detected (H).
Differential diagnosis
Wilson's disease and carbon monoxide intoxication were excluded with specific haematic laboratory tests.
Treatment
During the 6 h following admission, the patient's respiration was increasingly impaired and she was placed on mechanical ventilation in intensive care unit (ICU). She was treated with bicarbonates followed by rehydrant therapy with resolution of the ketoacidosis within 24 h.
Outcome and follow-up
The patient had progressive improvement of the consciousness state and muscle weakness and, after 4 days of ICU treatment, she returned to our department. That is when she revealed that during the previous 4 months, she had been on a strict slimming diet associated with the consumption of a 250 mL bottle per week (about 35 mL/day diluted in 30 mL of warm water) of a topical antiseptic solution (Lysoform Medical). She declared she had ingested it in order to lose weight for aesthetic reasons. The mentioned compound contains bronopol (0.02 g/100 g) among the excipients (non-ionic surfactant, perfume, colourants, sequestered, deionised water as needed to make 100 g).
Neuroimaging follow-up after 20 days confirmed the irreversible bilateral putaminal necrosis (figure 1E–G) and the normal appearance of the optic nerves (figure 1H). At hospital discharge after 20 days, the neurological and ophthalmological examinations were normal. Liver, pancreatic and muscular enzymes were reduced to normal range except for γ-GT at 160 U/L (nv 7–38).
Discussion
Alcohol-related intoxication can produce metabolic acidosis with high anion gap and hyperosmolality.6 7 Among alcohols, methanol is the most frequently associated with cerebral toxicity, causing retinal damage and putaminal necrosis.7 8 The latter rare condition is believed to be due to an accumulation of formic acid and/or to a blood flow reduction in the putamen.8
Our patient chronically ingested an antiseptic solution containing bronopol diluted in warm water. When diluted in aqueous solution, at warm temperature and/or at high pH, bronopol may release formaldehyde, which is converted into formic acid in the liver8 9 (figure 2). Formic acid is then metabolised into CO2 and H2O, a process that depends on liver tetrahydrofolate concentration. This pathway is easily saturable, contributing to the accumulation of formic acid in the blood.8 9 Therefore, bronopol could also act as a toxic substance if the particular condition of a transformation into formic acid occurs. In the present case, considering the concentration of 0.02/100 g in a 250 mg bottle, we estimated the patient had ingested a total amount of 400 mg of bronopol during the 4 months of consumption. Bronopol blood dosage was not performed because it had never been associated with this clinical and neuroradiological picture before, and because when we became aware of the patient's bronopol ingestion it was probably too late to detect it in her blood. However, with the benefit of hindsight, considering that the patient ingested bronopol, methanol and ethylene glycol blood levels were negative, as expected. Actually, the toxic dose of bronopol and its effects are not known as the present case is the first to be reported. Although, the toxic effects of formic acid, especially on putamina, are well known.8 In this patient, formic acid was produced from bronopol because some particular conditions (high temperature and pH) occurred.2 3
Figure 2.
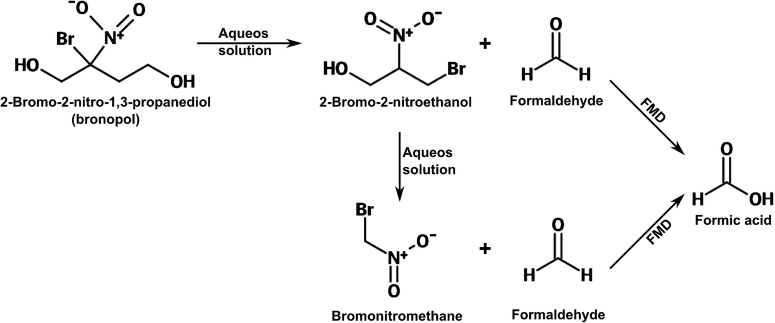
In aqueous solutions, bronopol rapidly degrades to various transformation products, consisting of 2-bromo-2-nitroethanol, bromonitromethane and formaldehyde. Formaldehyde is then rapidly metabolised into formic acid, largely in the liver, by the catalytic action of formaldehyde dehydrogenase; this conversion has an estimated half life of 1.5 min. Modified from Cui et al.2
Moreover, our patient was on a strict slimming diet for a long period, a process known to induce ketoacidosis.10 11 Starvation-induced ketoacidosis is generally mild and not life-threatening, however, putaminal MRI signal abnormalities due to lactate accumulation, oedema and neuronal death were described as complications of a therapeutic ketogenic diet.11 However, MRI putaminal abnormalities were reported to be transient and reversible after starvation-induced ketoacidosis remission.11
In the described case, it is possible to hypothesise a convergence of two factors, starvation-induced ketoacidosis and bronopol chronic ingestion, in order to determine putaminal lesions. Available data on basal ganglia damage due to starvation-induced ketoacidosis report a reversibility of the damage.11 Conversely, the presence of a stable and permanent necrotic lesion of putamina, as in our patient, seems to be ascribed to the accumulation of formic acid derived from bronopol. To the best of our knowledge, no cases of bronopol intoxication due to chronic ingestion are reported in the literature.4 Bronopol withdrawal, bicarbonate infusion and a rehydrant therapy allowed our patient to have a progressive clinical recovery despite the putaminal bilateral necrosis remaining stable, as reported in most of the cases of alcohol intoxication.7 12 13 Nevertheless, reasons of remission/persistence of clinical neurological findings are not clearly known.
Learning points.
Bilateral putaminal necrosis is frequently associated with methanol,7–9 ethylene glycol7 and cyanide14 intoxications, and more rarely with metabolic acidosis11 and infectious diseases.15 However, it can also be associated with other substances, such as bronopol, which share the same metabolic pathway.
Bronopol ingestion may cause cerebral basal ganglia damage because of formic acid production.
Starvation-induced ketoacidosis is a possible cause of reversible basal ganglia damage. In the present case, it could have enhanced the toxic effect of bronopol.
Acknowledgments
The authors thank Dr Anna Lepore of the Poison Control Centre of Riuniti Hospital of Foggia and Dr Cosma Andreula of the neuroradiology Unit of Anthea Hospital of Bari for their suggestions. Dr Gianpaolo Grilli for performing the cerebral MRI, Dr Donato Melchionda for choosing the images and commenting on them, and Dr Maria Grazia Pascarella and Giuseppe d'Orsi for their contribution in revising the article. They also thank Professor Silvia Gliatta for the English editing.
Footnotes
Contributors: All the authors actively participated in article drafting. MT, EC and TM followed the case and collaborated in writing the article. MT and TM prepared figures. LMS revised the article, and supervised and coordinated the team work.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed
References
- 1.Legin GY. 2-Bromo-2-nitro-1,3-propanediol(bronopol) and its derivatives: synthesis, properties, and application. Pharm Chem J 1996;30:273–84. 10.1007/BF02218777 [DOI] [Google Scholar]
- 2.Cui N, Zhang X, Xie Q et al. Toxicity profile of labile preservative bronopol in water: the role of more persistent and toxic transformation products. Environ Pollut 2011;159:609–15. 10.1016/j.envpol.2010.09.036 [DOI] [PubMed] [Google Scholar]
- 3.Kajimura K, Tagami T, Yamamoto T et al. The release of formaldehyde upon decomposition of 2-bromo-2-nitropropan-1, 3-diol (bronopol). J of Health Sci 2008;54:488–92. 10.1248/jhs.54.488 [DOI] [Google Scholar]
- 4.Peters MS, Connolly SM, Schroeter AL. Bronopol allergic contact dermatitis. Contact Dermatitis 1983;9:397–401. 10.1111/j.1600-0536.1983.tb04436.x [DOI] [PubMed] [Google Scholar]
- 5.Grasso D, Borreggine C, Perfetto F et al. Lentiform fork sign: a magnetic resonance finding in a case of acute metabolic acidosis. Neuroradiol J 2014;27:288–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Höjer J. Severe metabolic acidosis in the alcoholic: differential diagnosis and management. Hum Exp Toxicol 1996;15:482–8. 10.1177/096032719601500604 [DOI] [PubMed] [Google Scholar]
- 7.Kraut JA, Kurtz I. Toxic alcohol ingestions: clinical features, diagnosis, and management. Clin J Am Soc Nephrol 2008;3:208–25. 10.2215/CJN.03220807 [DOI] [PubMed] [Google Scholar]
- 8.Barceloux DG, Bond GR, Krenzelok EP et al. American academy of clinical toxicology ad hoc committee on the treatment guidelines for methanol poisoning. American Academy of Clinical Toxicology practice guide-lines on the treatment of methanol poisoning. J Toxicol Clin Toxicol 2002;40:415–46. 10.1081/CLT-120006745 [DOI] [PubMed] [Google Scholar]
- 9.Kerns W, Tomaszewski C, McMartin K et al. ; META Study Group. Methylpyrazole for toxic alcohols. Formate kinetics in methanol poisoning. J Toxicol Clin Toxicol 2002;40:137–43. 10.1081/CLT-120004401 [DOI] [PubMed] [Google Scholar]
- 10.Cartwright MM, Hajja W, Al-Khatib S et al. Toxigenic and metabolic. Causes of ketosis and ketoacidotic syndromes. Crit Care Clin 2012;28:601–31. 10.1016/j.ccc.2012.07.001 [DOI] [PubMed] [Google Scholar]
- 11.Erickson JC, Jabbari B, Difazio MP. Basal ganglia injury as a complication of the ketogenic diet. Mov Disord 2003;18:448–51. 10.1002/mds.10372 [DOI] [PubMed] [Google Scholar]
- 12.Hantson P, Duprez T, Mahieu P. Neurotoxicity to the basal ganglia shown by magnetic resonance imaging (MRI) following poisoning by methanol and other substances. J Toxicol Clin Toxicol 1997;35:151–61. 10.3109/15563659709001186 [DOI] [PubMed] [Google Scholar]
- 13.Naraqi S, Dethlefs RF, Slobodniuk RA et al. An outbreak of acute methyl alcohol intoxication. Aust N Z J Med 1979;9:65–8. 10.1111/j.1445-5994.1979.tb04116.x [DOI] [PubMed] [Google Scholar]
- 14.Grandas F, Artieda J, Obeso JA. Clinical and CT scan findings in a case of cyanide intoxication. Mov Disord 1989;4:188–93. 10.1002/mds.870040211 [DOI] [PubMed] [Google Scholar]
- 15.Cambonie G, Houdon L, Rivier F et al. Infantile bilateral striatal necrosis following measles. Brain Dev 2000;22:221–3. 10.1016/S0387-7604(00)00105-4 [DOI] [PubMed] [Google Scholar]