Abstract
This case series reviews two cases where elderly patients were found to have pneumatosis intestinalis on imaging. The two clinical presentations differed from one another, however, both were managed conservatively to good effect. In case one the patient presented with abdominal pain, a change in bowel habit and weight loss. In case two the patient presented with problematic diarrhoea, reduced oral intake, lethargy and weight loss. Both patients were haemodynamically stable and neither had an abnormal abdominal examination. Case 2 was started on oral metronidazole and by day 11 of treatment there was resolution of the pneumatosis on her abdominal X-ray and her diarrhoea had settled. These two cases illustrate the benefit of conservative management and avoidance of unnecessary surgical intervention in primary pneumatosis intestinalis. However, it is important to distinguish between these benign causes of pneumatosis intestinalis and those which are life-threatening in which surgery may be necessary.
Background
Pneumatosis is rare, affecting only 0.03% of the population.1 However, it is a radiological finding seen increasingly with more frequent and improved imaging. The severity of pneumatosis encompasses a wide spectrum from benign forms to life-threatening causes.2 Primary pneumatosis accounts for 15% of cases and is benign.2 The primary form usually presents with no associated symptoms and is an incidental finding.1 The secondary form accounts for 85% of cases, it is associated with chronic obstructive pulmonary disease (COPD) as well as obstructive and necrotic gastrointestinal disease.2 Even more uncommon is pneumatosis presenting as a pneumoperitoneum, occurring in less than 3% of cases.3 As of yet there are no clear management guidelines for pneumatosis intestinalis including when to consider surgery. It has been suggested up to 27% of those presenting with benign pneumatosis intestinalis may undergo avoidable surgery.3 4 However, there is a role for surgery. Life-threatening causes including bowel ischaemia, risk perforation of the bowel and the patient may die with conservative management.
Case 1 is of interest due to the marked difference between the radiological findings and clinical presentation. This case is a clear example of the importance of data interpretation in the clinical context. In case 2 the patient appeared to benefit from metronidazole therapy, the mechanism of which is not fully understood. It is unclear whether the clinical and radiological improvement was due to treatment with metronidazole or if the patient would have improved in time anyway.
Case presentation
Case 1
An 86-year-old woman presented with an acute onset of abdominal pain located in the suprapubic and right iliac fossa regions, radiating round her side to her back. The pain was sharp in nature, intermittent and severe. Associated with the pain was some chronic weight loss of approximately one stone over 12 months. The patient had noticed a change in her bowel movements but described this as an improvement, normally suffering from constipation, for the last week she had been opening her bowels twice daily.
The medical history, to note, included a cholecystectomy in 1992 and there was a family history of asthma and bowel cancer. Her drug history included thryoxine for hypothyroidism as well as morphine sulfate and biannual cortisone injections for osteoarthritis.
The patient lived with her daughter who helped with her care and was independent in her activities of daily living, mobilising with a zimmer frame. She did not have a smoking history and did not drink alcohol.
On examination she was haemodynamically stable and abdominal examination was normal.
The patient underwent routine blood tests. She was screened for infection which included a urine dipstick. She had a chest X-ray, an abdominal X-ray and a CT scan.
Case 2
An 86-year-old woman presented with diarrhoea, reduced oral intake, lethargy and reduced mobility. She did not report of any pain. She had notable weight loss of 2.5 stone over 1 month. She had presented 1 week earlier with similar symptoms, at this time she was started on loperamide and discharged following a normal CT scan. During her admission it became apparent her diarrhoea was overflow secondary to constipation. She was severely dehydrated from the diarrhoea and poor oral intake was an ongoing problem.
The medical history included Alzheimer's disease diagnosed in 2011 with a marked decline in the past 6 months. She also suffered from hypothyroidism. Her drug history included thyroxine, loperamide, ranitidine and nutritional supplements.
The patient lived with her daughter and son-in-law. Prior to this admission she was independent in her activities of daily living. She was an overseas patient from Brazil.
On examination she was haemodynamically stable. Some hard stool could be felt in the region of the transverse colon, the rest of her abdomen was soft and non-tender.
The patient's blood tests were monitored throughout her admission for electrolyte imbalance and dehydration secondary to diarrhoea. Infection was excluded by cultures of blood, urine and stool. She also had a chest X-ray to look for respiratory pathology. Immunosupression was investigated for with an immunology screen. An abdominal X-ray was performed to assess the extent of faecal impaction and following the results of this a CT scan was repeated (figures 1 and 2).
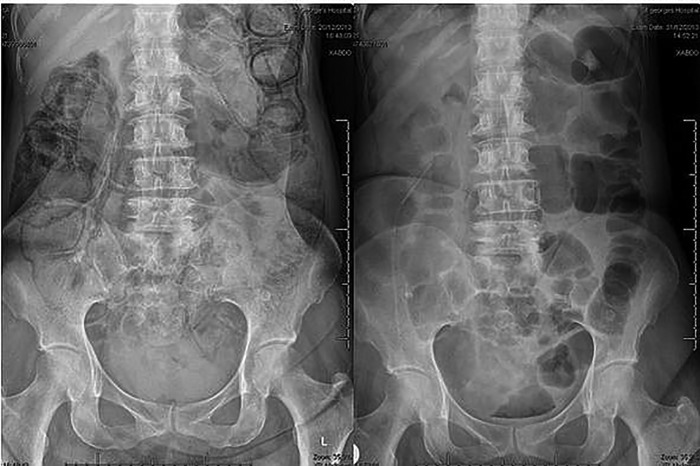
Figure 1.
Abdominal X-rays of case two. The one on the left showing pneumatosis and the one on the right showing its resolution.
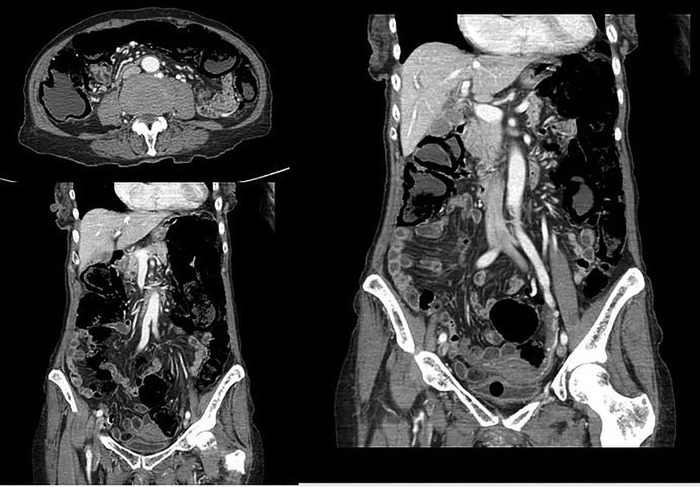
Figure 2.
CT scans of case two illustrating pneumatosis.
Investigations
Case 1
There were no abnormalities in her blood test results and her urine dipstick was negative. A chest X-ray showed free gas under diaphragm. Abdominal and pelvic CT scan showed dramatic small bowel pneumatosis and a large volume of intraperitoneal free gas concentrated in anterior abdomen, unusually in the absence of symptoms. There was gas tracking into adjacent small bowel mesentery. There was a volvulus of the small bowel mesentery which could have precipitated the small bowel changes and subsequent perforation if the patient was symptomatic at an earlier point. Small bowel loops were dilated, particularly in the left abdomen. These loops gradually tapered to collapse in the right abdomen, the terminal ileum had collapsed. There was no definitive transition point to suggested mechanical obstruction and no thickening of small bowel loops. The small bowel had an old appearance with multiple fluid filled proximal and mid small bowel loops. There was a trace of fluid in pelvis. There was no portal venous gas. The major mesenteric vessels were patent. There were scattered sigmoid diverticulosis, with no adjacent inflammation or colonic masses. The common bile duct was enlarged (13 mm) but this remained unchanged from a previous scan. The pancreas was atrophic. There were no destructive bone lesions.
Case 2
On admission the patient had marked electrolyte disturbance, raised inflammatory markers and hypercalcaemia. At the time of discharge her inflammatory markers had improved, she had an iron deficiency anaemia and her electrolytes had almost normalised with slight hypernatraemia and slight hypokalaemia. Her immunology screen was negative. There was no culture growth. Abdominal ultrasound did not reveal any masses. Abdominal X-ray showed faecal material throughout the length of the bowel and linear pneumatosis (figure 1). A repeat CT scan was recommended at this point. The CT scan showed pneumatosis affecting the whole length of the colon and terminal ileum (figure 2).
Differential diagnosis
Case 1
The large amount of pneumoperitoneum identified on imaging had no obvious cause. The presence of pneumatosis in the small bowel was likely to point to a small bowel cause, however, this was not confirmed.
Case 2
Differential diagnoses included ischaemic bowel, malignancy and infective diarrhoea.
Treatment
Both patients were managed conservatively. Case 1 was admitted for observation and given intravenous fluids. Case 2 was given a 1-month course of metronidazole (400 mg three times daily) as advised by gastroenterology. Less than half way through her treatment she began to pass more formed stool and the pneumatosis had resolved on her abdominal X-ray (figure 1). Case 2 also underwent a strict fluid balance and was consequently catheterised and fluid resuscitated to good effect. She was reviewed by the speech and language team to ensure there were no swallowing difficulties preventing her oral intake. She was also followed up by a dietician to maximise her calorie intake. Physiotherapists reviewed her mobility.
Outcome and follow-up
Case 1
The pain settled rapidly and she was discharged without any surgery. She was reviewed in clinic in May 2013 and reported to have been very well since discharge. She was pain free, opening her bowels regularly, her appetite had improved and she had gained weight. It was recommended she should have a small bowel meal to evaluate the small bowel. Owing to her age and clinical improvement she declined at this stage.
Case 2
As she was an overseas patient no formal follow-up was arranged, she remained well following discharge and has not had any further hospital admissions.
Discussion
There are four well-documented theories for the pathogenesis of pneumatosis. These are bowel necrosis, mucosal disruption, increased mucosal permeability and pulmonary disease.3 5 Koss6 preformed a clinical review in 1952 which considered 255 case reports and attributes the finding of pneumatosis to numerous underlying pathologies. Similarly in 1998 Pear performed a review and found at least 58 causative factors.5 However, these were condensed to the four major clinical and diagnostic imaging considerations.5 The most common and life-threatening of these is the result of bowel necrosis often due to bowel ischaemia, infarction, necrotising enterocolitis, neutropenic colitis, volvulus or sepsis.5 These situations represent surgical emergencies. In these cases the patient is likely to be clinically unwell, haemodynamically unstable, they may have an acute abdomen and are likely to be in severe pain.3 The clinical presentation and imaging findings are important in the differentiation of transient pneumatosis from these life-threatening causes.5 Treatment depends on the extent of the disruption and the underlying cause.
Pneumatosis intestinalis found secondary to mucosal disruption can have different origins. This includes over distention from a peptic ulcer, pyloric stenosis, annular pancreas or a more distal obstruction.5 Disruption can also be caused by ulceration, erosions, or trauma, including the trauma of child abuse.5 Additionally it can be iatrogenic from intracatheter jejunal feeding tubes, stent perforation, sclerotherapy, surgical trauma or endoscopic trauma.5 A more subtle form may occur due to mucosal erosions and defects in intestinal crypts secondary to acute and subclinical enteritides that allow intraluminal bacterial gas under pressure to infiltrate into the bowel wall layers, particularly into the submucosa.5 Additionally pneumatosis is seen with increased frequency in patients who are immunocompromised because of steroids, chemotherapy, radiation therapy or AIDS.5 In these cases the pneumatosis may result from intraluminal bacterial gas entering the bowel wall due to increased mucosal permeability caused by defects in bowel wall lymphoid tissue.5
A pulmonary cause must be considered in cases of COPD, asthma and cystic fibrosis. Pneumatosis intestinalis can occur with barotrauma, after chest tube placement, in addition to increased intrathoracic pressure associated with retching and vomiting.
Mucosal integrity, intraluminal pressure, bacterial flora and intraluminal gas all interact in the formation of pneumatosis intestinalis.7 There are many cases where there has been unnecessary surgical intervention.7–9 Surgical management is more often adopted in patients who present initially more unwell. It is likely if the patient is unwell at the point of presentation the pathogenesis will be thought to be secondary to a life-threatening cause such as bowel ischaemia, perforation or peritonitis and without surgical intervention these patients would not survive. There also are a number of cases where patients have been managed conservatively and recovered well.10–12 Where possible conservative management should be pursued in most patients found to have pneumatosis intestinalis alongside treating their underlying illness.9
Learning points.
Pneumatosis intestinalis is an unusual radiological finding associated with numerous pathologies from benign to life-threatening.
Radiographic findings must always be interpreted in the context of the clinical presentation.
A finding of pneumatosis may lead to unnecessary surgical intervention.
No current algorithm exists for management of pneumatosis intestinalis which can present a dilemma to the surgeon.
Conservative management must be considered in those thought to have primary pneumatosis intestinalis.
Surgery may be a lifesaving option in individuals presenting with pneumatosis secondary to bowel necrosis who are at risk of perforation. These patients will be clinically unwell at the point of presentation.
Footnotes
Contributors: The guarantors HAB and RB accept full responsibility for the work, had access to the data, and controlled the decision to publish, planning, conduct, and reporting of the work described in the article and are responsible for the overall content as guarantors. RB had the idea for the article and made ammendments and suggestions contributing to the article. HAB performed the literature search and wrote the article. Both HAB and RB accept full responsibility for the finished article. Case 1 was identified by HAB and RB. Case 1 was managed by RB and his team. Case 2 was identified by HAB and managed by HAB’s team. RA provided imaging reports for case 1
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Slesser AA, Patel PH, Das SC et al. A rare case of segmental small bowel pneumatosis intestinalis: a case report. Int J Surg Case Rep 2011;2:185–7. 10.1016/j.ijscr.2011.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goyal SK. Pneumatosis intestinalis imaging. Medscape 2013. http://emedicine.medscape.com/article/371955-overview (accessed 21 Oct 2013). [Google Scholar]
- 3.Akhtar S, Dawe N. Pneumatosis intestinalis presenting with a pneumoperitoneum in a patient with chronic bronchiectasis: a delayed diagnosis of superior mesenteric artery ischaemia. BMJ Case Reports 2010;2010 pii: bcr0120102622 (accessed 2 Oct 2013). 10.1136/bcr.01.2010.2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jamart J. Pneumatosis cystoides intestinalis, a statistical study of 919 cases. Acta Hepatogastroenterol (Stuttg) 1979;26:419–22. [PubMed] [Google Scholar]
- 5.Pear BL. Pneumatosis intestinalis: a review. Radiology 1998;207:13–19. 10.1148/radiology.207.1.9530294 [DOI] [PubMed] [Google Scholar]
- 6.Koss LG. Abdominal gas cysts (pneumatosis cystoides intestinorum hominis); an analysis with a report of a case and a critical review of the literature. Arch Pathol 1952;53:523–49. [PubMed] [Google Scholar]
- 7.St Peter SD, Abbas MA, Kelly KA. The spectrum of pneumatosis intestinalis. Arch Surg 2003;138:68–75 (accessed 21 Oct 2013). 10.1001/archsurg.138.1.68 [DOI] [PubMed] [Google Scholar]
- 8.Hsueh KC, Tsou SS, Tan KT. Pneumatosis intestinalis and pneumoperitoneum on computed tomography: beware of non-therapeutic laparotomy. World J Gastrointest Surg 2011;3:86–8 (accessed 21 Oct 2013). 10.4240/wjgs.v3.i6.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nathan H, Singhal S, Cameron JL. Benign pneumatosis intestinalis in the setting of coeliac disease. J Gastrointest Surg 2006;10:890–4 (accessed 21 Oct 2013). 10.1016/j.gassur.2005.11.004 [DOI] [PubMed] [Google Scholar]
- 10.Brill SE, Skipworth J, Stoker DL. Conservative management of pneumatosis intestinalis and massive pneumoperitoneum in the acute abdomen: a case report. Ann R Coll Surg Engl 2008;90:W11–13 (accessed 21 Oct 2013). 10.1308/147870808X257193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devgun P, Hassan H. Pneumatosis cystoids intestinalis: a rare benign cause of pneumoperitoneum. Case Rep Radiol 2013;2013:353245 (accessed 21 Oct 2013). 10.1155/2013/353245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tak PP, Van Duinen CM, Bun P et al. Pneumatosis cystoides intestinalis in intestinal pseodoobstruction. Resolution after therapy with metronidazole. Dig Dis Sci 1992;37:949–54 (accessed 21 Oct 2013). 10.1007/BF01300397 [DOI] [PubMed] [Google Scholar]