Abstract
CT scan and ultrasonography images revealed two small uniformly low-density and hypoechoic lesions in the liver, respectively, 7 years after curative resection of rectal cancer, in a 74-year-old man. The area of the liver including the two lesions was segmentally resected. Two lesions were histopathologically confirmed as early but active stage alveolar echinococcosis (AE) caused by accidental ingestion of eggs of the fox tapeworm, Echinococcus multilocularis. This case is very unique and rare, since early stage hepatic AE cases have only accidentally been confirmed from cases in which malignant hepatic tumours were suspected, and because two independent AE lesions were detected. Abdominal MRI showed two isointense tumour lesions with small areas of high-signal intensity in their centres on T2-weighted images. MRI findings appear to reflect the macroscopic view and microscopic findings of early stage AE with active cyst in the centre of each hepatic lesion well.
Background
Alveolar echinococcosis (AE) causes occupational and invasive lesions such as malignant tumours mainly in the liver. As early stage AE is asymptomatic, it is difficult or impossible for clinicians to detect it before it reaches an advanced stage; however, accidental differentiation of a misdiagnosed malignant tumour, as shown in this case, is a possibility.1–4 However, recent advances in diagnostic imaging techniques have greatly improved the chances of asymptomatic hepatic tumour detection.3–5 Diagnosis of AE is based on partial calcification of cysts in several diagnostic imaging findings and serological diagnosis using methods such as Em18 in advanced AE.6–12 As image diagnosis of early stage AE has not been possible until now, there are few reports of early stage AE.5 We experienced one AE case with two small independent early stage lesions in the liver. MRIs are expected to be highly useful for differential diagnosis of early but active stage AE with hydatid cyst fluid in the centre of hepatic lesion.
Case presentation
Two small low-density lesions at Cournand's segments 6 and 7 of the liver were detected by a CT scan and suspected to be metastasis of rectal cancer in a 74-year-old man living in Hokkaido, Japan, who had undergone rectal cancer surgery 7 years earlier. Abdominal MRI showed two isointense tumour lesions with small areas of high-signal intensity in their centres on T2-weighted images. These cystic lesions were histopathologically confirmed to be AE cysts caused by accidental ingestion of Echinococcus multilocularis eggs.
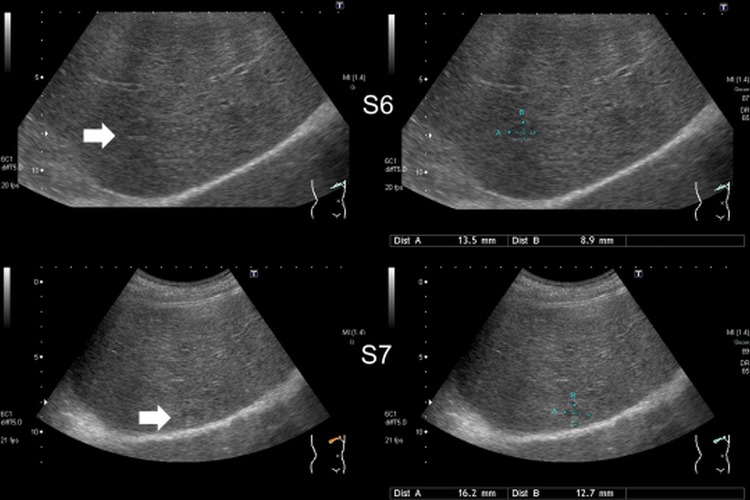
The patient was a crane operator but worked temporarily in the mink breeding industry in Hokkaido, but had no particular symptoms. Low anterior resection with lymph node dissection (pT3, pN0, M0, Stage IIA of the UICC (Union for International Cancer Control) classification) was performed for rectal cancer in January 2004. Ultrasonography (US) showed two hypoechoic lesions (figure 1) and CT scan showed two uniformly circular low-density lesions of 16.2×12.7 and 13.5×8.9 mm in segments 6 and 7, respectively (figure 2). MRI revealed two small lesions of high-signal intensity in their centres on T2-weighted images (figure 3). Cyst size inside the solid host tissues were 2.5×3.1 mm and 3.7×4.1 mm respectively. As there was no calcified lesion, which is a typical indicator of advanced AE, metastatic liver tumours and other hepatic diseases were suspected. Two lesions (figure 4) completely resected in January 2012 were histopathologically confirmed as early but active stage AE: two cysts with laminated and germinal layers inside were surrounded by fibrous tissue and leucocyte layers (figure 5). Mitochondrial DNA analysis revealed histological specimens as the Asian genotype of E. multilocularis.13–15 As we did not expect AE, we failed in keeping serum samples until 16 days after surgery. Serology by confirmative Western blottings using crude E. multilocularis antigens16 carried out at Hokkaido Institute of Public Health, Sapporo, and an affinity purified Em18 and a recombinant Em18 carried out at Asahikawa Medical University,7 17 were negative when a serum sample prepared 16 days after the surgery was examined (figures 6A, B). However, Western blotting using crude E. multilocularis antigens showed several bands (figure 6C), but it was unclear if any of these bands were specific to AE or not. Furthermore, it was unclear if this case was seropositive to Em18 if preoperative serum sample was examined, since most recent work showed antibody responses to Em18 drastically dropped within a few days after curative surgery.9 12 17 18
Figure 1.
Ultrasonography showing two hypoechoic lesions in the liver (S6, segment 6).
Figure 2.
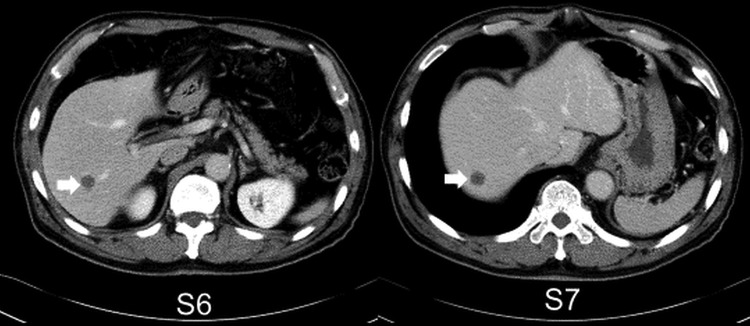
CT scan showing two low-density lesions in segment 6 (S6) and S7.
Figure 3.
MRI showing two isointense tumours with high intensity in their centres on T2-weighted imaging in segment 6 (S6) and S7.
Figure 4.
Resected specimen.
Figure 5.
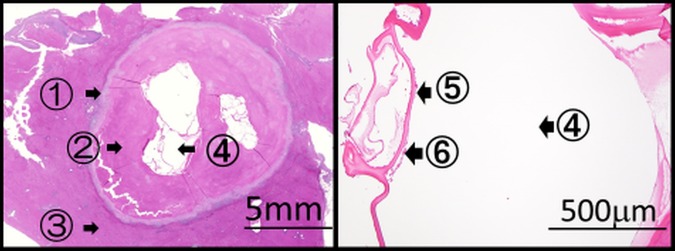
Histopathology showing an alveolar echinococcosis cyst of 3.7×4.1 mm in size. ①: Leucocyte layer, ②: fibrous layer, ③: normal liver tissue, ④: multilocular cysts, ⑤: laminated layer and ⑥: germinal layer.
Figure 6.
Western blotting (WB) figures using affinity purified Em18 (A) and recombinant Em18 (B), which are both negative, and crude antigens (C) illustrating several bands that appear to differ from several other bands, which have been expected to be specific or unique to echinococcosis.16 17
Outcome and follow-up
The patient's postoperative course is favourable. He is healthy and without signs of illness 3 years after liver surgery.
Discussion
AE causes occupational and invasive lesions, such as malignant tumours, mainly in the liver.1–4 Recent advances in studies of imaging figures including US, CT, MRI, fluorodeoxyglucose-positron emission tomography1–5 and serology to detect antibody responses to a specific antigen such as Em18, predominant in active but late or advanced stage AE,6–12 17 can differentiate almost all AE cases of lesions >3–5 cm with or without partial calcification.1–4 However, there is no tool suitable for detection of early stage AE with lesions <1 or even <3 cm except needle biopsy.19 20 However, most patients do not allow an invasive needle biopsy. Therefore, early stage AE cases have never been identified as AE before surgery, but have exclusively and exceptionally been confirmed by histopathological examination of the resected lesions after surgery in cases where malignant tumours were suspected.5
MRI figures revealed two cysts of early AE (liquid phase) as highly contrasted phase surrounded by a solid phase of host tissues. These findings clearly reflect the macroscopic view of excised specimens (figure 4). MRI is superior in density resolution and can detect a difference in the density distribution more clearly than CT.
MRIs of AE cases have been classified into five types.5 The earliest is type 1, showing multiple small round cysts without a solid component confirmed from a cyst <3 cm in diameter.5 However, the present case corresponds to none of the 5 types.5
This is the first report demonstrating two independent early stage hepatic AE lesions <5 mm by MRI. As one AE cyst is expected to be caused by one oncosphere invading the liver, this patient accidentally got infection with two oncospheres, which developed into two cysts in different segments. In general, multiple lesions either in the liver or in multiple organs, have been conceived to be due to metastasis of the primary hepatic AE.1–3 However, multiple primary infections may often occur in endemic areas.21 Nonetheless, as there is no tool to identify or suspect early stage AE cases, there is no report demonstrating such multiple primary infections. This may be the first report demonstrating two independent early stage AE lesions in the liver.
We would like to put forth the hypothesis that such MRI findings are unique to early stage hepatic AE. We expect MRI diagnosis of cyst(s) ≤1 cm or even <3 cm without calcification may be highly useful for detection of early stage hepatic AE cases. The MRI findings described in the text would be the important imaging point to differentiate between early stage AE and other lesions, including malignant tumours.
The imaging findings in early stage and advanced stage AE cases crucially differ from each other, since a small active hydatid cyst (liquid phase) in the centre of early stage AE lesion with outer solid cysts without calcification differs from a big necrotic cyst (abscess phase) in advanced stage AE with big outer solid cysts with partial calcification.
Learning points.
Diagnosis of early stage alveolar echinococcosis (AE) is very difficult for diagnostic imaging, especially without calcification.
MRI findings reflect early stage hepatic AE more clearly than CT or ultrasonography imaging.
T2-weighted imaging MRI introduces immediate diagnosis for early stage hepatic AE.
Acknowledgments
The authors sincerely thank K Ishibashi, H Takahashi, M Hashimoto and S Inaba, who saw this case at Department of Surgery, Hokkaido Kouseiren Engaru-Kousei General Hospital, and T Yanagida, K Nakaya and Y Sako, who performed laboratory diagnostics at Department of Parasitology, Asahikawa Medical University.
Footnotes
Contributors: TA, MH and HY saw this case in Department of Surgery. AI confirmed this case as AE. TA, MH and AI wrote the case report. TA obtained consent from the patient. All the authors critically appraised the manuscript and contributed to make necessary changes in the article.
Funding: This study was supported in part by a Grant-in-Aid for scientific research (No. 24256002) from Japan Society for the Promotion of Science, the Hokkaido Translational Research Fund (2007-2011) and the Special Coordination Fund for promoting science and technology (2010-2012) from the Ministry of Education, Culture, Sports, Science & Technology in Japan (MEXT) to AI.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Eckert J, Gemmell MA, Meslin FX et al. WHO/OIE Manual on echinococcosis in humans and animals: a public health problem of global concern. Paris: OIE, 2001:1–265. [Google Scholar]
- 2.Brunetti E, Kern P, Vuitton DA et al. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop 2010;114:1–16. 10.1016/j.actatropica.2009.11.001 [DOI] [PubMed] [Google Scholar]
- 3.Bresson-Hadni S, Delabrousse E, Blagosklonov O et al. Imaging aspects and non-surgical interventional treatment in human alveolar echinococcosis. Parasitol Int 2006;55:S267–72. 10.1016/j.parint.2005.11.053 [DOI] [PubMed] [Google Scholar]
- 4.Kern P, Wen H, Sato N et al. WHO classification of alveolar echinococcosis: principles and application. Parasitol Int 2006;55:S283–7. 10.1016/j.parint.2005.11.041 [DOI] [PubMed] [Google Scholar]
- 5.Kodama Y, Fujita N, Shimizu T et al. Alveolar echinococcosis: MR findings in the liver. Radiology 2003;228:172–7. 10.1148/radiol.2281020323 [DOI] [PubMed] [Google Scholar]
- 6.Ito A, Schantz PM, Wilson JF. Em18, a new serodiagnostic marker for differentiation of active and inactive cases of alveolar hydatid disease. Am J Trop Med Hyg 1995;52:41–4. [DOI] [PubMed] [Google Scholar]
- 7.Ito A, Nakao M, Sako Y. Echinococcosis: serological detection of patients and molecular identification of parasites. Future Microbiol 2007;2:439–49. 10.2217/17460913.2.4.439 [DOI] [PubMed] [Google Scholar]
- 8.Sako Y, Fukuda K, Kobayashi Y et al. Development of an immunochromatographic test to detect antibodies against recombinant Em18 for diagnosis of alveolar echinococcosis. J Clin Microbiol 2009;47:252–4. 10.1128/JCM.01476-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tappe D, Frosch M, Sako Y et al. Close relationship between clinical regression and specific serology in the follow-up of patients with alveolar echinococcosis in different clinical stages. Am J Trop Med Hyg 2009;80:792–7. [PubMed] [Google Scholar]
- 10.Tappe D, Sako Y, Itoh S et al. Immunoglobulin G subclass responses to recombinant Em18 in the follow-up of patients with alveolar echinococcosis in different clinical stages. Clin Vaccine Immunol 2010;17:944–8. 10.1128/CVI.00026-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knapp J, Sako Y, Grenouillet F et al. Comparison of the serological tests ICT and ELISA for the diagnosis of alveolar echinococcosis in France. Parasite 2014;21:34 10.1051/parasite/2014037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujimoto Y, Ito A, Ishikawa Y et al. Usefulness of recombinant Em18-ELISA to evaluate efficacy of treatment in patients with alveolar echinococcosis. J Gastroenterol 2005;40:426–31. 10.1007/s00535-004-1559-7 [DOI] [PubMed] [Google Scholar]
- 13.Nakao M, Xiao N, Okamoto M et al. Geographic pattern of genetic variation in the fox tapeworm Echinococcus multilocularis. Parasitol Int 2009;58:384–9. 10.1016/j.parint.2009.07.010 [DOI] [PubMed] [Google Scholar]
- 14.Ito A, Agvaandaram G, Bat-Ochir OE et al. Histopathological, serological, and molecular confirmation of indigenous alveolar echinococcosis cases in Mongolia. Am J Trop Med Hyg 2010;82:266–9. 10.4269/ajtmh.2010.09-0520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konyaev SV, Yanagida T, Nakao M et al. Genetic diversity of Echinococcus spp. in Russia. Parasitology 2013;140:1637–47. 10.1017/S0031182013001340 [DOI] [PubMed] [Google Scholar]
- 16.Liance M, Janin V, Bresson-Hadni S et al. Immunodiagnosis of Echinococcus infections: confirmatory testing and species differentiation by a new commercial western blot. J Clin Microbiol 2000;38:3718–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito A. Nothing is perfect! Trouble-shooting in immunological and molecular studies of cestode infections. Parasitology 2013;140:1551–65. 10.1017/S0031182013000966 [DOI] [PubMed] [Google Scholar]
- 18.Akabane H, Nakano S, Inagaki M et al. Postoperative course which used imaging and serodiagnosis related to a case of surgery for hepatic echinococcosis at Hokkaido P.W.F.A.C. Asahikawa-Kosei General Hospital, and an evaluation of said course. J Hokkaido Prefectural Welf Federation Agric Cooperatives 2012;44:38–44. (in Japanese) [Google Scholar]
- 19.Kern P, Frosch P, Helbig M et al. Diagnosis of Echinococcus multilocularis infection by reverse-transcription polymerase chain reaction. Gastroenterology 1995;109:596–600. 10.1016/0016-5085(95)90350-X [DOI] [PubMed] [Google Scholar]
- 20.Kawakami H, Kuwatani M, Sakamoto N. Hepatobiliary alveolar echinococcosis infiltration of the hepatic hilum diagnosed by endoscopic ultrasonography-guided fine-needle aspiration. Dig Endosc 2013;25:339–40. 10.1111/den.12034 [DOI] [PubMed] [Google Scholar]
- 21.Gottstein B, Saucy F, Deplazes P et al. Is high prevalence of Echinococcus multilocularis in wild and domestic animals associated with disease incidence in humans? Emerg Infect Dis 2001;7:408–12. 10.3201/eid0703.017307 [DOI] [PMC free article] [PubMed] [Google Scholar]