Abstract
Total mesorectal excision (TME) is the current optimal surgical treatment for patients with rectal carcinoma. A complete TME is related to lower local recurrence rates and increased patient survival. Many confounding factors in the patient's anatomy and prior therapy can make it difficult to obtain a perfect plane, and thus a complete TME. The resection specimen can be thoroughly evaluated, grossly and microscopically, to identify substandard surgical outcomes and increased risk of local recurrence. Complete and accurate data reporting is critical for patient care and helps surgeons improve their technique.
Keywords: abdominoperineal resection, circumferential resection margin, rectal carcinoma, total mesorectal excision
Total mesorectal excision (TME) has become the standard and preferred surgical technique for the excision of rectal cancer. The completeness of the mesorectal dissection and importance of an adequate circumferential margin for decreasing local recurrence in rectal cancer have been well demonstrated.1 2 3 4 5 6 7 As the pathologic grading of TME becomes more standardized,5 8 9 10 surgeons must also become familiar with the caveats of current TNM (tumor nodes metastases) staging, margin assessment, and grossing techniques to easily interpret pathology reports and understand the prognostic implications of certain pathologic findings.
Factors Associated with an Incomplete Total Mesorectal Excision
The technique of TME is based on sharp dissection of the mesorectal fascial envelope. The procedure is technically challenging for many reasons including small working space in the pelvis, complicated anatomy, and multiple dissection planes.11 12 13 14 A more difficult dissection may be anticipated in patients with previous pelvic surgery, such as hysterectomy or prostatectomy, and in patients with a large uterus.11 12 13 15 16 17 18 19 20 Dissection along the seminal vesicles in males can be technically challenging. An avascular plane between the seminal vesicles anteriorly and Denonvilliers fascia exists whereby the fascia can be retained on the specimen to ensure the mesorectal capsule is left intact in this plane. As the dissection is continued posteriorly and laterally, a point is reached at the distal rectum where the mesorectum thins out and in some patients may become nonexistent.13
An inability to achieve a mesorectal/complete plane on patients is not based solely on the skill of the surgeon.19 Abnormal body mass index (BMI), including obese and underweight individuals, is a predictive factor for an incomplete mesorectal excision.12 Obesity can make exposure and other technical aspects of the surgery more difficult, whereas there may be minimal tissue between tumor and the mesorectal envelope in underweight individuals. Narrow pelvic diameter has also been demonstrated to be associated with poorer quality TME specimens. Baik et al showed in a series of 100 patients who underwent magnetic resonance imaging (MRI) pelvimetry analysis that a narrow obstetric conjugate and shorter interspinous distance were related to an inadequate quality of the mesorectum specimen.21 Bosch and Nagtegaal performed a meta-analysis of 18 studies involving more than 4,000 patients in 2012, examining the achieved resection planes and confounding variables. There is no significant difference in the surgical planes achieved when laparoscopic or robot-assisted techniques are used.8 Prior chemoradiation effects no statistically measurable change, but abnormal BMI and abdominoperineal resection (APR) instead of anterior resection result in more muscularis propria/incomplete planes.8 The Belgian PROCARE study, a multidisciplinary project on rectal cancer, showed that the following factors were related to intramesorectal/nearly complete and muscularis propria/incomplete planes in 266 cases on univariate analysis: surgeon, female gender, abnormal BMI, low rectal cancer, absence of downstaging after long-course chemoradiation, laparoscopic and converted laparoscopic surgery, APR, and negative clinical nodal status. Multivariate analysis found only BMI, absence of downstaging, and laparoscopic surgery to be independently associated with incomplete excisions.12
Assessment and Processing of the Resection Specimen
Receipt and Evaluation of the Fresh Specimen
TME specimens must be received unopened to preserve the intactness of the mesorectum and allow for proper assessment and handling of the specimen.
Upon receipt, the TME specimen is measured and the initial assessment of the mesorectum noted. The length and diameter of the rectum are documented as is the length of the mesosigmoid colon, if present. This is measured from the wall of the sigmoid to the vascular pedicle. If received fixed and not pinned to a cork board, there is usually significant shrinkage artifact, which can lead to discrepancies between in vivo and ex vivo margin measurements. Goldstein et al have shown that a 5-cm length of colorectum in vivo is equivalent to 3 cm after resection and 2.2 cm after fixation.22 The external surface of the TME should be inspected and graded as mesorectal/complete, intramesorectal/nearly complete, or muscularis propria/incomplete (Table 1).8 9 10 23 24 The anal sphincters are also categorized in APR specimens (Table 1). Oriented gross photographs of the anterior and posterior aspects are taken before, or if necessary, after fixation.
Table 1. Evaluating the surgical plane: mesorectum and sphincter complex.
| Mesorectum | |
| Mesorectal plane (complete) | Intact mesorectum with only minor irregularities |
| No defects deeper than 5 mm | |
| No coning toward the distal margin of the resection specimen | |
| Smooth CRM on transverse sections | |
| Intramesorectal plane (nearly complete) | Moderate bulk to the mesorectum |
| One or more defects greater than 5 mm deep within the mesorectum | |
| Moderate coning | |
| No visible muscularis propria | |
| Irregular CRM on transverse sections | |
| Muscularis propria plane (incomplete) | Exposed muscularis propria |
| Moderate to marked coning | |
| Irregular CRM on transverse sections | |
| Sphincteric complex | |
| Extralevator | Cylindrical specimen with no waist effect |
| Levators removed en bloc | |
| Sphincteric plane | Slight waist effect |
| No significant defects or perforations | |
| Intrasphincteric/submucosal plane | Significant waist effect |
| Perforation or missing areas of muscularis propria | |
Abbreviation: CRM, circumferential resection margin (radial margin).
Grading of the Mesorectum and Sphincter Complex
In keeping with the guidelines of the College of American Pathologists (CAP), the terms “complete,” “nearly complete,” or “incomplete” qualify the mesorectum completeness. In addition, we include the more descriptive evaluation of the mesorectum based on the surgical plane of resection: the mesorectal plane, the intramesorectal plane, or the muscularis propria plane. These are explained in Table 1 and illustrated in Figs. 1 to 3.
Fig. 1.
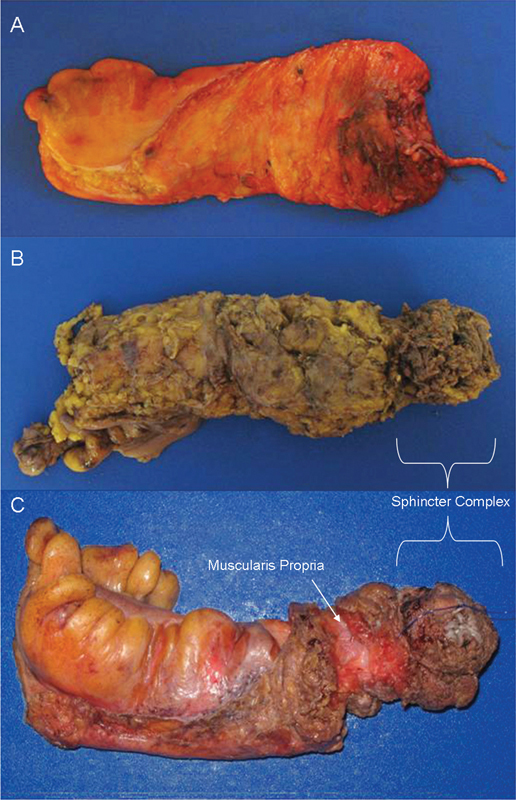
Mesorectal grading: (A) mesorectal plane; (B) intramesorectal plane; and (C) muscularis propria plane with arrow denoting the exposed muscularis propria. Note: Specimens B and C are APR resections with the brackets identifying the sphincter complex. APR, abdominoperineal resection.
Fig. 3.
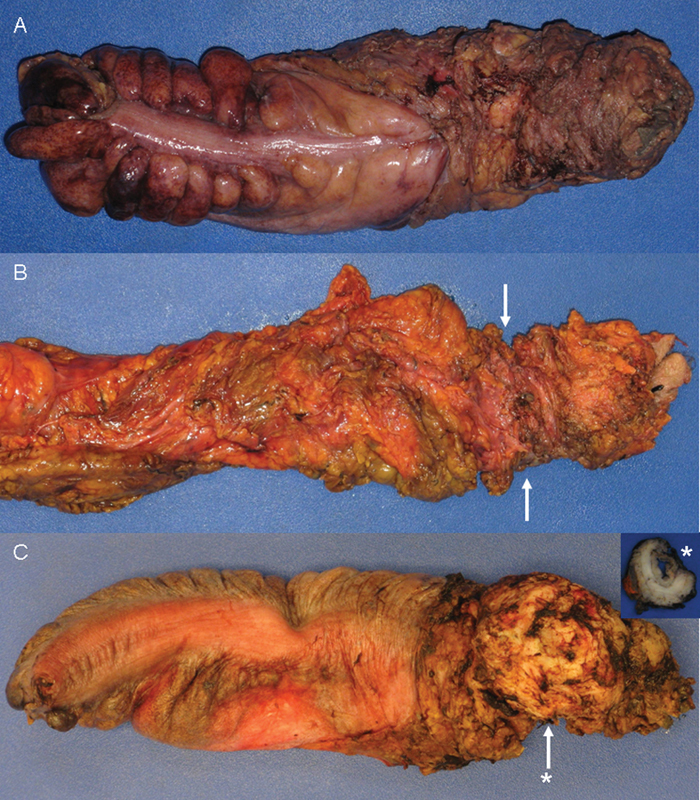
Sphincter grading: (A) extralevator; (B) sphincteric with arrows denoting “waist effect”; and (C) intrasphincteric with arrow denoting sphincteric defect and asterisk (*) indicating level of cross section (inset).
Fig. 2.
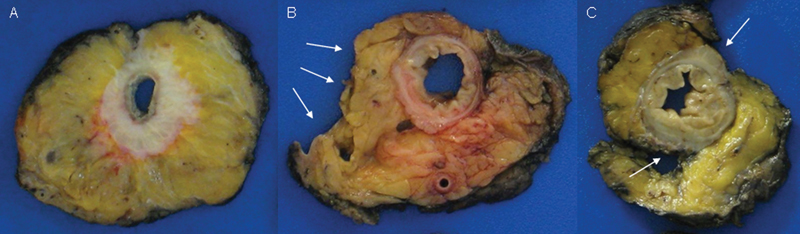
Mesorectal grading—transverse sections (A) mesorectal plane; (B) intramesorectal plane with arrows denoting mesorectal defects; and (C) muscularis propria plane with arrows denoting the exposed muscularis propria.
Orientation and Inking
The nonperitonealized surfaces (radial margin) of the TME specimen are inked black. To maintain orientation of the specimen postsectioning, a stripe of orange ink along the right lateral aspect is applied. Areas grossly suspicious for peritoneal tumor involvement are differentially inked blue. If any attached structures are present with the specimen (e.g., seminal vesicles, coccyx), these are differentially inked as well.
Opening and Fixation
The stapled proximal and distal mucosal margins are transected, leaving them loosely attached to the specimen. The specimen is opened longitudinally, beginning at its proximal end along the anterior aspect. The specimen is opened to 2 cm. above the tumor. If the tumor is identifiable, documentation includes the distance to the proximal and distal margins as well as the location of the tumor relative to the anterior peritoneal reflection. Due to preoperative treatment effect, the intraluminal tumor can be difficult to visualize when the specimen is intact, and in these cases, the measurements will be documented after transverse serial sectioning. Tumor size is measured both in the cephalad to caudal plane and in circumference. Other than transecting the distal mucosal margin, we typically do not open the TME specimen distal to the tumor until after fixation. In APR specimens, we remove the purse string suture to allow evacuation of luminal contents and formalin fixation. The specimen is placed in an appropriately sized container with the proper amount of formalin, which allows the specimen to lie straight. The specimen is submerged in formalin with the lumen purged of all air. Gauze is not placed in the lumen as it tends to compress and distort the mucosa. Specimens are fixed for 24 to 48 hours before sectioning.
Sectioning and Sampling
The TME specimen is transversely sectioned beginning at the distal end at approximately 3- to 5-mm intervals. Maintaining orientation, the slices are placed on a clean cutting board for photographic documentation. The slices are positioned with the top left of the photograph being the most proximal transverse section and the bottom right the most distal transverse section, demonstrated in Fig. 4. In addition, the slices are oriented relative to the patient's anatomic position to correlate with MRI findings (Fig. 5). A ruler is placed in the photograph for scale. A photograph of the slices is taken, with close-ups of individual slices when needed. The intactness of the mesorectum is reassessed postsectioning since in some cases, significant surgical defects have been noted that resulted in a change to the grading of the mesorectum.
Fig. 4.
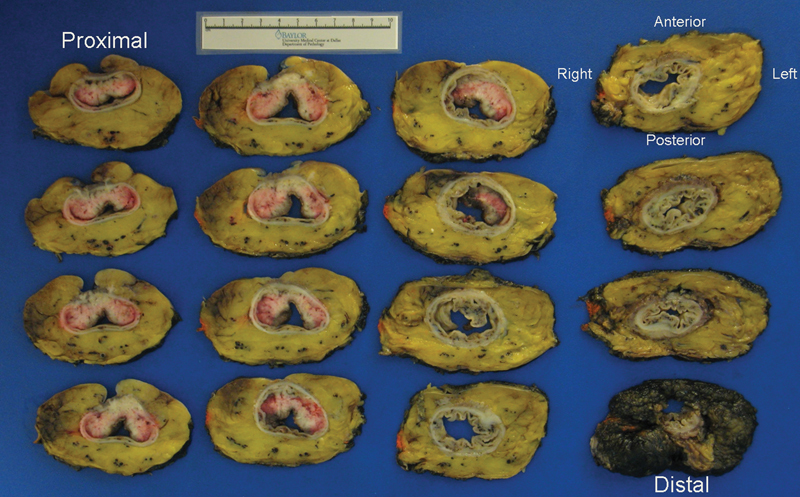
Specimen orientation key.
Fig. 5.
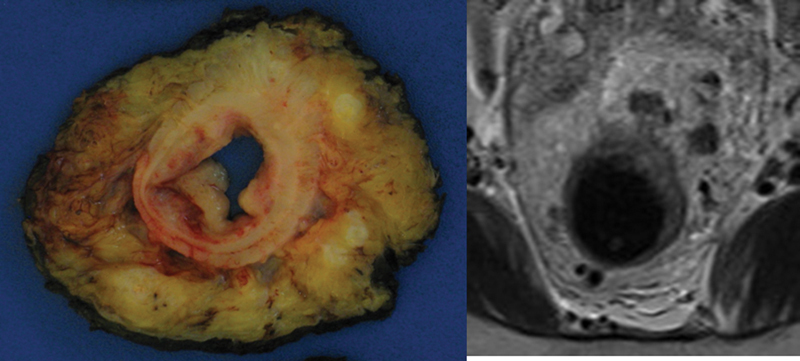
Comparison of magnetic resonance imaging image with gross cross section. MRI, magnetic resonance imaging.
Correlations of MRI findings with gross pathologic findings are documented in the pathology report and sections are submitted accordingly. Transverse slices are inspected to note the following: (1) extent of tumor and the closest distance of the tumor to the circumferential resection margin (CRM) (the closest point is specified as anterior, posterior, right/left lateral); (2) areas suspicious for extramural vascular invasion (EMVI), often correlating with “stranding” noted on the MRI25; (3) any positive lymph nodes and their relationship to the CRM (the location of positive node is specified in a clock face manner); and (4) other pertinent pathologic findings (polyps, diverticula, adhesions, etc.).
Routine sections include the following:
En face proximal and distal resection margins
Three sections of tumor showing the closest CRM
One section of tumor showing the luminal aspect
Two sections of tumor abutting peritonealized surface when applicable
Section(s) of areas suggestive for EMVI
All lymph nodes.
Lymph nodes abutting the CRM should be submitted illustrating the relationship to the CRM. Any lymph nodes specifically noted on MRI findings are submitted and designated as such.26 Since the anatomic landmarks seen on imaging are not available with the resected specimen, clock face positions are used to identify suspicious lymph nodes. If the apical node is identified (Dukes), it is submitted separately and designated as such.27 Whole mount sections can be helpful to determine depth of extension of T3 tumors, but this can be well demonstrated with carefully considered sampling of the tumor. Whole mount tissue processing is not used in our institution, and no tissue is routinely frozen for further studies. Some institutions do not routinely submit proximal margins if they are grossly greater than 5 cm, but all proximal and distal margins are examined microscopically at this institution.
Criteria for Pathologic Staging
American Joint Committee on Cancer (AJCC) Pathologic Staging
Rectal cancer must be properly staged to direct postoperative care and treatment.28 29 The standard method of pathologic staging in rectal cancer is the TNM staging system, which classifies patients into relatively homogenous groups based on depth of primary tumor invasion, presence of metastasis to regional lymph nodes, and presence of distant histologically confirmed metastases.7 28 30 31 These factors among others have been shown to correlate with clinical outcomes.29 ypStaging of rectal cancers show that ypStage I and ypStage 0 cancers have similar prognoses in the current TNM 7 classification.32
Depth of Invasion
Depth of invasion in rectal cancer is an important indicator of prognosis, and this is particularly true of extramural tumor extension.7 30 33 The depth of invasion can be evaluated preoperatively by high-resolution MRI and postoperatively by gross and microscopic pathologic analysis. In the TNM system, depth of invasion is designated as Tis-T4.30 33 Tis designates carcinoma in situ. T1 designates submucosal invasion. T2 designates involvement of the muscularis propria. T3 designates invasion through the muscularis propria and involvement of the pericolorectal tissues. T4 designates tumors that invade the visceral peritoneum, invade adjacent structure, or are adherent to adjacent structures/organs.30
Lymph Nodes
The number of positive lymph nodes identified within a TME specimen is an important prognostic factor in TME for rectal cancer.34 35 The TNM staging system designates tumors that involve one to three nodes as N1. These tumors are further classified as N1a, indicating metastasis to one regional lymph node, or as N1b, indicating metastasis to two to three regional lymph nodes. Tumors with metastasis in more than four regional nodes are designated as N2, with metastasis to four to six nodes specified as N2a, and metastasis to seven or more regional lymph nodes specified as N2b.30 After preoperative treatment, lymph nodes may show fibrosis or mucin pools without identifiable tumor cells. Although there is no current evidence-based guidance available, these lymph nodes are currently considered to be negative.36
According to the seventh edition of the AJCC Cancer Staging Manual, tumor deposits in the pericolic/perirectal fat or in mesocolic fat with no residual lymph node tissue, but within the area of lymph drainage, are designated as N1c.30 This definition of tumor deposits is a new development in the seventh edition of AJCC guidelines and has proven to be somewhat controversial, with some expressing concern that this change may increase upstaging and thus unnecessary treatment.29 37 Irregularly shaped tumor deposits may represent EMVI or direct, yet discontinuous tumor extension, although those with a rounded appearance may represent a completely replaced lymph node. Discerning the true nature of these deposits has serious implications for the patient.36 The TNM 5 designated all deposits greater than 3 mm as positive lymph nodes, whereas TNM 6 relied on contours: smooth deposits were declared positive lymph nodes while irregular deposits were declared venous invasion. TNM 7 leaves it up to the pathologist, suggesting that deposits with round contours be considered a completely replaced lymph node but offering no true guidance.36 37 Neoadjuvant chemoradiation can cause fragmentation of the tumor and leave pockets of viable tumor, which may be overinterpreted as a tumor deposit and consequently upstage the patient. This is unsettling, since many patients then are considered a stage III and subject to potentially toxic treatments.36 37
The TNM system requires that at least 12 lymph nodes be identified for accurate staging. An increase in survival has been associated with this number of lymph nodes, regardless of whether these nodes were negative or positive.28 30 38 39 Identification of fewer nodes has been associated with an increased risk of understaging, with the assumption that this increases the likelihood of missing positive nodes.38 The lymph node count has been selected by the National Quality Forum as a quality measure and as such may be related to funding in the future.40 41 Factors associated with increased lymph node yield include the extent and quality of surgical resection, use of chemical fixatives that make adipose tissue translucent, identification of nodes by multidisciplinary teams at high-volume teaching hospitals, and laparoscopic surgical approach.34 35 41 Methylene blue injection facilitates identification of sentinel nodes, but it is not currently used on colorectal cancers in our institution.
Margins
The importance of and ideal distance to distal margins are widely debated.34 42 Five centimeters of tumor-free margin is often considered ideal, but the minimal acceptable tumor-free margin varies from 1 to 5 mm between different authors.40 42 Where possible these measurements should be done on a fresh specimen, as 30 to 50% shrinkage can occur with fixation.40
A minimal circumferential margin of at least 1 mm is generally accepted.4 This has been shown in multiple studies to correlate with improved local recurrence and cancer-specific survival rates.4 Another important prognostic factor is tumor extension into the mesorectum. This has been shown to be an independent prognostic factor in T3 rectal tumors, and mesorectal extension > 5 mm has been shown to have a negative prognostic effect.7 APR approach and choice of resection plane are associated with perforation and positive CRM. CRM positivity is not necessarily a reflection of the quality of the surgery, as TNM stage, tumor size, low tumor position, and tumor characteristics such as differentiation and invasive growth pattern have all been associated with CRM positivity.8 43
With new developments in robotic technologies, there is increased interest in incorporating these technologies into the surgical treatment of rectal cancer, in particular, for TMEs.44 This may prove useful due to improved visualization and increased dexterity when compared with traditional laparoscopic instrumentation.16 44 Research into operative outcomes with robot-assisted technique has shown that surgical outcomes after robotic-assisted TME are not inferior to a more traditional laparoscopic or open approach.16 44
Perineural Invasion
Perineural invasion by rectal carcinoma is a negative prognostic factor.30 45 46 It has been reported that perineural invasion may be underreported due to the technical difficulty in its identification, and significant interobserver variability has been noted.45 46
Extramural Vascular Invasion
Venous invasion beyond the muscularis propria is predictive of visceral metastasis, relapse, and decreased survival in colorectal cancer.28 47 48 49 50 Thus, preoperative MRI can be valuable in identifying high-risk patients who may benefit from neoadjuvant treatment. Identified on T2-weighted images, radiologists can note whether EMVI is present near the mesorectal fascia.25 51 When EMVI is noted on MRI but not found histologically, it may indicate the need for taking additional sections and further assessment. Elastic stains are not routinely used to look for EMVI, but studies have shown a marked increase in EMVI detection when used. Some suggest that all tumor blocks be stained with elastic stains to increase the sensitivity of venous invasion detection and, accordingly, to improve the prediction of cancer-specific survival.50 52 53
Differentiation
The grade of tumor cell differentiation in rectal cancer has consistently shown prognostic significance. However, no universal grading system is agreed upon for the differentiation of rectal cancer.45 54 Some systems use the worst area and other use the most common area There is also variability between observers.55 Two of the most common systems for grading tumor differentiation rely solely on the percentage of tumor that is making glands.54 Our institution follows the CAP recommendations, with low-grade tumors having > 50% gland formation and high-grade tumors having < 50% gland formation.
Tumor budding, or dedifferentiation, at the invasive front of rectal cancers is defined as single or small groups (less than 5 cells) of invasive tumor cells. When present, dedifferentiation has been shown to be an independent prognostic indicator.54 56 This has been correlated with lymph node metastasis, distant metastasis (liver metastasis in particular), and local recurrence.46 56 In general, tumor budding has been associated with higher grade tumors.54
Tumor Regression Scoring
Many systems exist to grade the amount of tumor regression following neoadjuvant therapy, and complete regression is associated with an improved prognosis.57 Most of these systems are based on the percentage of remaining viable tumor cells, often with four to five different “levels” of regression.30 46 57 58 These systems are all somewhat subjective, and significant interobserver variability has been demonstrated.58 When assessing tumor regression, it is important to exclude scars, fibrosis, acellular mucin pools, or granulation tissue as tumor.30 33 Acellular mucin pools are seen in patients with and without mucinous histology. They are not associated with worse outcomes and should not be considered when assessing residual tumor.59 Only intact tumor cells should be considered.55 Residual tumor cells tend to be located at the invasive front of the tumor, and some small studies suggest the tumor is preferentially eradicated from the mucosa and submucosa but remain in the deeper layers of bowel wall.60
It is difficult to differentiate between complete (no residual cancer) and near complete response (< 5% residual tumor cells) or between major (5–50% residual tumor cells) and minor (> 50% residual tumor cells) response. Determining minor response from no response is also a diagnostic challenge. Despite this, there seems to be a difference between complete response and minor response in terms of survival. Many studies have shown complete tumor regression to be associated with increased cancer-free and overall survival, and partial regression has been associated with improvement in cancer-free survival.61 62
Ancillary Studies
In our institution, all colorectal carcinomas are routinely tested for microsatellite instability (MSI) via polymerase chain reaction and mismatch repair proteins via immunohistochemical staining. Patients with known metastases are also tested for KRAS and BRAF mutations on representative sections of primary tumor.
The likelihood of finding MSI-high tumors (lower metastatic potential) decreases along the colon, being more common in the midgut and less common in the hindgut.63 CpG island methylator phenotype–high tumors, which seem to correspond with the serrated polyp pathway, confer a poorer prognosis and have a similar distribution.64 Chromosomally unstable tumors are therefore the most common type of rectal cancer, understanding that there is occasional overlap of the three molecular pathways.65 Although initial studies have suggested that MSI-high tumors are resistant to 5-FU therapy,66 a randomized trial of 1,800 patients showed no difference in chemotherapy efficacy in regard to MSI-high, BRAF, or KRAS mutation status.63
Significance of Common Pathologic Findings
Positive Circumferential Resection Margin
A microscopic positive margin, with tumor cells either on inked surface or less than 1 mm from the margin, is predictive of local recurrence, distant metastasis, and decreased cancer-specific survival.19 67 The positive margin may be due to direct or discontinuous tumor spread, lymphatic or venous invasion, positive lymph nodes, or perineural invasion.43 68 CRM positivity is much more likely in muscularis propria planes (19–29%) compared with mesorectal planes (1.6–14.6%).8 As tumor less than 1 mm from the inked soft tissue margin is considered positive, some cases are CRM+ but R0, as R1 denotes transected tumor.69
Local recurrence is decreased by 50% by neoadjuvant chemoradiation, regardless of which plane is achieved.19 As postoperative chemoradiation is not adequate compensation based on recurrence data, second surgeries to achieve R0 status may be warranted.2 Local recurrence does develop even in R0 patients, albeit at a much lower rate.1 4 49
Positive Lymph Nodes at Circumferential Resection Margin
CRM positivity due to tumor in lymph nodes is not specifically reported in most studies, therefore, the clinical implications are less clear. The study by Nagtegaal et al for the Dutch Colorectal Cancer Group in 2002 did examine this variable and found that CRM positivity due to lymph nodes was independent of achieving a mesorectal/complete plane of excision.23 Only two small studies to date have specifically examined this situation, suggesting that local recurrence rates are not as high as when direct tumor spread is present at the margin.43 68 70 Further studies are needed for prognostic clarification and appropriate treatment.
Distal Margin < 1 cm
A “close shave” margin, although preferably avoided, has not shown to have a higher rate of local recurrence than larger distal margin distances in some studies, particularly if the patient received neoadjuvant chemoradiation.3 5
Insufficient Lymph Nodes (Less than 12)
Although 12 lymph nodes have been the benchmark by which adequate excision and/or pathology grossing has been held to since 2000 by the National Comprehensive Cancer Network, data show that this is not always achievable or reasonable, particularly in rectal cancers with neoadjuvant treatment.55 71 The Belgian PROCARE study showed only 11 lymph nodes on average in pretreated TME specimens compared with 14 in naïve TME specimens.55 Higher numbers of lymph nodes are found at teaching institutions with greater colorectal surgical volumes.41 A study at the Cleveland Clinic showed a significant difference in lymph nodes based on approach, with an average of 26 from laparoscopic rectal cases versus 21 in open rectal cases.34 Mayo Clinic's prospective Clinical Outcomes of Surgical Therapy (COST) trial did not demonstrate any relationship between increased lymph node numbers and improved overall survival, which directly contradicts numerous studies.40 72 73 Although an accurate lymph node count is often considered an important factor in rectal cancer prognosis, Mathis et al suggest that total lymph node count is not, in fact, predictive of overall or disease-free survival.40
An alternate method of determining prognosis based on lymph nodes is the calculation of the metastatic lymph node ratio (MLNR). This is calculated by dividing the number of lymph nodes positive for metastasis by the total number of retrieved nodes.35 This can be particularly useful in cases where the patient has received neoadjuvant chemoradiotherapy, which can decrease the number of identifiable regional lymph nodes.35 One of the shortfalls of MLNR is that there is no universal cutoff, with recommended cutoffs ranging from 0.17 to 0.69.28 35 MLNR has been suggested as a viable alternative to the TNM lymph node assessment and has been shown in both prospective and retrospective studies to be an effective predictor of recurrence and survival, even in cases where less than 12 lymph nodes are identified.28 35 38 However, a large retrospective study of 10,000 cases from 1995 to 2010 (Quirke, unpublished data) shows the MLNR to be dependent on the number of lymph nodes found, suggesting it is a poor indicator of outcome. Consequently, the total number of lymph nodes remains paramount for correct staging.
Treatment Effect in Lymph Nodes
In patients who receive neoadjuvant chemoradiation, evidence of treated, nonviable tumor may be seen within lymph nodes. Lakes of mucin, necrotic tumor cells, or fibrosis may involve or replace the lymph node. It appears that ypN0 has statistically similar outcomes to pN0 in regard to local recurrence, metastases, and overall survival.74 When residual viable tumor is present in treated lymph nodes (ypN+), micrometastases did not impact survival, but macrometastic disease (> 0.2 cm) reduced disease-free and overall survival when compared with ypN0 patients in a single study.27
Conclusion
It is vital that pathologists report colorectal cancer to a high standard as their reports directly influence treatment and outcomes. Surgeons need to understand the standardized pathology report regarding their rectal resections. Careful evaluation of the mesorectal envelope has important implications for local recurrence and reflects the technical capability of the surgeon in most instances. The pathology report contains useful information regarding the nature of the tumor and the patient's risk for recurrence, which can be evaluated in tandem with molecular studies. Discussion of a patient's pathology as part of a multidisciplinary team approach is imperative regarding risk stratification, correlation with imaging studies, and recommendations for further testing and treatment.
References
- 1.Engelen S M, Maas M, Lahaye M J. et al. Modern multidisciplinary treatment of rectal cancer based on staging with magnetic resonance imaging leads to excellent local control, but distant control remains a challenge. Eur J Cancer. 2013;49(10):2311–2320. doi: 10.1016/j.ejca.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Fekete Z, Muntean A, Irimie A. et al. What is the significance of a microscopically positive resection margin in the curative-intent treatment of rectal adenocarcinoma? A retrospective study. J BUON. 2013;18(4):989–995. [PubMed] [Google Scholar]
- 3.Lim J W, Chew M H, Lim K H, Tang C L. Close distal margins do not increase rectal cancer recurrence after sphincter-saving surgery without neoadjuvant therapy. Int J Colorectal Dis. 2012;27(10):1285–1294. doi: 10.1007/s00384-012-1467-x. [DOI] [PubMed] [Google Scholar]
- 4.Lin H H, Lin J K, Lin C C. et al. Circumferential margin plays an independent impact on the outcome of rectal cancer patients receiving curative total mesorectal excision. Am J Surg. 2013;206(5):771–777. doi: 10.1016/j.amjsurg.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Mezhir J J, Shia J, Riedel E. et al. Whole-mount pathologic analysis of rectal cancer following neoadjuvant therapy: implications of margin status on long-term oncologic outcome. Ann Surg. 2012;256(2):274–279. doi: 10.1097/SLA.0b013e31825c13d5. [DOI] [PubMed] [Google Scholar]
- 6.Shimada Y, Takii Y. Clinical impact of mesorectal extranodal cancer tissue in rectal cancer: detailed pathological assessment using whole-mount sections. Dis Colon Rectum. 2010;53(5):771–778. doi: 10.1007/DCR.0b013e3181cf7fd8. [DOI] [PubMed] [Google Scholar]
- 7.Shin R, Jeong S Y, Yoo H Y. et al. Depth of mesorectal extension has prognostic significance in patients with T3 rectal cancer. Dis Colon Rectum. 2012;55(12):1220–1228. doi: 10.1097/DCR.0b013e31826fea6a. [DOI] [PubMed] [Google Scholar]
- 8.Bosch S L, Nagtegaal I D. The Importance of the Pathologist's Role in Assessment of the Quality of the Mesorectum. Curr Colorectal Cancer Rep. 2012;8(2):90–98. doi: 10.1007/s11888-012-0124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enker W E, Levi G S. Macroscopic assessment of mesorectal excision. Cancer. 2009;115(21):4890–4894. doi: 10.1002/cncr.24655. [DOI] [PubMed] [Google Scholar]
- 10.García-Granero E, Faiz O, Muñoz E. et al. Macroscopic assessment of mesorectal excision in rectal cancer: a useful tool for improving quality control in a multidisciplinary team. Cancer. 2009;115(15):3400–3411. doi: 10.1002/cncr.24387. [DOI] [PubMed] [Google Scholar]
- 11.Havenga K, Grossmann I, DeRuiter M, Wiggers T. Definition of total mesorectal excision, including the perineal phase: technical considerations. Dig Dis. 2007;25(1):44–50. doi: 10.1159/000099169. [DOI] [PubMed] [Google Scholar]
- 12.Leonard D, Penninckx F, Fieuws S. et al. Factors predicting the quality of total mesorectal excision for rectal cancer. Ann Surg. 2010;252(6):982–988. doi: 10.1097/SLA.0b013e3181efc142. [DOI] [PubMed] [Google Scholar]
- 13.Phang P T. Total mesorectal excision: technical aspects. Can J Surg. 2004;47(2):130–137. [PMC free article] [PubMed] [Google Scholar]
- 14.West N P, Hohenberger W, Finan P J, Quirke P. Mesocolic plane surgery: an old but forgotten technique? Colorectal Dis. 2009;11(9):988–989. doi: 10.1111/j.1463-1318.2009.01968.x. [DOI] [PubMed] [Google Scholar]
- 15.Akiyoshi T Ueno M Watanabe T Laparoscopic total mesorectal excision for rectal cancer: is it the predictive factor for incomplete mesorectal excision? Ann Surg 20112545835–836., author reply 836 [DOI] [PubMed] [Google Scholar]
- 16.Baik S H, Kim N K, Lim D R, Hur H, Min B S, Lee K Y. Oncologic outcomes and perioperative clinicopathologic results after robot-assisted tumor-specific mesorectal excision for rectal cancer. Ann Surg Oncol. 2013;20(8):2625–2632. doi: 10.1245/s10434-013-2895-8. [DOI] [PubMed] [Google Scholar]
- 17.Bondeven P, Hagemann-Madsen R H, Laurberg S, Pedersen B G. Extent and completeness of mesorectal excision evaluated by postoperative magnetic resonance imaging. Br J Surg. 2013;100(10):1357–1367. doi: 10.1002/bjs.9225. [DOI] [PubMed] [Google Scholar]
- 18.Kauff D W, Kempski O, Huppert S. et al. Total mesorectal excision—does the choice of dissection technique have an impact on pelvic autonomic nerve preservation? J Gastrointest Surg. 2012;16(6):1218–1224. doi: 10.1007/s11605-012-1870-1. [DOI] [PubMed] [Google Scholar]
- 19.Quirke P, Steele R, Monson J. et al. MRC CR07/NCIC-CTG CO16 Trial Investigators; NCRI Colorectal Cancer Study Group. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet. 2009;373(9666):821–828. doi: 10.1016/S0140-6736(09)60485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart D B, Dietz D W. Total mesorectal excision: what are we doing? Clin Colon Rectal Surg. 2007;20(3):190–202. doi: 10.1055/s-2007-984863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baik S H, Kim N K, Lee K Y. et al. Factors influencing pathologic results after total mesorectal excision for rectal cancer: analysis of consecutive 100 cases. Ann Surg Oncol. 2008;15(3):721–728. doi: 10.1245/s10434-007-9706-z. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein N S, Soman A, Sacksner J. Disparate surgical margin lengths of colorectal resection specimens between in vivo and in vitro measurements. The effects of surgical resection and formalin fixation on organ shrinkage. Am J Clin Pathol. 1999;111(3):349–351. doi: 10.1093/ajcp/111.3.349. [DOI] [PubMed] [Google Scholar]
- 23.Nagtegaal I D, van de Velde C J, van der Worp E, Kapiteijn E, Quirke P, van Krieken J H. Cooperative Clinical Investigators of the Dutch Colorectal Cancer Group. Macroscopic evaluation of rectal cancer resection specimen: clinical significance of the pathologist in quality control. J Clin Oncol. 2002;20(7):1729–1734. doi: 10.1200/JCO.2002.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Parfitt J R, Driman D K. The total mesorectal excision specimen for rectal cancer: a review of its pathological assessment. J Clin Pathol. 2007;60(8):849–855. doi: 10.1136/jcp.2006.043802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith N J, Shihab O, Arnaout A, Swift R I, Brown G. MRI for detection of extramural vascular invasion in rectal cancer. AJR Am J Roentgenol. 2008;191(5):1517–1522. doi: 10.2214/AJR.08.1298. [DOI] [PubMed] [Google Scholar]
- 26.Park J S, Jang Y J, Choi G S. et al. Accuracy of preoperative MRI in predicting pathology stage in rectal cancers: node-for-node matched histopathology validation of MRI features. Dis Colon Rectum. 2014;57(1):32–38. doi: 10.1097/DCR.0000000000000004. [DOI] [PubMed] [Google Scholar]
- 27.Sprenger T, Rothe H, Becker H. et al. Lymph node metastases in rectal cancer after preoperative radiochemotherapy: impact of intramesorectal distribution and residual micrometastatic involvement. Am J Surg Pathol. 2013;37(8):1283–1289. doi: 10.1097/PAS.0b013e3182886ced. [DOI] [PubMed] [Google Scholar]
- 28.Madbouly K M, Abbas K S, Hussein A M. Metastatic lymph node ratio in stage III rectal carcinoma is a valuable prognostic factor even with less than 12 lymph nodes retrieved: a prospective study. Am J Surg. 2014;207(6):824–831. doi: 10.1016/j.amjsurg.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 29.Quirke P, Williams G T, Ectors N, Ensari A, Piard F, Nagtegaal I. The future of the TNM staging system in colorectal cancer: time for a debate? Lancet Oncol. 2007;8(7):651–657. doi: 10.1016/S1470-2045(07)70205-X. [DOI] [PubMed] [Google Scholar]
- 30.Edge S Byrd D R Compton C C Fritz A G Greene F L Trotti A eds. AJCC Cancer Staging Manual 7th ed. New York: Springer; 2010 [Google Scholar]
- 31.Sobin L H Gospodarowicz M K Wittekind C eds. TNM Classification of Malignant Tumors 7th ed. New York: Wiley-Blackwell; 2009 [Google Scholar]
- 32.Moon S H, Kim D Y, Park J W. et al. Can the new American Joint Committee on Cancer staging system predict survival in rectal cancer patients treated with curative surgery following preoperative chemoradiotherapy? Cancer. 2012;118(20):4961–4968. doi: 10.1002/cncr.27507. [DOI] [PubMed] [Google Scholar]
- 33.Dieguez A. Rectal cancer staging: focus on the prognostic significance of the findings described by high-resolution magnetic resonance imaging. Cancer Imaging. 2013;13(2):277–297. doi: 10.1102/1470-7330.2013.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boutros M, Hippalgaonkar N, Silva E, Allende D, Wexner S D, Berho M. Laparoscopic resection of rectal cancer results in higher lymph node yield and better short-term outcomes than open surgery: a large single-center comparative study. Dis Colon Rectum. 2013;56(6):679–688. doi: 10.1097/DCR.0b013e318287c594. [DOI] [PubMed] [Google Scholar]
- 35.Tayyab M, Sharma A, Macdonald A W, Gunn J, Hartley J E, Monson J R. Prognostic significance of lymph node ratio in patients undergoing abdominoperineal resection of rectum. Langenbecks Arch Surg. 2012;397(7):1053–1057. doi: 10.1007/s00423-012-0986-9. [DOI] [PubMed] [Google Scholar]
- 36.Nagtegaal I D, Quirke P, Schmoll H J. Has the new TNM classification for colorectal cancer improved care? Nat Rev Clin Oncol. 2012;9(2):119–123. doi: 10.1038/nrclinonc.2011.157. [DOI] [PubMed] [Google Scholar]
- 37.Nagtegaal I D, Tot T, Jayne D G. et al. Lymph nodes, tumor deposits, and TNM: are we getting better? J Clin Oncol. 2011;29(18):2487–2492. doi: 10.1200/JCO.2011.34.6429. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, Lv L, Ye Y, Jiang K, Shen Z, Wang S. Comparison of metastatic lymph node ratio staging system with the 7th AJCC system for colorectal cancer. J Cancer Res Clin Oncol. 2013;139(11):1947–1953. doi: 10.1007/s00432-013-1525-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le Voyer T E, Sigurdson E R, Hanlon A L. et al. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol. 2003;21(15):2912–2919. doi: 10.1200/JCO.2003.05.062. [DOI] [PubMed] [Google Scholar]
- 40.Mathis K L, Green E M, Sargent D J, Delaney C, Simmang C L, Nelson H. Surgical quality surrogates do not predict colon cancer survival in the setting of technical credentialing: a report from the prospective COST trial. Ann Surg. 2013;257(1):102–107. doi: 10.1097/SLA.0b013e318260a8e6. [DOI] [PubMed] [Google Scholar]
- 41.Rhoads K F, Ackerson L K, Ngo J V, Gray-Hazard F K, Subramanian S V, Dudley R A. Adequacy of lymph node examination in colorectal surgery: contribution of the hospital versus the surgeon. Med Care. 2013;51(12):1055–1062. doi: 10.1097/MLR.0b013e3182a53d72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blenkinsopp W K, Stewart-Brown S, Blesovsky L, Kearney G, Fielding L P. Histopathology reporting in large bowel cancer. J Clin Pathol. 1981;34(5):509–513. doi: 10.1136/jcp.34.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagtegaal I D, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol. 2008;26(2):303–312. doi: 10.1200/JCO.2007.12.7027. [DOI] [PubMed] [Google Scholar]
- 44.Pucci M J, Beekley A C. Use of robotics in colon and rectal surgery. Clin Colon Rectal Surg. 2013;26(1):39–46. doi: 10.1055/s-0033-1333660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White M, Foulis A K, Smith G, Horgan P G, Roxburgh C S. The role of S100 staining in the pathological assessment of perineural invasion in rectal cancer. Colorectal Dis. 2014;16(1):71–72. doi: 10.1111/codi.12471. [DOI] [PubMed] [Google Scholar]
- 46.Compton C C. Colorectal carcinoma: diagnostic, prognostic, and molecular features. Mod Pathol. 2003;16(4):376–388. doi: 10.1097/01.MP.0000062859.46942.93. [DOI] [PubMed] [Google Scholar]
- 47.Smith N J, Barbachano Y, Norman A R, Swift R I, Abulafi A M, Brown G. Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg. 2008;95(2):229–236. doi: 10.1002/bjs.5917. [DOI] [PubMed] [Google Scholar]
- 48.Koh D M, Smith N J, Swift R I, Brown G. The relationship between MR demonstration of extramural venous invasion and nodal disease in rectal cancer. Clin Med Oncol. 2008;2:267–273. doi: 10.4137/cmo.s370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martling A, Singnomklao T, Holm T, Rutqvist L E, Cedermark B. Prognostic significance of both surgical and pathological assessment of curative resection for rectal cancer. Br J Surg. 2004;91(8):1040–1045. doi: 10.1002/bjs.4557. [DOI] [PubMed] [Google Scholar]
- 50.Roxburgh C S, McMillan D C, Anderson J H, McKee R F, Horgan P G, Foulis A K. Elastica staining for venous invasion results in superior prediction of cancer-specific survival in colorectal cancer. Ann Surg. 2010;252(6):989–997. doi: 10.1097/SLA.0b013e3181f1c60d. [DOI] [PubMed] [Google Scholar]
- 51.Taylor F G, Swift R I, Blomqvist L, Brown G. A systematic approach to the interpretation of preoperative staging MRI for rectal cancer. AJR Am J Roentgenol. 2008;191(6):1827–1835. doi: 10.2214/AJR.08.1004. [DOI] [PubMed] [Google Scholar]
- 52.Vass D G, Ainsworth R, Anderson J H, Murray D, Foulis A K. The value of an elastic tissue stain in detecting venous invasion in colorectal cancer. J Clin Pathol. 2004;57(7):769–772. doi: 10.1136/jcp.2003.015826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Messenger D E, Driman D K, McLeod R S, Riddell R H, Kirsch R. Current practice patterns among pathologists in the assessment of venous invasion in colorectal cancer. J Clin Pathol. 2011;64(11):983–989. doi: 10.1136/jclinpath-2011-200156. [DOI] [PubMed] [Google Scholar]
- 54.Turner R R, Li C, Compton C C. Newer pathologic assessment techniques for colorectal carcinoma. Clin Cancer Res. 2007;13(22, Pt 2):6871s–6876s. doi: 10.1158/1078-0432.CCR-07-1151. [DOI] [PubMed] [Google Scholar]
- 55.Demetter P, Vandendael T, Sempoux C. et al. PROCARE. Need for objective and reproducible criteria in histopathological assessment of total mesorectal excision specimens: lessons from a national improvement project. Colorectal Dis. 2013;15(11):1351–1358. doi: 10.1111/codi.12362. [DOI] [PubMed] [Google Scholar]
- 56.Zlobec I, Hädrich M, Dawson H. et al. Intratumoural budding (ITB) in preoperative biopsies predicts the presence of lymph node and distant metastases in colon and rectal cancer patients. Br J Cancer. 2014;110(4):1008–1013. doi: 10.1038/bjc.2013.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vallböhmer D, Bollschweiler E, Brabender J. et al. Evaluation of histological regression grading systems in the neoadjuvant therapy of rectal cancer: do they have prognostic impact? Int J Colorectal Dis. 2012;27(10):1295–1301. doi: 10.1007/s00384-012-1487-6. [DOI] [PubMed] [Google Scholar]
- 58.Chetty R, Gill P, Govender D. et al. International study group on rectal cancer regression grading: interobserver variability with commonly used regression grading systems. Hum Pathol. 2012;43(11):1917–1923. doi: 10.1016/j.humpath.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 59.Lim S B, Hong S M, Yu C S. et al. Prevalence and clinical significance of acellular mucin in locally advanced rectal cancer patients showing pathologic complete response to preoperative chemoradiotherapy. Am J Surg Pathol. 2013;37(1):47–52. doi: 10.1097/PAS.0b013e3182657186. [DOI] [PubMed] [Google Scholar]
- 60.Duldulao M P, Lee W, Streja L. et al. Distribution of residual cancer cells in the bowel wall after neoadjuvant chemoradiation in patients with rectal cancer. Dis Colon Rectum. 2013;56(2):142–149. doi: 10.1097/DCR.0b013e31827541e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zorcolo L, Rosman A S, Restivo A. et al. Complete pathologic response after combined modality treatment for rectal cancer and long-term survival: a meta-analysis. Ann Surg Oncol. 2012;19(9):2822–2832. doi: 10.1245/s10434-011-2209-y. [DOI] [PubMed] [Google Scholar]
- 62.Lee Y C, Hsieh C C, Chuang J P. Prognostic significance of partial tumor regression after preoperative chemoradiotherapy for rectal cancer: a meta-analysis. Dis Colon Rectum. 2013;56(9):1093–1101. doi: 10.1097/DCR.0b013e318298e36b. [DOI] [PubMed] [Google Scholar]
- 63.Hutchins G, Southward K, Handley K. et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol. 2011;29(10):1261–1270. doi: 10.1200/JCO.2010.30.1366. [DOI] [PubMed] [Google Scholar]
- 64.Bae J M, Kim J H, Cho N Y, Kim T Y, Kang G H. Prognostic implication of the CpG island methylator phenotype in colorectal cancers depends on tumour location. Br J Cancer. 2013;109(4):1004–1012. doi: 10.1038/bjc.2013.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Sousa E Melo F, Wang X, Jansen M. et al. Poor-prognosis colon cancer is defined by a molecularly distinct subtype and develops from serrated precursor lesions. Nat Med. 2013;19(5):614–618. doi: 10.1038/nm.3174. [DOI] [PubMed] [Google Scholar]
- 66.Guastadisegni C, Colafranceschi M, Ottini L, Dogliotti E. Microsatellite instability as a marker of prognosis and response to therapy: a meta-analysis of colorectal cancer survival data. Eur J Cancer. 2010;46(15):2788–2798. doi: 10.1016/j.ejca.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 67.Amajoyi R Lee Y Recio P J Kondylis P D Neoadjuvant therapy for rectal cancer decreases the number of lymph nodes harvested in operative specimens Am J Surg 20132053289–292., discussion 292 [DOI] [PubMed] [Google Scholar]
- 68.Birbeck K F, Macklin C P, Tiffin N J. et al. Rates of circumferential resection margin involvement vary between surgeons and predict outcomes in rectal cancer surgery. Ann Surg. 2002;235(4):449–457. doi: 10.1097/00000658-200204000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wittekind C, Compton C, Quirke P. et al. A uniform residual tumor (R) classification: integration of the R classification and the circumferential margin status. Cancer. 2009;115(15):3483–3488. doi: 10.1002/cncr.24320. [DOI] [PubMed] [Google Scholar]
- 70.Nagtegaal I D, Marijnen C A, Kranenbarg E K, van de Velde C J, van Krieken J H. Pathology Review Committee; Cooperative Clinical Investigators. Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: not one millimeter but two millimeters is the limit. Am J Surg Pathol. 2002;26(3):350–357. doi: 10.1097/00000478-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 71.Miller E D, Robb B W, Cummings O W, Johnstone P A. The effects of preoperative chemoradiotherapy on lymph node sampling in rectal cancer. Dis Colon Rectum. 2012;55(9):1002–1007. doi: 10.1097/DCR.0b013e3182536d70. [DOI] [PubMed] [Google Scholar]
- 72.Kidner T B Ozao-Choy J J Yoon J Bilchik A J Should quality measures for lymph node dissection in colon cancer be extrapolated to rectal cancer? Am J Surg 20122046843–847., discussion 847–848 [DOI] [PubMed] [Google Scholar]
- 73.Vather R, Sammour T, Kahokehr A, Connolly A, Hill A. Quantitative lymph node evaluation as an independent marker of long-term prognosis in stage III rectal cancer. ANZ J Surg. 2011;81(12):883–888. doi: 10.1111/j.1445-2197.2010.05595.x. [DOI] [PubMed] [Google Scholar]
- 74.Erlenbach-Wünsch K, Semrau S, Fietkau R. et al. ypN0 nodal status after neoadjuvant chemoradiotherapy for rectal carcinoma is not associated with adverse prognosis as compared with pN0 after primary surgery. Int J Colorectal Dis. 2014;29(2):231–237. doi: 10.1007/s00384-013-1790-x. [DOI] [PubMed] [Google Scholar]


