Abstract
Studies that have used structural magnetic resonance imaging (MRI) suggest that individuals with psychoses have brain alterations, particularly in frontal and temporal cortices, and in the white matter tracts that connect them. Furthermore, these studies suggest that brain alterations may be particularly prominent, already at illness onset, in those individuals more likely to have poorer outcomes (eg, higher number of hospital admissions, and poorer symptom remission, level of functioning, and response to the first treatment with antipsychotic drugs). The fact that, even when present, these brain alterations are subtle and distributed in nature, has limited, until now, the utility of MRI in the clinical management of these disorders. More recently, MRI approaches, such as machine learning, have suggested that these neuroanatomical biomarkers can be used for direct clinical benefits. For example, using support vector machine, MRI data obtained at illness onset have been used to predict, with significant accuracy, whether a specific individual is likely to experience a remission of symptoms later on in the course of the illness. Taken together, this evidence suggests that validated, strong neuroanatomical markers could be used not only to inform tailored intervention strategies in a single individual, but also to allow patient stratification in clinical trials for new treatments.
Keywords: first-episode schizophrenia, magnetic resonance imaging, psychosis
Abstract
Los estudios que han empleado imágenes de resonancia magnética estructural (IRM) sugieren que los sujetos con psicosis tienen alteraciones cerebrates, especialmente en las cortezas frontal y temporal, y en los tractos de sustancia blanca que las conecta. Además, dichos estudios sugieren que las alteraciones cerebrales pueden ser muy importantes, ya al inicio de la enfermedad, en aquellos individuos con más probabilidades de tener peor pronóstico (por ejemplo, mayor número de hospitalizaciones, peor remisión sintomática, menor nivel de funcionamiento y peor respuesta al primer tratamiento con antipsicóticos). De hecho, cuando estas alteraciones cerebrales están presentes, ellas son leves y se distribuyen en cualquier parte, por lo que hasta la fecha la utilidad de la IRM es limitada en el manejo clínico de estos trastornos. Recientemente los planteamientos de la IRM, a partir de las técnicas aprendízaje máquina, han sugerido que estos biomarcadores neuroanatómicos pueden ser empleados con beneficíos clínicos directos. Por ejemplo, empleando las técnicas de máquinas soporte de vectores para regresión, los datos obtenidos con IRM al comienzo de la enfermedad han sido útiles para predetir, con precisión significativa, si un sujeto determinado puede experimentar más adelante una remisión sintomática durante el curso de la enfermedad. En su conjunto esta evidencia sugiere que los marcadores bien establecidos y validados podrían emplearse no solo para informar sobre estrategias de intervención a la medida en un paciente individual, sino también para asignar la estratificación de pacientes en ensayos clínicos de nuevos tratamientos.
Abstract
Selon les études de la structure cérébrale avec l'imagerie par résonance magnétique (IRM), les sujets psychotiques présentent des modifications, particulièrement dans les cortex frontal et pariétal, et dans les faisceaux de substance blanche qui les relient. Au début de la maladie, ces modifications seraient prédominates chez les sujets plus susceptibles d'évoluer défavorablement (par exemple, un plus grand nombre d'hospitalisations, une moins bonne rémission des symptômes, un niveau de fonctionnement et une réponse moins bons au premier traitement par antipsychotiques). Le fait que ces modifications cérébrales, même lorsqu'elles sont présentes, sont discrètes et de nature diffuse a limité jusqu'à présent l'utilité de l'IRM dans la prise en charge clinique de ces maladies. Plus récemment, des techniques d'IRM comme celles fondées sur un auto-apprentissage par la machine, ont suggéré que ces biomarqueurs neuro-anatomiques peuvent être utilisés en vue de bénéfices cliniques. Par exemple, à l'aide d'une machine à vecteurs de support, les données IRM obtenues au début de la maladie ont permis de prédire, avec une précision significative, chez un patient donné, la probabilité d'une rémission plus ou moins tardive des symptômes au cours de l'évolution de la maladie. L'ensemble de ces données suggèrent que des marqueurs neuro-anatomiques validés seraient utiles non seulement pour individualiser le traitement chez un patient donné, mais aussi pour stratifier les patients dans des groupes lors des études cliniques de nouveaux traitements.
Introduction
Why have we not been able to use neuroimaging in the clinical management of psychoses? After 30 years of using this approach, we have established that structural brain alterations are present in individuals with psychoses. While this information has advanced academic knowledge on the pathophysiology of these disorders, it has had limited utility in clinical practice. The main reason can be found in the nature of brain alterations in psychoses: they are subtle and spatially distributed. Still, the time may now have come when quick and noninvasive structural neuroimaging abandons the academic solitude in which it has been operating, and brings its novel methods and findings into the clinical arena. This paper aims to appraise this evidence, and discuss it in the context of potential future applications of magnetic resonance imaging (MRI) in the clinical management of psychoses.
The first part of this review will present evidence on the presence of neuroanatomical alterations in psychoses. It will then discuss studies that have examined the relationship between brain alterations and various medium- to long-term clinical and functional outcomes. In the third section, it will discuss more recent evidence on the relationship between brain alterations and early outcomes, with a particular focus on the relationship between symptomatic responses and initial treatment with antipsychotic drugs. The paper will then present a discussion of more novel approaches for the analysis of MRI data, such as support vector machine. Findings from these approaches have started to suggest that MRI has the potential to provide clinically meaningful information. Finally, it will discuss the implications of having strong and valid neuroimaging markers of psychosis outcomes in developing targeted, as well as novel, treatment interventions for these disorders.
Structural brain alterations in psychosis
Moving from the first evidence that individuals with established schizophrenia have subtle structural brain alterations, most notably an enlargement of the lateral ventricles, we have been studying individuals earlier and earlier along the disease course. More than 10 years ago, the seminal work by Pantelis and colleagues1 showed that individuals at high risk of developing psychosis, but not ill yet, already show smaller volumes of the same brain areas that have been reported as smaller in individuals with an established psychosis, namely frontal and temporal cortices.1 Even more novel, however, was their report that this is particularly the case for individuals who then actually develop the illness, and that some of these alterations could be specific to the type of psychosis that these subjects developed.1,2 For example, they found that subjects who develop an affective type of psychosis showed reductions in the subgenual anterior cingulate cortex, an area involved in the generation of affective symptoms. Complementary to these gray-matter changes, ultra-high-risk individuals also have white matter alterations similar to, albeit less extensive than, first-episode patients. These include poorer integrity of the major associative fibers that connect fronto-parieto-temporal (superior longitudinal fasciculus) and fronto-parieto-occipital (inferior fronto-occipital fasciculus) regions, commissural fibers (corpus callosum), and cortico-subcortical pathways (corona radiata, corticospinal tract, and corticopontine tract).3 These findings, integrated with evidence from many reviews and meta-analyses, suggest that brain alterations become more extensive at the time of a first full psychotic episode, and then possibly even more marked over the years, when the illness becomes established.4-6 Still, even in later stages, alterations remain subtle and distributed. These are indeed two main factors that have limited our ability to use this information about brain alterations in the clinical management of the individual patient.
Therefore, many studies have tried to establish whether these distributed brain changes are more likely to occur in individuals with a more severe illness course or a worse clinical and functional outcome. The definitions for course and outcome have varied across studies. For example, a poorer clinical outcome has been variously defined as response to early treatment, number of subsequent episodes, severity of symptoms, hospitalizations, or duration of remissions; while functional outcome has been defined as the ability to live independently, maintain employment, or be in a relationship. Nevertheless, many studies have tried to establish if structural brain alterations are related to illness characteristics by comparing brain structure in patients and healthy controls and then evaluating the relationship with outcomes at a single time point; or conducting repeated evaluations of brain structure, and then investigating the relationship between longitudinal brain changes and outcomes. Most of these studies have evaluated the relationship with medium to long-term outcomes, and only more recently has the attention shifted to the evaluation of the relationship between brain alterations and early outcomes such as response to the first treatment. The next two sections will cover this evidence in the same chronological order.
Brain structure and its relationship with medium- to long-term clinical and functional outcomes
Studies that have evaluated brain structure at the time of illness onset and again following treatment, have often reported smaller volumes of gray matter in association with indicators of subsequent poorer clinical outcomes (eg, higher number of admissions or worse symptom severity), and with worse social or occupational outcomes. For example, smaller volumes of prefrontal areas have been found to predict poorer functioning (defined as a combination of social, occupational, and psychological functioning) 1 year later in a small sample of first-episode psychosis patients.7 Similarly, in a sample of patients with schizophrenia, drawn from an unselected general population sample, Jääskeläinen and colleagues8 found that individuals showing higher density (an indirect measure of volume) of frontal and limbic areas had an overall better clinical (defined as a lower number of hospitalizations) and functional (defined as not being on a disability pension) outcome in the subsequent 16 years. Wassink and colleagues9 reported an association between smaller volumes of another brain area, the cerebellum, and longer negative and psychotic symptom duration, as well as psychosocial impairment (eg, quality of relationships, sexual activity, enjoyment of recreation, and work performance) in individuals with schizophrenia spectrum disorders after a 7-year follow-up. Still, others have not found an association between brain volumes at onset and subsequent outcomes. For example, the prospective study by van Ilaren and colleagues10 found no relationship between brain volume measurements and symptom severity, level of functioning (defined as the ability to maintain a variety of social roles), need for care, and illness course after 2 years. White matter has also been evaluated as a marker of poor outcome. Mitelman and colleagues11 examined white matter integrity using diffusion tensor imaging (DTI), in a group of 104 patients with an average 4-year schizophrenia duration. They classified them as having a poor or good outcome, based on both symptom severity and level of functioning at this time point. They found that although both patient groups showed reduced overall white matter integrity of the prefrontal and temporal areas (as a measure of fractional anisotropy [FA]), these alterations were more extensive in patients with a poor outcome than in those with a good outcome.
Longitudinal studies that have evaluated brain structure at multiple time points after illness onset can help clarify if brain alterations, evident at illness onset, progress differently in individuals who then developed a poorer outcome. For example, a larger decrease in the volume of the lingual gyrus, insula, and cerebellum has been reported in patients with a worse functional outcome (a combination of social, occupational, and psychological functioning) 4 years after the first psychotic episode.12 With a similar duration of follow-up, van Ilaren and colleagues also reported a greater gray matter volume reduction, and a greater thinning of temporal and frontal cortices in individuals who had poorer outcomes (including a higher number of hospitalizations and a lower level of social and personal functioning) 5 years after illness onset.13-14 Similarly, in a sample of children and adolescents with early onset first-episode schizophrenia, Arango and colleagues15 showed that more weeks of hospitalization and higher severity of negative symptoms were correlated with greater left frontal gray matter volume loss and greater cerebral spinal fluid increase, respectively. Dynamic changes in relation to outcomes were also observed by our group in a 6-year follow-up study of hippocampal volume in first-episode psychosis patients.16 In this sample, we specifically found that individuals who had an increase in hippocampal volume over the first 6 years of illness were the ones who showed a less severe illness course and lower symptom severity, as well as a better functional outcome at follow-up, thus suggesting an important role for brain neuroplasticity.
Taken together, these findings suggest that brain alterations are present at illness onset and may be associated with worse medium- to long-term illness outcomes and ability to function. Furthermore, brain alterations may particularly progress in individuals with more severe outcomes. Still, two important considerations need to be made in the interpretation of these findings. First, it is not possible to establish what the casual link is between brain structure and illness course. While brain alterations could be the substrate of a more severe illness, the more severe illness itself could affect brain plasticity by increasing exposure to substance abuse, worse living conditions, or a less stimulating environment. Second, these patients are all treated after illness onset. It is, therefore, possible that patients with a more severe illness are exposed to longer periods of treatment or higher doses of antipsychotics, which may themselves affect brain volumes.17 From a clinical perspective, it may be more informative to study the relationship between brain alterations at illness onset and outcome measures that occur earlier in the course of illness, which is the topic of the next section.
Brain structure and its relationship with early outcomes
If brain alterations could be used to predict, as early as possible, which patients are destined to have a poorer response to the first few weeks or months of treatment with antipsychotic medications (the main stake for the treatment of psychosis), we could implement interventional strategies targeted at this specific subgroup. Currently, we treat the first psychotic episode on a trialand-error basis, and we simply have no way of saying who will respond to the first antipsychotic drug and who might benefit from longer or different interventions. In fact, we know that only -≈55% of patients respond to antipsychotics in the first 12 months of treatment.18 Yet, this early response is thought to be one of the strongest predictors of subsequent functional and clinical outcomes in psychosis.19,20
A pooled analysis, published in 1992, examined the relationship between response to antipsychotics and brain measures, such as ventricular-brain ratio, sulcal prominence, third ventricles, and other brain measures.21 The authors found that the composite effect sizes were very small and did not reach statistical significance. However, recent studies have lent renewed support to the notion that alterations in both gray and white matter may be associated with a poorer response to antipsychotics, possibly because they used more homogeneous populations, or in some cases, better imaging acquisition approaches and more sophisticated methods of analysis. For example, Zipursky and colleagues22 examined global tissue volumes in firstepisode psychosis patients, and found that subjects with a poorer response (defined as having a lower reduction in symptom severity from baseline) to the antipsychotic haloperidol in the first month of treatment had smaller cortical gray matter volume than those who showed symptomatic improvement. Relatively consistent data have been reported on the relationship between response to clozapine and brain structure. Cortical measures, such as sulcal enlargement, particularly in the prefrontal cortex, have been frequently related to poor response to clozapine in patients with chronic schizophrenia.23-25 In a double-blind, 10-week trial of clozapine and haloperidol, Arango and colleagues26 found that a larger prefrontal gray matter volume was associated with better treatment response in clozapine-treated patients, but with a poor response in haloperidol- treated patients. They also found no relationship between response to these drugs and hippocampal and caudate nucleus volumes. The authors suggested that the presence of larger brain volumes might characterize patients who are more likely to benefit from clozapine. This finding is also consistent with evidence from a study in patients with treatment-resistant schizophrenia who were treated for with clozapine 6 months.27 Patients with a larger baseline volume of the dorsolateral-prefrontal cortex were more likely to show improvement in their negative symptoms. Interestingly, amelioration of positive symptoms was related to a larger temporal cortex volume, and amelioration of disorganization symptoms was inversely related to hippocampal volume. Another temporal area has been reported as being related to early outcome—the parahippocampal gyrus. Using voxel-based morphometry, Bodnar and colleagues28 found that the parahippocampal gyrus was significantly smaller in first-episode psychosis patients who, after 6 months of antipsychotic treatment, had not remitted, compared with those who had achieved remission. Furthermore, by building a classification model using parahippocampal gray matter concentration, they were able to correctly classify remission status in 79% of the cases. In another study of patients with established schizophrenia treated with olanzapine or risperidone, 3-week response to these antipsychotics was associated with smaller volumes of other areas, such as the insula and rectal gyrus, and larger volumes of the basal ganglia.29
White matter has also been associated with treatment response, but the evidence is considerably more scant. Response to antipsychotics was evaluated by Garver and colleagues in a small sample of patients with schizophrenia after 28 days of treatment.30 Here, somewhat counterintuitively, the 8 patients who responded to antipsychotics showed worse white matter microstructural integrity than the 5 patients who did not respond to medication. Of note, individuals, in this study, had an established illness, and therefore, had taken other antipsychotics prior to this treatment. In contrast, studies in antipsychotic naive or minimally treated patients have the advantage that any structural brain alterations would be less likely to reflect the effect of antipsychotics on brain structure,17 and more likely to be a marker of illness. Luck and colleagues31 analyzed a sample of first-episode psychosis patients using DTI, and then reassessed them at 6 months, which is when they classified them as having either a poor or good treatment outcome. They specifically examined three white matter tracts connecting frontal and temporal regions: the cingulum, the superior longitudinal fasciculus, and the uncinate fasciculus. Patients with a poor outcome showed greater alterations in white matter integrity in the superior longitudinal fasciculus and uncinate than patients with a good outcome, suggesting that abnormal fronto-temporal connectivity may represent an early marker of short-term clinical outcomes.
Our group has also recently focused on the evaluation of the relationship between neuroanatomical markers in white and gray matter at the time of presentation, and response to treatment at 12 weeks.32,33 This is a time when all patients would have received at least one full therapeutic course of antipsychotic medication. We examined white matter integrity, using DTI and tractbased spatial statistics, to assess FA in a large sample of patients at their first episode of any psychosis.33 We operationalized response to treatment as having achieved symptomatic remission according to the criteria of the Schizophrenia Working Group.34 We found that already at illness onset, patients who subsequently did not respond (n=40) had lower FA than healthy controls in the uncinate, cingulum, and corpus callosum. Furthermore, they also had lower FA in these regions than patients who responded to treatment (n=40; Figure 1, Tables I and II).
Figure 1. White matter maps of differences between nonresponders and responders. The figure shows areas of significantly decreased fractional anisotropy in nonresponders when compared with responders, at baseline (A) (P<0.05; Family-Wise Error [FEW]-corrected) and at 1 2-week follow-up (B) (P<0.05; FWE-corrected). Fractional anisotropy white matter skeleton is represented by green voxels displayed on a background image that corresponds to the mean fractional anisotropy image in standard Montreal Neurological Institute (MNI) MNI152 brain space (radiological view), with the coordinate number on top. Red-yellow voxels represent regions in which the fractional anisotropy was significantly lower in the nonresponder group relative to the responder group. From reference 33: Reis Marques TR, Taylor H, Chaddock CA, et al White matter integrity as predictor of response to treatment in first episode psychosis. Brain. 2014;137:172-182. © 2013, Oxford University Press.
Table I. White matter regions of fractional anisotropy reduction between nonresponders and responders at baseline. JHU, Johns Hopkins University; MNI, Montreal Neurological Institute. a Obtained by dividing the number of significant voxels within each regional mask by the total number of voxels in that mask. From reference 33: Reis Marques TR, Taylor H, Chaddock CA, et al White matter integrity as predictor of response to treatment in first episode psychosis. Brain. 2014;137:172-182. © 2013, Oxford University Press.
| JHU white matter atlas region | Significant cluster size (n° voxels) | Region significant (%)a | t-statistic | MNI coordinates of peak voxel (mm) | ||
| X | Y | Z | ||||
| Uncinate | ||||||
| Left | 70 | 91% | 3.38 | -33 | -6 | -14 |
| Right | 59 | 86% | 2.24 | 35 | -4 | -14 |
| Fornix | ||||||
| Left stria terminalis | 270 | 76% | 2.98 | -34 | -11 | -15 |
| Right stria terminalis | 11 | 4% | 2.64 | 34 | -11 | -15 |
| Corticospinal | ||||||
| Left | 203 | 75% | 1.92 | 9 | -29 | -23 |
| Right | 135 | 51% | 3.02 | 10 | -27 | -27 |
| Cerebral peduncle | ||||||
| Left | 436 | 72% | 2.35 | -9 | -29 | -20 |
| Right | 360 | 59% | 3.40 | 10 | -28 | -19 |
| External capsule | ||||||
| Right | 781 | 67% | 2.48 | 36 | -7 | -13 |
| Left | 790 | 61% | 4.36 | -35 | -8 | -13 |
| Internal capsule | ||||||
| Left posterior limb | 575 | 66% | 2.64 | -20 | -14 | 0 |
| Right anterior limb | 480 | 58% | 3.24 | 10 | 1 | 1 |
| Left retrolenticular limb | 372 | 47% | 2.44 | -24 | -19 | 0 |
| Left anterior limb | 361 | 43% | 3.00 | -13 | -1 | 4 |
| Corpus callosum | ||||||
| Body | 1985 | 63% | 2.70 | -13 | 17 | 24 |
| Genu | 1039 | 60% | 3.55 | -15 | 32 | 14 |
| Splenium | 1145 | 46% | 2.13 | 1 | -31 | 21 |
| Corona radiata | ||||||
| Right anterior | 1005 | 63% | 3.26 | 27 | 28 | 10 |
| Left anterior | 942 | 56% | 3.75 | -15 | 33 | 15 |
| Left posterior | 364 | 48% | 1.27 | -21 | -44 | 35 |
| Left superior | 578 | 42% | 2.36 | -22 | -25 | 36 |
| Right superior | 591 | 42% | 3.21 | 21 | -16 | 36 |
| Right posterior | 153 | 20% | 2.67 | 30 | -60 | 19 |
| Cerebellar peduncle | ||||||
| Left superior | 99 | 48% | 2.16 | -5 | -30 | -21 |
| Right superior | 90 | 36% | 2.46 | 7 | -31 | -16 |
| Left inferior | 66 | 33% | 1.97 | -13 | -45 | -32 |
| Middle | 753 | 33% | 3.19 | -23 | -46 | -36 |
| Pontine cross tract | 98 | 25% | 2.35 | -2 | -30 | -30 |
| Medial lemniscus | ||||||
| Left | 61 | 24% | 2.13 | -4 | -38 | -37 |
| Right | 30 | 12% | 2.10 | 6 | -34 | -25 |
| Superior frontal occipital | ||||||
| Right | 18 | 23% | 1.52 | 23 | 2 | 19 |
| Left | 9 | 10% | 1.45 | -21 | 5 | 19 |
Table II. White matter regions of fractional anisotropy reduction between nonresponders and responders at 12-weeks follow-up. JHU, Johns Hopkins University; MNI, Montreal Neurological Institute. -'Obtained by dividing the number of significant voxels within each regional mask by the total number of voxels in that mask. From reference 33: Reis Marques TR, Taylor H, Chaddock CA, et al White matter integrity as predictor of response to treatment in first episode psychosis. Brain. 2014;137:172-182. © 2013, Oxford University Press.
| JHU white matter atlas region | Significant cluster size (n° voxels) | Region significant (%)a | t-statistic | MNI coordinates of peak voxel (mm) | ||
| X | Y | Z | ||||
| Uncinate | ||||||
| Left | 58 | 81% | 2.48 | -34 | -6 | -14 |
| Right | 65 | 96% | 1.88 | 36 | -3 | -16 |
| External capsule | ||||||
| Left | 831 | 64% | 4.61 | -33 | -6 | -13 |
| Right | 341 | 29% | 2.55 | 36 | -7 | -13 |
| Corona radiata | ||||||
| Left anterior | 1014 | 59% | 2.70 | -15 | 36 | -1 |
| Right anterior | 978 | 59% | 3.49 | 23 | 19 | 18 |
| Left superior | 760 | 54% | 3.56 | -22 | -25 | 35 |
| Right superior | 292 | 21% | 3.47 | 23 | -19 | 38 |
| Left posterior | 406 | 53% | 2.19 | -26 | -30 | 27 |
| Right posterior | 21 | 3% | 1.76 | 19 | -26 | 35 |
| Fornix | ||||||
| Left stria terminalis | 202 | 58% | 2.55 | -28 | -26 | -8 |
| Right stria terminalis | 10 | 3% | 2.63 | 34 | -11 | -15 |
| Internal capsule | ||||||
| Left anterior limb | 446 | 53% | 2.51 | -17 | 6 | 9 |
| Right anterior limb | 327 | 39% | 3.13 | 21 | 4 | 15 |
| Left retrolenticular limb | 286 | 36% | 2.01 | -26 | -24 | 8 |
| Left posterior limb | 160 | 18% | 1.42 | -22 | -9 | 11 |
| Right posterior limb | 88 | 10% | 2.25 | 20 | -4 | 10 |
| Corpus callosum | ||||||
| Genu | 806 | 46% | 2.91 | -13 | 33 | -3 |
| Body | 725 | 23% | 3.01 | 15 | 17 | 25 |
| Splenium | 377 | 15% | 2.10 | -23 | -54 | 17 |
| Superior frontal occipital | ||||||
| Left | 34 | 35% | 2.25 | -21 | 14 | 19 |
| Right | 26 | 32% | 1.27 | 23 | 2 | 19 |
| Superior longitudinal fasciculus | ||||||
| Left | 331 | 24% | 2.36 | -35 | -25 | 35 |
| Right | 367 | 24% | 1.89 | 38 | -39 | 28 |
| Thalamic radiation | ||||||
| Left posterior | 207 | 19% | 1.73 | -28 | -53 | 18 |
| Inferior longitudinal fasciculus | ||||||
| Left sagittal stratum | 38 | 8% | 2.54 | -35 | -11 | -14 |
| Right sagittal stratum | 7 | 1% | 2.19 | 36 | -11 | -14 |
| Cingulum | ||||||
| Right | 25 | 7% | 2.30 | 10 | 17 | 26 |
One of the most striking findings of this work was that the responders were indistinguishable from the healthy control subjects, suggesting that the original clinical sample was biologically rather heterogeneous. We rescanned these subjects at the 12-week clinical evaluation, and found that the same regional differences seen at baseline between the two patient groups were also present at 12 weeks, although the differences appeared less widespread. Furthermore, although there was some increase in FA over time, this was in the same direction in the two patient groups, with no significant time vs group interaction. We interpreted these findings to indicate that white matter integrity and brain connectivity are important moderators of response to antipsychotics. It is intriguing to speculate whether these alterations are established early on, as a neurodevelopmental deviation. The presence of a neurodevelopmental alteration would find further support in our gray matter findings from this sample.32 In these individuals, we performed the first 3D evaluation of cortical gyrification, a marker potentially indicative of early neurodevelopmental disturbances,35,36 in relation to subsequent treatment response. Gyrification defects have been associated with exposure to obstetric complications37,38 and with psychotic symptoms resistant to treatment.39 Interestingly, the earlier neuroimaging studies in treatment-resistant patients that were mentioned in the previous section, found that sulcal enlargement (which could lead to hypogyria) was associated with a poorer response to clozapine.23,25 In our samples, we found that, already at illness onset, patients who subsequently did not respond to treatment had significant cortical folding defects (hypogyria) of several frontotemporal regions and the insula when compared with the responders. They also had widespread deficits in gyrification extending to the precuneus, angular gyrus, and lingual gyrus when compared with healthy controls (Figure 2, Table III).
Figure 2. Voxel clusters showing differences in gyrification among responders, nonresponders, and healthy controls. Clusters of spatially contiguous suprathreshold voxels indicating higher gyrification for each between-group comparison are displayed in red, yellow, and purple. There were no clusters of reduced gyrification in these between-group comparisons. All clusters are displayed on a reconstructed average white matter surface (fsaverage in the FreeSurfer software) and sun/ived multiple testing using Monte Carlo simulation with a cluster inclusion criterion of P=0.05. The left hemisphere is on the left side of the image, and the right hemisphere is on the right side. The exact values of dusterwise probability for the clusters are presented in Table III. From reference 32: Paianiyappan L, Marques TR, Taylor H, et al Cortical folding defects as markers of poor treatment response in first-episode psychosis. JAMA Psychiatry. 2013;70:1031-1040. Copyright © 2013, American Medical Association.
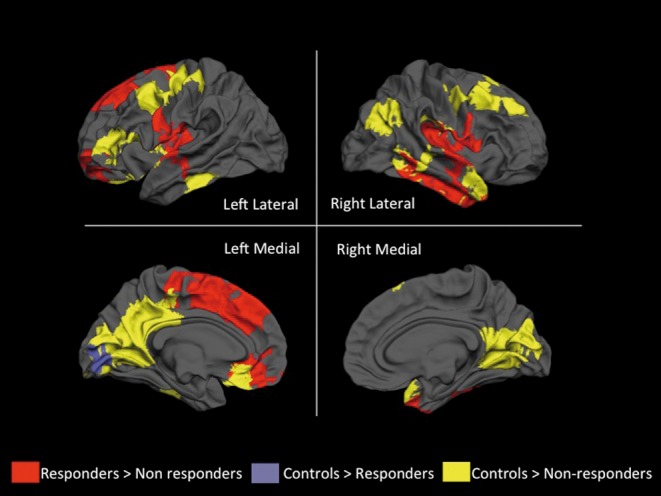
Table III. Group differences in gyrification among responders, nonresponders and healthy controls. The threshold for inclusion into a duster is P<0.05. From reference 33: Reis Marques TR, Taylor H, Chaddock CA, et al White matter integrity as predictor of response to treatment in first episode psychosis. Brain. 2014;137:172-182. © 2013, Oxford University Press.
| Cortical region | Talairach coordinates of the peak | Cluster size (mm2) | Clusterwise probability |
| Healthy controls > all patients | |||
| Left posterior cingulate/precuneus | -8,-40,41 | 8304 | 0.0001 |
| Left precentral | -41,-12,48 | 3589 | 0.0001 |
| Left middle/inferior frontal | -35,26,12 | 1167 | 0.0440 |
| Right mid die frontal cortex | 33,23,43 | 1304 | 0.0003 |
| Right inferior parietal | 45,-58,36 | 755 | 0.0364 |
| Responders > nonresponders | |||
| Left insula/central sulcus | -57,-8,10 | 3697 | 0.0001 |
| Right insuIa/lateral temporal | 37,-13,2 | 6703 | 0.0001 |
| Left superior frontal/mid-cingulate | -9,32,27 | 5080 | 0.0001 |
| Left middle frontal gyrus | -35,51,-1 | 2581 | 0.0001 |
| Healthy controls > nonresponders | |||
| Left posterior cingulate/precuneus | -9,-49,26 | 7219 | 0.0001 |
| Left precentral | -37,7,37 | 3775 | 0.0001 |
| Right superior/middle frontal | 20,33,34 | 3415 | 0.0001 |
| Right superior temporal | 53,-30,3 | 1811 | 0.0001 |
| Right cuneus/linguaI gyrus | 10,-58,2 | 4382 | 0.0001 |
| Right insula/lateral temporal | 37,-13,2 | 4502 | 0.0001 |
| Left middle frontal | -38,43,5 | 2330 | 0.0001 |
| Right angular gyrus | 45,-59,36 | 1415 | 0.0020 |
| Left inferior temporal | -53,-28,-24 | 792 | 0.244 |
| Left orbitofrontal | -33,20,-17 | 1077 | 0.0030 |
| Healthy controls > responders | |||
| Left lingual | -4,-82,1 | 862 | 0.0151 |
| Non-affective psychosis < affective psychosis | |||
| Right insula/superior temporal | 37,-12,2 | 5778 | 0.0001 |
| Right inferior temporal | 51,-27,-22 | 7245 | 0.0001 |
| Left insula/inferior frontal | -42,19,7 | 5870 | 0.0001 |
| Left middle frontal | -22,44,20 | 1037 | 0.0049 |
| Right subgenual anterior cingulate | 7,15,-17 | 1245 | 0.0007 |
| Right posterior cingulate/precuneus | 41,-81,6 | 1809 | 0.0001 |
| Left lateral occipitotemporal | -49,-62,-2 | 4116 | 0.0001 |
| Left posterior cingulate | -4,-,15,31 | 1310 | 0.0004 |
In contrast, and similarly to what we saw for white matter, patients who subsequently responded were virtually indistinguishable from the healthy controls. These findings are very consistent with the evidence described earlier that only the nonresponders have reduced integrity in the white matter tracts that connect these cortical regions.33 It is also interesting that the alterations in gyrification and white matter were evident in nonresponders with both affective (bipolar disorder or major depression with psychotic symptoms) and nonaffective (schizophrenia, schizophreniform disorder, schizoaffective disorder and psychosis not otherwise specified) psychosis, suggesting that as a group, nonresponders are likely to have a more homogeneous pathophysiological process underlying psychoses than the responders. Taken together, these findings suggest that individuals who are less likely to respond to treatment represent either a more severely affected group, or a group that has experienced a pathophysiological insult at an early neurodevelopmental stage than those who respond to treatment, possibly with a differential exposure to factors that determine axonal integrity (genetic or molecular), which, in turn, influences brain connectivity and gyrification.
Although crucial to our understanding of psychosis, these group findings need to be extended if they are to inform clinical outcomes at the level of a single individual.
Moving toward using brain structure at illness onset in the individual prediction of outcome
Structural imaging has strong potential for clinical applicability as it is: (i) noninvasive; (ii) quick to acquire; (iii) widely available; and finally, (iv) cheap compared with other imaging methods such as positron emission tomography. Therefore, what progress have we made toward its clinical applicability?
Over the last few years, the application of novel approaches to the analysis of structural imaging data has made us feel closer to their potential use in clinical management. To this end, machine-learning methods, such as support vector machine, have shown promise in differentiating patients or ultra-high-risk individuals from healthy controls, based on structural MRI data.40-42 Obviously, an even more interesting translational question would be: can we use structural MRI data at illness onset to predict which individuals will develop worse outcomes or poorer responses to treatment? About 2 years ago, our group published the first paper showing that structural brain scans at the first episode could be used to predict, with significant accuracy, outcomes at 6 years. In this study, we found that the MRI scan obtained when patients first presented to services could be used to predict which patients developed a continuous nonremitting illness course; distinguishing them from both healthy controls (sensitivity, 71; specificity, 61) and from patients who had an episodic, more benign illness (sensitivity, 71; specificity, 68). Consistent with our recent data, discussed in the previous section, we were not able to distinguish the patients who went on to have an episodic course from the healthy controls.43 Other papers have since confirmed, in different clinical populations, the potential of machine-learning approaches to discriminate individual patients in predicting the onset and subsequent severity of psychosis in ultra-high-risk individuals.44,45 While promising, these approaches need replication in larger samples of patients at the same illness stages and treated with the same pharmacological interventions, and also need validation for scans obtained using different scanners. These are just some of the aims of an ongoing, large, multicenter study funded by the FP7 Programme of the European Commission, OPTiMiSE (Optimization of Treatment and Management of Schizophrenia in Europe, www.optimisetrial.eu). Across various centers in Europe, we are acquiring MRI scans from a very large sample of patients with first-episode schizophrenia, who are then all treated with the same antipsychotic, amisulpride, for 4 weeks, when their symptomatic response is evaluated. This study will use both univariate and multivariate image analysis approaches, ie, support vector machine, to establish whether it is possible to predict the 4-week response based on the MRI scan obtained at the first episode, alone or in combination with, other biological markers, including magnetic resonance spectroscopy.
Conclusion
We know that the outcome and response to available treatments for schizophrenia, and for psychosis in general, is heterogeneous. Still, we do not have strong biological markers that could help us disentangle this heterogeneity and be useful in clinical practice. There are, however, structural brain alterations that, if refined, have potential for use early in the stratification of patients with psychosis. For this to be achieved, a number of steps still have to take place. For example, validation with very large datasets could help establish a reference group (a “databank”), which clinical imaging centers could access for an automated classification of their patients' MRIs in order to obtain an estimate of the likelihood of a specific outcome. For this to be achieved, it would be essential to have standardized definitions of outcomes within the clinical academic community that are relevant to clinicians, patients, and their careers.
Such a system could then be used to assign a patient to targeted assertive case management at first presentation to services, in the optimization of pharmacological treatment, or in cognitive and family interventions.
These have all been shown to improve treatment adherence and reduce relapse rates,46,47 eventually improving outcome. At the same time, they could help identify those patients most likely to have a good remitting illness after their first episode, who could then avoid long-term exposure to antipsychotic medication. Furthermore, the identification of gray matter and connectivity markers that characterize poor response poses the question of whether these markers could be used in patient stratification in clinical trials of new treatment strategies, or even to inform drug development of agents that can enhance and restore brain connectivity, which may elicit a better response in those who currently do not respond to available antipsychotics.
REFERENCES
- 1.Pantelis C., Velakoulis D., McGorry PD., et al Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 2.Dazzan P., Soulsby B., Mechelli A., et al Volumetric abnormalities predating the onset of schizophrenia and affective psychoses: an MRI study in subjects at ultrahigh risk of psychosis. Schizophr Bull. 2012;38:1083–1091. doi: 10.1093/schbul/sbr035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carletti F., Woolley JB., Bhattacharyya S., et al Alterations in white matter evident before the onset of psychosis. Schizophr Bull. 2012;38:1170–1179. doi: 10.1093/schbul/sbs053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellison-Wright I., Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res. 2009;108:3–10. doi: 10.1016/j.schres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 5.Ellison-Wright I., Glahn DC., Laird AR., Thelen SM., Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165:1015–1023. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fusar-Poli P., Radua J., McGuire P., Borgwardt S. Neuroanatomical maps of psychosis onset: voxel-wise meta-analysis of antipsychotic-naive VBM studies. Schizophr Bull. 2012;38:1297–1307. doi: 10.1093/schbul/sbr134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kasparek T., Prikryl R., Schwarz D., et al Gray matter morphology and the level of functioning in one-year follow-up of first-episode schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1438–1446. doi: 10.1016/j.pnpbp.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 8.Jääskeläinen E., Juola P., Kurtti J., et al Associations between brain morphology and outcome in schizophrenia in a general population sample. Eur Psychiatry. 2014;29:456–462. doi: 10.1016/j.eurpsy.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Wassink TH., Andreasen NC., Nopoulos P., Flaum M. Cerebellar morphology as a predictor of symptom and psychosocial outcome in schizophrenia. Biol Psychiatry. 1999;45:41–48. doi: 10.1016/s0006-3223(98)00175-9. [DOI] [PubMed] [Google Scholar]
- 10.van Haren NE., Cahn W., Hulshoff Pol HE., et al Brain volumes as predictor of outcome in recent-onset schizophrenia: a multi-center MRI study. Schizophr Res. 2003;64:41–52. doi: 10.1016/s0920-9964(03)00018-5. [DOI] [PubMed] [Google Scholar]
- 11.Mitelman SA., Newmark RE., Torosjan Y., et al White matter fractional anisotropy and outcome in schizophrenia. Schizophr Res. 2006;87:138–159. doi: 10.1016/j.schres.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Mane A., Falcon C., Mateos JJ., et al Progressive gray matter changes in first episode schizophrenia: a 4-year longitudinal magnetic resonance study using VBM. Schizophr Res. 2009;114:136–143. doi: 10.1016/j.schres.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 13.van Haren NE., Hulshoff Pol HE., Schnack HG., et al Focal gray matter changes in schizophrenia across the course of the illness: a 5-year follow-up study. Neuropsychopharmacology. 2007;32:2057–2066. doi: 10.1038/sj.npp.1301347. [DOI] [PubMed] [Google Scholar]
- 14.van Haren NE., Schnack HG., Cahn W., et al Changes in cortical thickness during the course of illness in schizophrenia. Arch Gen Psychiatry. 2011;68:871–880. doi: 10.1001/archgenpsychiatry.2011.88. [DOI] [PubMed] [Google Scholar]
- 15.Arango C., Rapado-Castro M., Reig S., et al Progressive brain changes in children and adolescents with first-episode psychosis. Arch Gen Psychiatry. 2012;69:16–26. doi: 10.1001/archgenpsychiatry.2011.150. [DOI] [PubMed] [Google Scholar]
- 16.Lappin JM., Morgan C., Chalavi S., et al Bilateral hippocampal increase following first-episode psychosis is associated with good clinical, functional and cognitive outcomes. Psychol Med. 2013:1–13. doi: 10.1017/S0033291713001712. [DOI] [PubMed] [Google Scholar]
- 17.Navari S., Dazzan P. Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings. Psychol Med. 2009;39:1763–1777. doi: 10.1017/S0033291709005315. [DOI] [PubMed] [Google Scholar]
- 18.Boter H., Peuskens J., Libiger J., et al EUFEST study group. Effectiveness of antipsychotics in first-episode schizophrenia and schizophreniform disorder on response and remission: an open randomized clinical trial (EUFEST). Schizophr Res. 2009;115:97–103. doi: 10.1016/j.schres.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Emsley R., Rabinowitz J., Medori R. Remission in early psychosis: rates, predictors, and clinical and functional outcome correlates. Schizophr Res. 2007;89:129–139. doi: 10.1016/j.schres.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Lambert M., Naber D., Schacht A., et al Rates and predictors of remission and recovery during 3 years in 392 never-treated patients with schizophrenia. Acta Psychiatr Scand. 2008;118:220–229. doi: 10.1111/j.1600-0447.2008.01213.x. [DOI] [PubMed] [Google Scholar]
- 21.Friedman L., Lys C., Schulz SC. The relationship of structural brain imaging parameters to antipsychotic treatment response: a review. J Psychiatry Neurosci. 1992;17:42–54. [PMC free article] [PubMed] [Google Scholar]
- 22.Zipursky RB., Zhang-Wong J., Lambe EK., Bean G., Beiser M. MRI correlates of treatment response in first episode psychosis. Schizophr Res. 1998;30:81–90. doi: 10.1016/s0920-9964(97)00126-6. [DOI] [PubMed] [Google Scholar]
- 23.Honer WG., Smith GN., Lapointe JS., MacEwan GW., Kopala L., Altman S. Regional cortical anatomy and clozapine response in refractory schizophrenia. Neuropsychopharmacology. 1995;13:85–87. doi: 10.1016/0893-133X(95)00017-8. [DOI] [PubMed] [Google Scholar]
- 24.Konicki PE., Kwon KY., Steele V., et al Prefrontal cortical sulcal widening associated with poor treatment response to clozapine. Schizophr Res. 2001;48:173–176. doi: 10.1016/s0920-9964(00)00130-4. [DOI] [PubMed] [Google Scholar]
- 25.Friedman L., Knutson L., Shurell M., Meltzer HY. Prefrontal sulcal prominence is inversely related to response to clozapine in schizophrenia. Biol Psychiatry. 1991;29:865–877. doi: 10.1016/0006-3223(91)90053-o. [DOI] [PubMed] [Google Scholar]
- 26.Arango C., Breier A., McMahon R., Carpenter WT. Jr, Buchanan RW. The relationship of clozapine and haloperidol treatment response to prefrontal, hippocampal, and caudate brain volumes. Am J Psychiatry. 2003;160:1421–1427. doi: 10.1176/appi.ajp.160.8.1421. [DOI] [PubMed] [Google Scholar]
- 27.Molina V., Reig S., Sarramea F., et al Anatomical and functional brain variables associated with clozapine response in treatment-resistant schizophrenia. Psychiatry Res. 2003;124:153–161. doi: 10.1016/s0925-4927(03)00108-2. [DOI] [PubMed] [Google Scholar]
- 28.Bodnar M., Harvey PO., Malla AK., Joober R., Lepage M. The parahippocampal gyrus as a neural marker of early remission in first-episode psychosis: a voxel-based morphometry study. Clinical Schizophr Rel Psychoses. 2011;4:217–228. [PubMed] [Google Scholar]
- 29.Molina V., Martin C., Ballesteros A., de Herrera AG., Hernandez-Tamames JA. Optimized voxel brain morphometry: association between brain volumes and the response to atypical antipsychotics. Eur Arch Psychiatry Clin Neurosci. 2011;261:407–416. doi: 10.1007/s00406-010-0182-2. [DOI] [PubMed] [Google Scholar]
- 30.Garver DL., Holcomb JA., Christensen JD. Compromised myelin integrity during psychosis with repair during remission in drug-responding schizophrenia. Int J Neuropsychopharmacology. 2008;11:49–61. doi: 10.1017/S1461145707007730. [DOI] [PubMed] [Google Scholar]
- 31.Luck D., Buchy L., Czechowska Y., et al Fronto-temporal disconnectivity and clinical short-term outcome in first episode psychosis: a DTI-tractography study. J Psychiatr Res. 2011;45:369–377. doi: 10.1016/j.jpsychires.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Palaniyappan L., Marques TR., Taylor H., et al Cortical folding defects as markers of poor treatment response in first-episode psychosis. JAMA Psychiatry. 2013;70:1031–1040. doi: 10.1001/jamapsychiatry.2013.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reis Marques TR., Taylor H., Chaddock CA., et al White matter integrity as predictor of response to treatment in first episode psychosis. Brain. 2014;137:172–182. doi: 10.1093/brain/awt310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andreasen NC., Carpenter WT. Jr, Kane JM., Lasser RA., Marder SR., Weinberger DR. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162:441–449. doi: 10.1176/appi.ajp.162.3.441. [DOI] [PubMed] [Google Scholar]
- 35.Mangin JF., Jouvent E., Cachia A. In-vivo measurement of cortical morphology: means and meanings. Curr Opin Neurol. 2010;23:359–367. doi: 10.1097/WCO.0b013e32833a0afc. [DOI] [PubMed] [Google Scholar]
- 36.Palaniyappan L., Liddle PF. Aberrant cortical gyrification in schizophrenia: a surface-based morphometry study. J Psychiatry Neurosci. 2012;37:399–406. doi: 10.1503/jpn.110119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gimenez M., Junque C., Vendrell P., et al Abnormal orbitofrontal development due to prematurity. Neurology. 2006;67:1818–1822. doi: 10.1212/01.wnl.0000244485.51898.93. [DOI] [PubMed] [Google Scholar]
- 38.Haukvik UK., Schaer M., Nesvag R., et al Cortical folding in Broca's area relates to obstetric complications in schizophrenia patients and healthy controls. Psychol Med. 2012;42:1329–37. doi: 10.1017/S0033291711002315. [DOI] [PubMed] [Google Scholar]
- 39.Palaniyappan L., Liddle PF. Dissociable morphometric differences of the inferior parietal lobule in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2012;262:579–587. doi: 10.1007/s00406-012-0314-y. [DOI] [PubMed] [Google Scholar]
- 40.Koutsouleris N., Meisenzahl EM., Davatzikos C., et al Use of neuroanatomical pattern classification to identify subjects in at-risk mental states of psychosis and predict disease transition. Arch Gen Psychiatry. 2009;66:700–712. doi: 10.1001/archgenpsychiatry.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun D., van Erp TG., Thompson PM., et al Elucidating a magnetic resonance imaging-based neuroanatomic biomarker for psychosis: classification analysis using probabilistic brain atlas and machine learning algorithms. Biol Psychiatry. 2009;66:1055–1060. doi: 10.1016/j.biopsych.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pettersson-Yeo W., Benetti S., Marquand AF., et al Using genetic, cognitive and multi-modal neuroimaging data to identify ultra-high-risk and first-episode psychosis at the individual level. Psychol Med. 2013;43:2547–2562. doi: 10.1017/S003329171300024X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mourao-Miranda J., Reinders AA., Rocha-Rego V., et al Individualized prediction of illness course at the first psychotic episode: a support vector machine MRI study. Psychol Med. 2012;42:1037–1047. doi: 10.1017/S0033291711002005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koutsouleris N., Riecher-Rossler A., Meisenzahl EM., et al Detecting the psychosis prodrome across high-risk populations using neuroanatomical biomarkers. Schizophr Bull. 2014 Jun 9. [Epub ahead of print] doi: 10.1093/schbul/sbu078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tognin S., Pettersson-Yeo W., Valli I., et al Using structural neuroimaging to make quantitative predictions of symptom progression in individuals at ultra-high risk for psychosis. Front Psychiatry. 2013;4:187. doi: 10.3389/fpsyt.2013.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malla A., Norman R., Bechard-Evans L., Schmitz N., Manchanda R., Cassidy C. Factors influencing relapse during a 2-year follow-up of first-episode psychosis in a specialized early intervention service. Psychol Med. 2008;38:1585–1593. doi: 10.1017/S0033291707002656. [DOI] [PubMed] [Google Scholar]
- 47.Eack SM., Greenwald DP., Hogarty SS., Keshavan MS. [Google Scholar]



