Abstract
Antiplatelet therapies form the cornerstone of atherothrombosis prevention, reducing the morbidity and mortality associated with cardiovascular disease. Despite these benefits, there is still an unmet need for more effective and safer pharmacological agents. To expedite this process, biological platforms that better reflect the intravascular environment in humans will be required in order to shorten drug development time, enable better determination of dosing regimes, and aid in the design of clinical studies. This article focuses on a unique genetically modified animal model that predicts the in vivo response of anti-platelet agents in humans more accurately than is currently possible using conventional murine models of thrombosis.
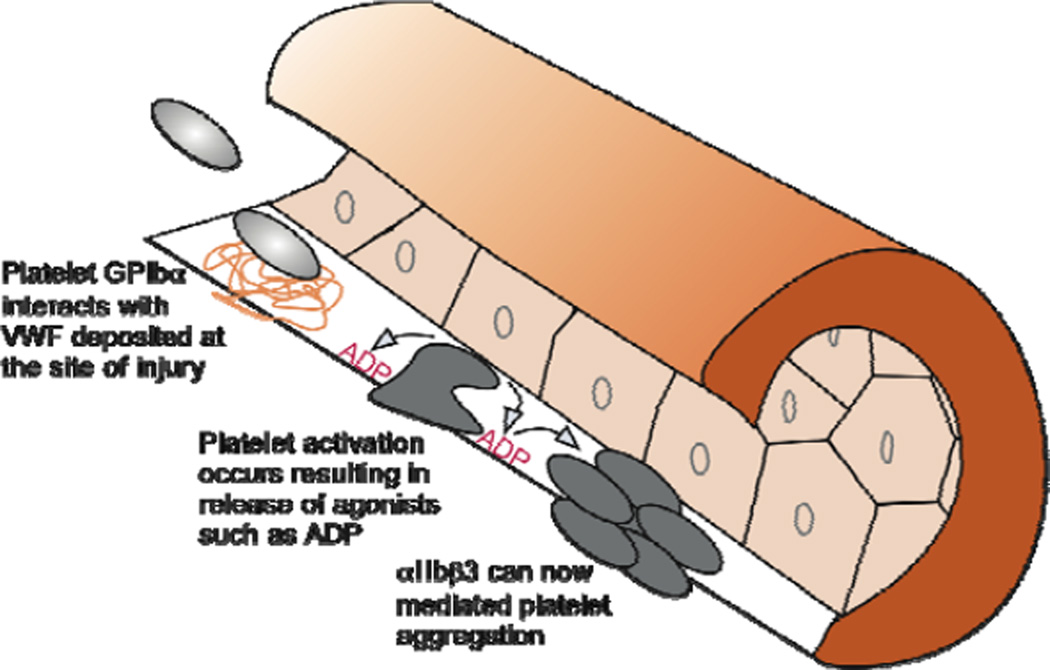
Pathological arterial thrombosis is the most-frequent cause of death worldwide (WHO. World Health Statistics 2010). Platelets are fundamental to this process by virtue of their ability to deposit at sites of vascular damage and subsequently form occlusive thrombi through a series of well-defined adhesive and cell signaling events (Figure 1) (van Zanten et al. 1994, Furie and Furie 2008, Ruggeri and Mendolicchio 2007). For instance, initiation of platelet contact with the injured vessel wall relies von Willebrand factor (VWF), a multidomain, multimeric plasma protein that forms an adhesive bridge between the platelet receptor glycoprotein (GP) Ibα and exposed components of the extracellular matrix (ECM) (Sakariassen et al. 1979). Stabilization of adhesion and thrombus growth, on the other hand, require the participation of the collagen and fibrinogen receptors α2β1 and αIIbβ3, respectively (Kasirer-Friede 2007). A prerequisite for these events is the activation of intracellular signaling pathways triggered by platelet interactions with the exposed ECM and in response to agonists released from platelets (e.g. ADP) or generated (e.g. thrombin) at the site of injury (Abrams 2005, Offermanns 2006). Transgenic mouse models have contributed substantially to our understanding of these processes and have permitted identification of targets amenable to antithrombotic therapy (Sachs and Nieswandt 2007). However, several limitations need to be addressed before small animal models can better aid in the development and preclinical testing of agents destined for use in patients.
Figure 1. Illustration depicting sequential steps required for intra-arterial thrombosis.
Upon vessel wall injury, due to mechanical trauma or rupture of an atherosclerotic plaque, circulating platelets rapidly adhere to exposed subendothelial-bound VWF. This is followed by platelet activation and release of granule contents such as ADP, which further enhances the activation process in an autocrine and paracrine fashion. Consequently, αIIbβ3 can promote and stabilize platelet-platelet interactions resulting in the formation of a thrombus.
Limitations of murine models of thrombosis
The cellular and soluble components of the hemostatic system in mice are similar to those in humans in many respects, but key differences in platelet adhesion and signaling receptors do exist. These include variations in the density, isoform, and structure of the proteins supporting these phenomena and thus these differences would directly impact on the classes of antiplatelet agents that could be tested in conventional mouse models of thrombosis. For instance, mouse platelets are approximately half the size of human platelets, a property impacting on the number of receptors that can be expressed on their surface (Ware et al. 1993). Furthermore subtle structural differences in cell surface receptors such as αIIbβ3 can affect drug responsiveness, resulting in a limited ability of inhibitors to reduce murine platelet aggregation and thrombus formation (Magallon et al. 2011). Additionally, human, but not murine platelets rely on the protease-activated receptor 1 (PAR1) for activation by thrombin. This precludes evaluation of the antithrombotic effect of PAR1 antagonists in mice (Coughlin 2000). Thus, the ideal situation would be to study the in vivo efficacy of antiplatelet agents directly against the target that they were designed to inhibit: receptors on human platelets. This would require generating an animal model that permits human but not murine platelet-mediated hemostasis and thrombosis. To achieve this goal, it would be necessary to first identify components of the hemostatic system requiring genetic alteration (humanization).
Humanizing murine VWF is key
Previously we have demonstrated that surface-immobilized murine VWF interacts poorly with human platelets under high flow conditions (Chen et al. 2008). In particular, this results from an inability of a region contained within VWF known as the A1 domain (VWF-A1) to support biologically relevant interactions with GPIbα on human platelets. Although human and murine VWF-A1 domains share high sequence (~86% identity) and structural similarity, there are minor differences in structure. Modeling of the interspecies complex, based on known atomic structures, revealed the difference that was potentially responsible for this phenomenon: a single amino acid difference at position 1326 in VWF-A1 (arginine (R) in mouse and histidine (H) in humans) (Chen et al. 2008). This difference results in an electrostatic clash between the positive charges of arginine at position 1326 in the mouse A1 domain and lysine at position 231 in human GPIbα, preventing sufficient interactions between these proteins (Figure 2). Based on these observations, we surmised that substituting histidine for arginine would favor the binding of human GPIbα, while reducing interactions of its murine counterpart. The latter would be due to the loss of a favorable electrostatic environment. This was initially confirmed by demonstrating that recombinant murine VWF-A1 protein containing the 1326R>H mutation supported human platelet attachment at levels equivalent to that observed for the recombinant human protein under identical flow conditions.
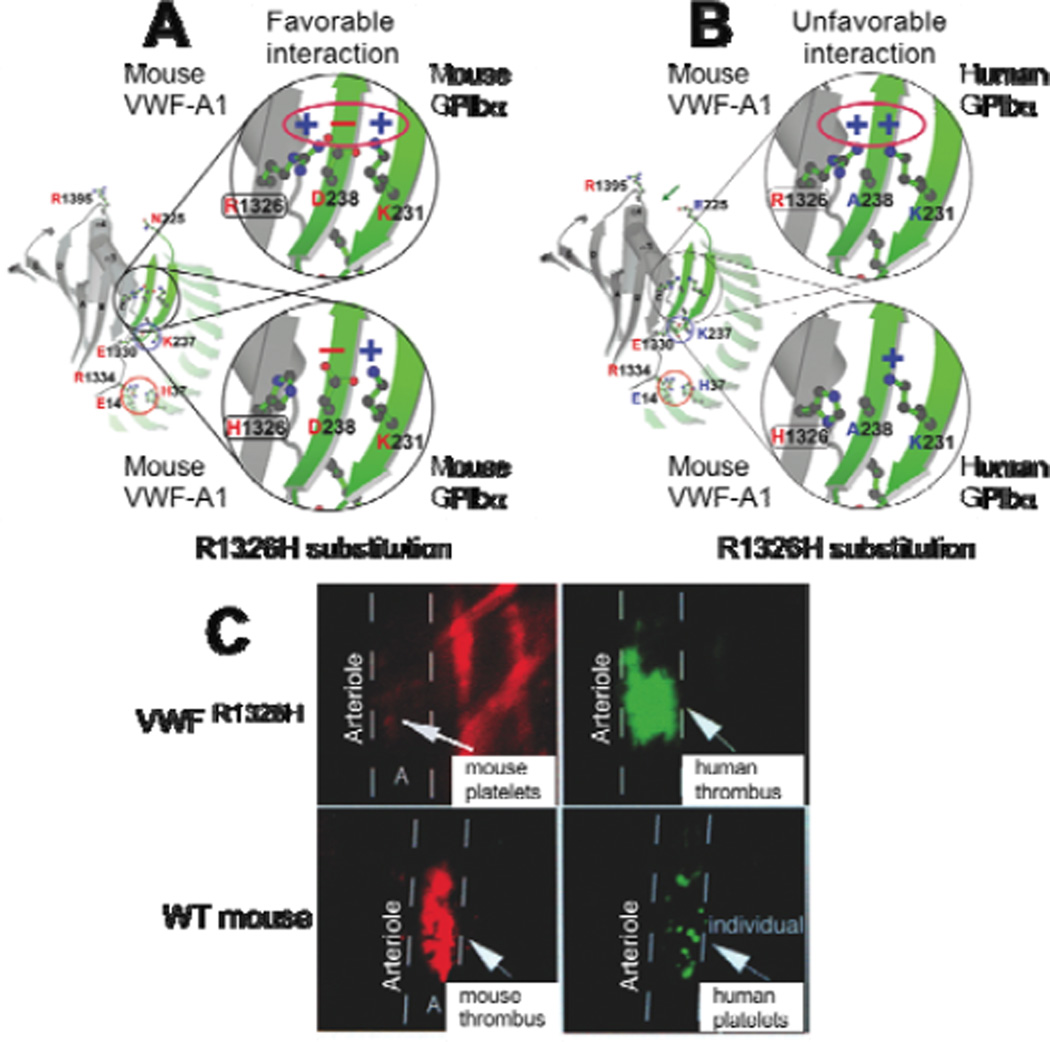
Figure 2. Effect of the arginine (R) to histidine (H) substitution in mouse VWF –A1 on the human and mouse GPIbα binding interface.
(A) Model of the mouse GPIbα–mouse VWF-A1 complex in its native state (upper panel) or with the R1326H mutation (lower panel). In the latter case, the amino acid substitution results in a loss of a favourable electrostatic interaction that supports contact between this receptor-ligand pair. (B) Model of the human GPIbα–mouse VWF-A1 interspecies complex in the absence (upper panel) and presence of (lower panel) the R1326H mutation. In the former case, there is an unfavorable electrostatic environment that would impair this interaction; the R1326H substitution prevents this from occurring and now closely resembles the human-human complex. (C) Representative photomicrographs of fluorescent images depicting mouse versus human platelet-mediated thrombus formation in VWF R1326H mutant (upper panels) or wild type (WT) mice (lower panels). Mouse platelets were labeled with rhodamine (red) and human platelets with carboxyfluorescein (green).
To provide definitive evidence that this single residue difference is critical for altering the binding preferences across species, we genetically modified the murine VWF genomic sequence so that it possessed the R1326H substitution (VWFR1326H). Animals homozygous for this point mutation were viable and had normal VWF antigen levels, multimer pattern, factor VIII function, and collagen binding. However, bleeding time was prolonged secondary to an impaired ability of mouse platelets to initiate contact and form thrombi at sites of vascular injury. Key to the use of this biological platform in preclinical drug testing was the demonstration that human platelets administrated to mutant mice restored hemostasis and thrombosis. In fact, thrombi that formed in injured arterioles were composed mainly of human platelets (>90%), and depending on the extent of vascular damage could occlude the entire vessel lumen (Figure 2C). Evidence that GPIbα plays an essential role in the initial platelet adhesion leading to arterial thrombosis was confirmed by the ability of a function-blocking antibody to this receptor to significantly impair this process. Taken together, these results not only demonstrate that the GPIbα–VWF axis supports human platelet contact with the injured vessel wall but that key downstream adhesion and signaling pathways must be involved in mediating arterial thrombosis.
Clinical utility of the humanized VWF mouse
As aforementioned, animal models have been instrumental in identifying targets for the development of antithrombotic drugs. Models include those in which the G protein-coupled ADP receptor P2Y12 (Andre et al. 2003, Foster et al. 2001) or the integrin receptor αIIbβ3 (Tronik-Le Roux et al. 2000, Hodivala-Dilke et al. 1999) have been genetically deleted, demonstrating the importance of these molecules in platelet activation and thrombus growth, respectively. These observations supported the development of oral inhibitors directed against the ADP receptor P2Y12 (i.e., ticlopidine, clopidogrel, prasugrel, and ticagrelor) and short-term use intravenous agents that prevent αIIbβ3-mediated platelet aggregation (i.e., abciximab, tirofiban, and eptifibatide) (Cattaneo 2010, Michelson 2010, Patrono 2008, Schrör 2003).
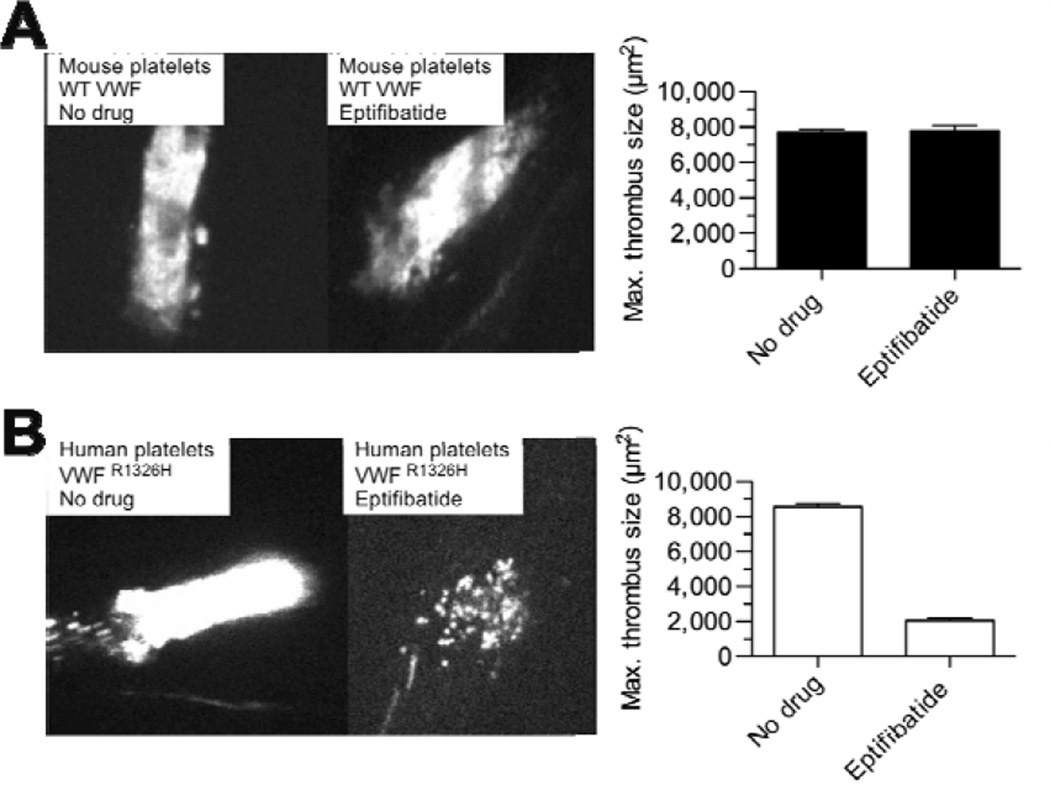
To validate the use of our humanized VWF mouse in preclinical testing and to demonstrate its benefits beyond conventional animal models, we evaluated the ability of several FDA-approved αIIbβ3 inhibitors to reduce human versus murine platelet-mediated thrombosis in response to laser-induced vascular injury. Consistent with the effectiveness of these agents in patients undergoing percutaneous coronary intervention (PCI), administration of abciximab, eptifibatide, or tirofiban at doses recommended by the by ACC/AHA for PCI (per kg of body weight) significantly reduced the ability of circulating human platelets to support thrombus formation in injured arterioles in our VWFR1326H mutant mouse (Kushner et al. 2009, Magallon et al. 2011) (Figure 3); no such effect was noted in wild type control animals even though only minor structural differences in αIIbβ3 exist between the species (Basani et al. 2009, Kamata et al. 2001).
Figure 3. Effect of the αIIbβ3 inhibitor eptifibatide on thrombus formation.
Representative photomicrographs evaluating the in vivo efficacy of eptifibatide in preventing (A) mouse versus (B) human platelet-mediated thrombosis in wild type (WT) or VWF R1326H mutant mice, respectively. Eptifibatide was administered as per ACC/AHA guidelines (Kushner et al. 2009): 180 µg/kg intravenous bolus followed by a continuous infusion of 2 µg/kg/min. Thrombus formation was induced by laser injury to arterioles contained within the microcirculation of the cremaster muscle. Data represent the mean ± SEM
However, what truly sets the VWFR1326H mutant mouse apart from standard small animal models of thrombosis is the ability to test the in vivo reactivity of human platelets purified from patients on drugs such as P2Y12 inhibitors. It has been well established that genetic polymorphisms in cytochrome P450 can have a major impact on the biotransformation of clopidogrel to its active metabolite, leading to diminished platelet inhibition and higher rates of adverse cardiovascular events (Mega et al. 2009). Thus, the ability to assess the in vivo effectiveness of such drugs after conversion to an active form in a human being has obvious clinical implications. To this end, we evaluated human platelet-mediated thrombus formation in the arterial circulation of VWFR1326H mutant mice pre- and post administration of clopidogrel to healthy volunteers. A single dose of this P2Y12 inhibition (300 mg) was effective in reducing the size of human thrombi as compared to those formed prior to drug administration. The importance of αIIbβ3 and ADP in this process was also confirmed by studying the thrombogenic nature of platelets from individuals with either Hermansky-Pudlak syndrome (lack dense granules where ADP is stored) or Glanzmann's thrombasthenia (lack αIIbβ3). Consistent with the known defects in hemostasis, platelets from these individuals failed to support significant thrombus formation in response to vascular injury in our humanized mouse model.
Based on these studies, we conclude that: 1) key adhesive and signaling pathways known to be essential for thrombus formation in humans are also required for this process to occur in our humanized VWF mouse, and 2) this biological platform is capable of assessing the in vivo efficacy of antiplatelet agents and yields the expected reduction in thrombus formation at doses that are therapeutic in humans. Thus the VWFR1326H mutant mouse model may have value not only in expediting drug development but in providing proof of concept for target modulation in the early phases of clinical trials.
Future Directions
In addition to drug development and testing, the generation of small animal models that better reflect the hemostatic system in humans could impact significantly on our understanding of various platelet-related disorders. These include, but are not limited to, the platelet storage lesion associated with blood banking (Cauwenberghs et al. 2007, Devine and Serrano, 2010), autoimmune disorders such as idiopathic thrombocytopenic purpura and arthropathies (Cines et al. 2009, Boilard et al. 2010), and the role of human platelets in inflammation and cancer (Gawaz et al. 2005, Labelle et al. 2011). Perhaps the most intriguing potential use of humanized animal models such as our VWFR1326H mutant mouse will be in recapitulating human thrombopoiesis in vivo and testing the functionality of platelets derived from pluripotent human stem cells (Fuentes et al. 2010, Takayama et al. 2010). The availability of immunodeficient murine models that enable engraftment of human stem cells and prolong the circulation lifetime of human platelets should facilitate this process (Macchiarini et al. 2005).
Acknowledgements
Work presented in this review was supported by funds from the New York State Foundation for Science, Technology and Innovation Faculty Development Program (NYSTAR), the Division of Neonatology at Columbia University Medical Center, and the National Institute of Health (HL097971-01 and HL103989-01). We are grateful to Michael Rosen for critical review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO. World Health Statistics. 2010 http://www.who.int/whosis/whostat/2010/en/index.html.
- 2.van Zanten GH, de Graaf S, Slootweg PJ, et al. Increased platelet deposition on atherosclerotic coronary arteries. J Clin Invest. 1994;93:615–632. doi: 10.1172/JCI117014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furie B, Furie BC. Mechanisms of thrombus formation. N Eng J Med. 2008;359:938–949. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 4.Ruggeri ZM, Mendolicchio GL. Adhesion mechanisms in platelet function. Circ Res. 2007;100:1673–1685. doi: 10.1161/01.RES.0000267878.97021.ab. [DOI] [PubMed] [Google Scholar]
- 5.Sakariassen KS, Bolhuis PA, Sixma JJ. Human blood platelet adhesion to artery subendothelium is mediated by factor VIII-von Willebrand factor bound to the subendothelium. Nature. 1979;279:636–638. doi: 10.1038/279636a0. [DOI] [PubMed] [Google Scholar]
- 6.Kasirer-Friede A, Kahn ML, Shattil SJ. Platelet integrins and immunoreceptors. Immunol Rev. 2007;218:247–264. doi: 10.1111/j.1600-065X.2007.00532.x. [DOI] [PubMed] [Google Scholar]
- 7.Abrams CS. Intracellular signaling in platelets. Curr Opin Hematol. 2005;12:401–405. doi: 10.1097/01.moh.0000176681.18710.e3. [DOI] [PubMed] [Google Scholar]
- 8.Offermanns S. Activation of Platelet Function Through G Protein–Coupled Receptors. Circulation Research. 2006;99:1293–1304. doi: 10.1161/01.RES.0000251742.71301.16. [DOI] [PubMed] [Google Scholar]
- 9.Sachs UJ, Nieswandt B. In vivo thrombus formation in murine models. Circ Res. 2007;100:979–991. doi: 10.1161/01.RES.0000261936.85776.5f. [DOI] [PubMed] [Google Scholar]
- 10.Ware J, Russell SR, Marchese P, Ruggeri ZM. Expression of human platelet glycoprotein Ib alpha in transgenic mice. J Biol Chem. 1993;268:8376–8382. [PubMed] [Google Scholar]
- 11.Magallon J, Chen JC, Rabbani L, et al. Humanized mouse model of thrombosis is predictive of the clinical efficacy of antiplatelet agents. Circulation. 2011;123:319–326. doi: 10.1161/CIRCULATIONAHA.110.951970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Tan K, Zhou H, et al. Modifying murine von Willebrand factor A1 domain for in vivo assessment of human platelet therapies. Nat Biotechnol. 2008;26:114–119. doi: 10.1038/nbt1373. [DOI] [PubMed] [Google Scholar]
- 14.Andre P, Delaney SM, LaRocca T, et al. P2Y12 regulates platelet adhesion/activation, thrombus growth, and thrombus stability in injured arteries. J Clin Invest. 2003;112:398–406. doi: 10.1172/JCI17864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster CJ, Prosser DM, Agans JM, et al. Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J Clin Invest. 2001;107:1591–1598. doi: 10.1172/JCI12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tronik-Le Roux D, Roullot V, Poujol C, Kortulewski T, Nurden P, Marguerie G. Thrombasthenic mice generated by replacement of the integrin alpha(IIb) gene: demonstration that transcriptional activation of this megakaryocytic locus precedes lineage commitment. Blood. 2000;96:1399–408. [PubMed] [Google Scholar]
- 17.Hodivala-Dilke KM, McHugh KP, Tsakiris DA, et al. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103:229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cattaneo M. New P2Y(12) inhibitors. Circulation. 2010;121:171–179. doi: 10.1161/CIRCULATIONAHA.109.853069. [DOI] [PubMed] [Google Scholar]
- 19.Michelson AD. Antiplatelet therapies for the treatment of cardiovascular disease. Nat Rev Drug Discov. 2010;9:154–169. doi: 10.1038/nrd2957. [DOI] [PubMed] [Google Scholar]
- 20.Patrono C, Baigent C, Hirsh J, Roth G. Antiplatelet drugs: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:199S–233S. doi: 10.1378/chest.08-0672. [DOI] [PubMed] [Google Scholar]
- 21.Schrör K, Weber AA. Comparative pharmacology of GP IIb/IIIa antagonist. J Thromb Thrombolysis. 2003;15:71–80. doi: 10.1023/b:thro.0000003308.63022.8d. [DOI] [PubMed] [Google Scholar]
- 22.Kushner FG, Hand M, Smith SC, Jr, et al. focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54:2205–2241. doi: 10.1016/j.jacc.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 23.Basani RB, Zhu H, Thornton MA, et al. Species differences in small molecule binding to alpha IIb beta 3 are the result of sequence differences in 2 loops of the alpha IIb beta propeller. Blood. 2009;113:902–910. doi: 10.1182/blood-2008-09-177337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamata T, Tieu KK, Irie A, Springer TA, Takada Y. Amino acid residues in the alpha IIb subunit that are critical for ligand binding to integrin alpha IIbbeta 3 are clustered in the beta-propeller model. J Biol Chem. 2001;276:44275–44283. doi: 10.1074/jbc.M107021200. [DOI] [PubMed] [Google Scholar]
- 25.Mega JL, Close SL, Wiviott SD, et al. Cytochrome P450 genetic polymorphisms and the response to prasugrel: relationship to pharmacokinetic, pharmacodynamic, and clinical outcomes. Circulation. 2009;119:2553–2560. doi: 10.1161/CIRCULATIONAHA.109.851949. [DOI] [PubMed] [Google Scholar]
- 26.Cauwenberghs S, van Pampus E, Curvers J, Akkerman JW, Heemskerk JW. Hemostatic and signaling functions of transfused platelets. Transfus Med Rev. 2007;21:287–294. doi: 10.1016/j.tmrv.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Devine DV, Serrano K. The platelet storage lesion. Clin Lab Med. 2010;30:475–487. doi: 10.1016/j.cll.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Cines DB, Bussel JB, Liebman HA, Luning Prak ET. The ITP syndrome: pathogenic and clinical diversity. Blood. 2009;113:6511–6521. doi: 10.1182/blood-2009-01-129155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boilard E, Nigrovic PA, Larabee K, et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327:580–583. doi: 10.1126/science.1181928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115:3378–3384. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;5:576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuentes R, Wang Y, Hirsch J, et al. Infusion of mature megakaryocytes into mice yields functional platelets. J Clin Invest. 2010;120:3917–3922. doi: 10.1172/JCI43326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takayama N, Nishimura S, Nakamura S, et al. Transient activation of c-MYC expression is critical for efficient platelet generation from human induced pluripotent stem cells. J Exp Med. 2010;207:2817–2830. doi: 10.1084/jem.20100844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macchiarini F, Manz MG, Palucka AK, Shultz LD. Humanized mice: are we there yet? J Exp Med. 2005;202:1307–1311. doi: 10.1084/jem.20051547. [DOI] [PMC free article] [PubMed] [Google Scholar]