Abstract
Adult, male, Sprague-Dawley rats were injected with granulocyte-macrophage colony-stimulating factor-transfected bone marrow stromal cells (GM-CSF-BMSCs) into the ischemic boundary zone at 24 hours after onset of middle cerebral artery occlusion. Results showed reduced infarct volume, decreased number of apoptotic cells, improved neurological functions, increased angiogenic factor expression, and increased vascular density in the ischemic boundary zone in rats that underwent GM-CSF-BMSCs transplantation compared with the BMSCs group. Experimental findings suggested that GM-CSF-BMSCs could serve as a potential therapeutic strategy for ischemic stroke and are superior to BMSCs alone.
Keywords: bone marrow stromal cells, granulocyte-macrophage colony-stimulating factor, gene transfection, ischemic stroke, transplantation, stem cells, neural regeneration
Abbreviations:
GM-CSF, granulocyte-macrophage colony-stimulating factor; BMSCs, bone marrow stromal cells; MCAO, middle cerebral artery occlusion; mNSS, modified neurological severity scores
INTRODUCTION
Bone marrow stromal cells (BMSCs) exhibit characteristics of multipotential stem cells. BMSCs can be expanded in vitro and induced to differentiate into osteogenic, chondrogenic, adipogenic, and other mesenchymal lineages[1], such as neuronal cells[2,3]. Recent studies have shown that BMSC transplantation results in therapeutic benefits following traumatic brain injury, ischemic brain injury, or spinal cord injury[4,5,6,7,8]. However, post-injury transplantations resulted in central nervous system protein expression in < 20% of transplanted cells[9]. BMSCs provide a stromal microenvironment for hematopoiesis and produce an array of trophic factors and cytokines that influence cell proliferation[10,11]. The mechanisms of functional recovery following BMSC transplantation are more likely due to the release of trophic factors that promote neural differentiation of BMSCs and cellular integration within injured ischemic sites[10,11].
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a well-known hematopoietic cytokine and was originally identified because of its ability to stimulate hematopoietic cell differentiation and function[12]. More recently, GM-CSF has attracted attention, because of its effects on neural cell proliferation and differentiation. Previous studies have shown that exogenous GM-CSF stimulates neuronal progenitor proliferation, prevents neuronal apoptosis, improves functional recovery following experimental spinal cord contusion injury, and provides neuroprotection in a rat transient middle cerebral artery occlusion (MCAO) model[13,14]. In addition, results from our previous study showed that GM-CSF increases neural differentiation of BMSCs in vitro[15]. These results suggest that GM-CSF plays an important role in neuroprotection following brain injury.
The present study intracerebrally transplanted GM-CSF-transfected BMSCs after ischemic stroke to determine the effects of GM-CSF-transfected BMSCs on functional recovery compared to BMSCs, as well as to explore the possible mechanisms of action.
RESULTS
Quantitative analysis of experimental animals
The MCAO model was established in a total of 105 Sprague-Dawley rats, and the rats were randomly and equally assigned to three groups: control, BMSCs, and BMSCs-GM-CSF. At 24 hours after MCAO model establishment, the rats were injected with serum-free α-MEM, untreated BMSCs, and GM-CSF gene-transfected BMSCs, respectively. A total of 18 rats died following MCAO modeling, and 15 rats did not meet the MCAO model standard; these rats were excluded from the experiment and were immediately supplemented. Subsequently, 105 rats, with 35 rats in each group, were included in the final analysis.
BMSC characteristics
BMSCs were harvested from rat bone marrow and were isolated following adherence to plastic. The cells were then passaged 2–3 more times. The BMSCs became comparatively homogeneous in appearance as the cells were passed. As the cells approached confluency, they assumed a relatively elongated or spindle shape (supplementary Figure 1 online).
GM-CSF transfection efficiency
To determine GM-CSF gene transfection efficiency, protein levels of GM-CSF at 0, 6, 12, 24, 48, and 96 hours after GM-CSF gene transfection in BMSCs were measured by western blot analysis. Western blot assay revealed that 6 hours after transfection, GM-CSF was expressed and reached a peak at 24 hours after transfection (Figures 1A, B). Based on these findings, BMSCs from 24 hours after GM-CSF transfection were utilized for transplantation in the following experiments.
Figure 1.
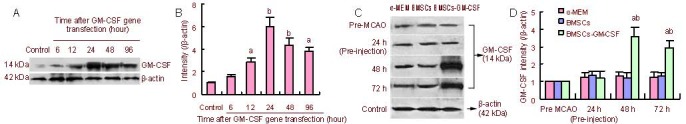
GM-CSF expression in GM-CSF-transfected BMSCs and the effect of BMSCs-GM-CSF on GM-CSF production in vivo.
(A) GM-CSF protein content in BMSCs was examined by western blot assay at 6, 12, 24, 48, and 96 hours after GM-CSF gene transfection.
(B) Bar graph shows GM-CSF protein levels at different time points after GM-CSF gene transfection in BMSCs. Data are expressed as mean ± SD. aP < 0.05, bP < 0.01, vs. control group, two-way analysis of variance followed by post-hoc Tukey test. The preMCAO group was standardized to 1.
(C) GM-CSF protein content was examined by western blot assay using protein samples from the ischemic hemisphere of rats treated with α-MEM, BMSCs, and BMSCs-GM-CSF at 24, 48, and 72 hours after MCAO and before MCAO. At 24 hours after MCAO, the right lateral corpus striatum was injected with BMSCs or BMSCs-GM-CSF. In the image, 24 h after MCAO refers to results before injection. 48, 72 hours after MCAO refer to 24, 48 hours after injection.
(D) Bar graph shows GM-CSF protein levels in the ischemic hemisphere of rats treated with α-MEM, BMSCs, and BMSCs-GM-CSF at 24, 48, and 72 hours after MCAO and before MCAO. Data are expressed as mean ± SD. aP < 0.01, vs. control (α-MEM) group; bP < 0.01, vs. BMSCs group, two-way analysis of variance followed by post-hoc Tukey test. The pre-MCAO group was standardized to 1.
GM-CSF: Granulocyte-macrophage colony-stimulating factor; BMSCs: bone marrow stromal cells; MCAO: middle cerebral artery occlusion.
In vivo GM-CSF production following BMSCs-GM- CSF transplantation
To further examine the effects of BMSCs-GM-CSF on in vivo production of GM-CSF, GM-CSF levels were measured in vivo before MCAO and at 24 (before injection), 48, and 72 hours after MCAO (after injection at 24 and 48 hours) in all rats by western blot. GM-CSF levels were significantly increased in the ischemic hemisphere of BMSCs-GM-CSF-transplanted rats at 48 and 72 hours after MCAO compared with the control and BMSC groups (P < 0.01 and P < 0.05, respectively). However, there was no significant difference between the control and BMSCs groups at 24, 48, and 72 hours after MCAO (P > 0.05; Figures 1C, D).
Transplantation of BMSCs-GM-CSF significantly improved nerve functions in rats
Prior to MCAO, as well as 1 day after MCAO (prior to BMSCs injection), there were no statistical differences in limb placement scores or modified neurological severity scores (mNSS) between groups (P > 0.05). However, at 8 and 15 days after MCAO, the BMSCs-GM-CSF group exhibited significantly greater limb placement scores and mNSS compared with control and BMSCs groups (P < 0.01 and P < 0.05, respectively). At 8 and 15 days after MCAO, there were no significant differences in limb placement scores and mNSS between control and BMSCs groups (P > 0.05; Figure 2).
Figure 2.
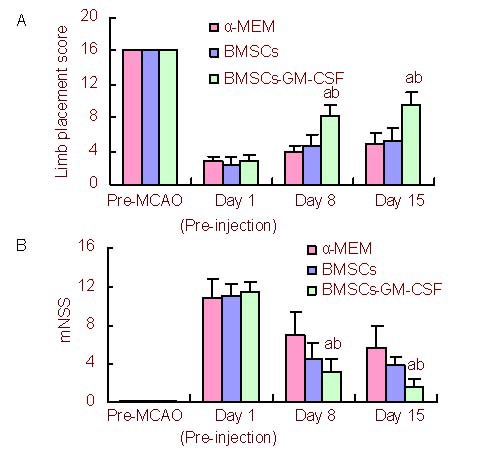
Functional recovery enhanced by intracerebral transplantation of BMSCs-GM-CSF.
Data are expressed as mean ± SD. aP < 0.01, vs. control (α-MEM) group; bP < 0.05, vs. BMSCs group, two-way analysis of variance followed by post-hoc Tukey test.
(A) Bar graph shows limb placement score at 1 day (pre-injection), as well as 8 and 15 days after MCAO and before MCAO. Score 0: severe neurological deficits; Score 16: no neurological deficits.
(B) Modified neurological severity scores (mNSS) behavioral assessment was analyzed at 1 day (pre-injection), as well as 8 and 15 days after MCAO and before MCAO. In the severity scores of injury, one score point was awarded for inability to perform the test or for the lack of a tested reflex. A higher score represented a more severe injury. A score of 18 was the maximum.
GM-CSF: Granulocyte-macrophage colony-stimulating factor; BMSCs: bone marrow stromal cells; MCAO: middle cerebral artery occlusion.
BMSCs-GM-CSF transplantation significantly reduced infarct volume in rats
In all groups, relative infarct volume decreased from day 1 (pre-injection) to day 15 after MCAO. However, at 8 and 15 days after MCAO, the relative infarct volume was significantly lower in the BMSCs-GM-CSF group compared with the control group (P < 0.01 and P < 0.05, respectively). The BMSCs group did not exhibit any significant recovery in relative infarct volume compared with the control group at 8 days after MCAO (P > 0.05). However, relative infarct volume significantly decreased in the BMSCs group compared with the control group at 15 days after MCAO (P < 0.01; Figure 3).
Figure 3.
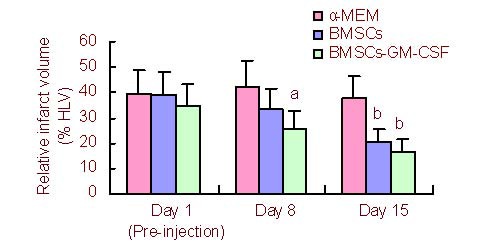
Infarct volume reduction following BMSCs-GM-CSF treatment.
The relative infarct volume is the percentage of lesion volume to contralateral hemispheric volume. Data are expressed as mean ± SD. aP < 0.05, bP < 0.01, vs. control group, two-way analysis of variance followed by post-hoc Tukey test.
HLV: Hemispheric lesion volume; GM-CSF: granulocyte-macrophage colony-stimulating factor; BMSCs: bone marrow stromal cells.
BMSCs-GM-CSF transplantation significantly decreased the percentage of apoptotic cells after MCAO
To determine the effect of BMSCs-GM-CSF on neural apoptosis after MCAO, the percentage of apoptosis- positive cells in the ischemic boundary zone was quantified. Results showed no significant differences in the number of apoptotic cells at 1 day (pre-injection) after MCAO between the three groups (P > 0.05). However, the percentage of apoptotic cells significantly decreased in the BMSCs-GM-CSF group compared with the control and BMSCs groups at 8 and 15 days after MCAO (P < 0.01 and P < 0.05, respectively). In addition, at 15 days after MCAO, there was a significant decrease in apoptotic cells in the BMSCs group compared with the control group (P < 0.01; Figures 4 A–D). Experimental findings indicate that BMSCs-GM-CSF significantly decreased the neural apoptosis in ischemic boundary zone relative to BMSCs.
Figure 4.
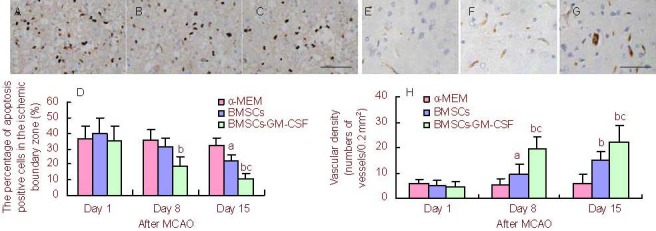
BMSCs-GM-CSF decreased neural apoptosis and increased vascular density after MCAO.
Data are expressed as mean ± SD. aP < 0.05, bP < 0.01, vs. control (α-MEM) group; cP < 0.05, vs. BMSCs group, two-way analysis of variance followed by post-hoc Tukey test.
(A–C) TUNEL-positive cells in the ischemic boundary zone of rats treated with α-MEM, BMSCs, or BMSCs-GM-CSF at 15 days after MCAO (scale bar: 100 μm).
(D) Bar graph shows percentage of apoptosis-positive cells in the ischemic boundary zone of rats treated with α-MEM, BMSCs, or BMSCs-GM-CSF at 1 day (pre-injection), as well as 8 and 15 days after MCAO.
(E–G) Immunohistochemical staining for factor VIII indicating blood vessels in the ischemic boundary zone of rats treated with α-MEM, BMSCs, or BMSCs-GM-CSF at 15 days after MCAO (scale bar: 100 μm).
(H) Bar graph shows vascular density of rats treated with α-MEM, BMSCs, or BMSCs-GM-CSF at 1 day (pre-injection), as well as 8 and 15 days after MCAO.
GM-CSF: Granulocyte-macrophage colony-stimulating factor; BMSCs: bone marrow stromal cells; MCAO: middle cerebral artery occlusion.
BMSCs-GM-CSF induced angiogenesis and increased angiogenic factor levels in the ischemic boundary zone
Rabbit anti-rat factor VIII-related antigen was used as a marker to determine vascular proliferation in the ischemic boundary zone on days 1 (pre-injection), 8, and 15 after MCAO. Immunohistochemical staining for factor VIII indicated the presence of blood vessels. Results showed no significant difference in vascular density on day 1 (pre-injection) after MCAO between groups (P > 0.05). However, at 8 and 15 days after MCAO, vascular density significantly increased in the BMSCs-GM-CSF group compared with control and BMSCs groups (P < 0.01 and P < 0.05, respectively). In addition, at 8 and 15 days after MCAO, vascular density significantly increased in the BMSCs group compared with the control group (P < 0.01; Figures 4E–H).
Reverse-transcription PCR analysis showed significantly increased vascular endothelial growth factor mRNA levels in the ischemic boundary zone of BMSCs-GM- CSF- treated rats compared with of α-MEM- and BMSCs- treated rats at 48 hours after injection (P < 0.05; Figure 5).
Figure 5.
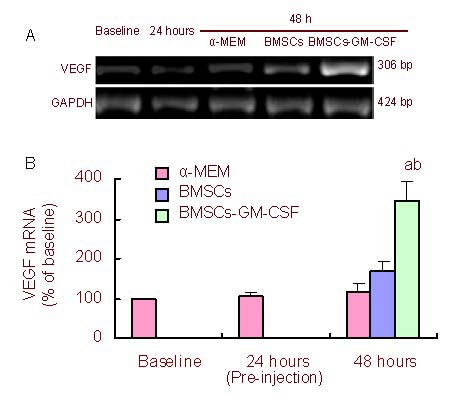
BMSCs-GM-CSF increased VEGF mRNA levels at 48 hours after MCAO.
(a) VEGF mRNA was measured by reverse transcription-PCR before MCAO (baseline), as well as at 24 hours after MCAO (pre-injection) and 48 hours after MCAO.
(b) Bar graph shows VEGF mRNA levels at different time points. VEGF mRNA expression is the percentage of VEGF mRNA to GAPDH mRNA/baseline. Baseline value was standardized to 1.
Data are expressed as mean ± SD. aP < 0.05, vs. control (α-MEM) group; bP < 0.05, vs. BMSCs group, two-way analysis of variance followed by post-hoc Tukey test. At 24 hours after MCAO, the right lateral corpus striatum was injected with BMSCs or BMSCs-GM-CSF. In the image, 24 hours after MCAO refers to results before injection.
GM-CSF: Granulocyte-macrophage colony-stimulating factor; BMSCs: bone marrow stromal cells; MCAO: middle cerebral artery occlusion; VEGF: vascular endothelial growth factor.
DISCUSSION
GM-CSF is a well-known hematopoietic cytokine that stimulates stem cell proliferation in the bone marrow. Recent results have shown that GM-CSF also stimulates proliferation of neuronal progenitor cells and microglia[16]. Results from the present study revealed GM-CSF expression in normal BMSCs.
However, to increase the cytokine effects of GM-CSF, BMSCs were transfected with the GM-CSF gene and neurological functional recovery was observed in stroke rats.
Behavioral testing results showed that BMSCs-GM-CSF transplantation induced significantly greater functional recovery following MCAO than α-MEM or BMSCs transplantation. Consistent with these results, in the BMSCs-GM-CSF group, infarct volume (% hemisphereic lesion volume) significantly decreased by 8 and 15 days after MCAO. Results demonstrated that intracerebral transplantation of BMSCs-GM-CSF significantly improved functional neurological recovery following focal cerebral ischemia. Results also suggested that the combined therapy of BMSCs-GM-CSF was more therapeutically efficient than BMSCs therapy alone. In addition, results suggested that BMSCs could function as a promising carrier for gene therapy.
In addition to neural differentiation, GM-CSF inhibits cell death in a variety of cell types[17,18,19]. A recent study showed that GM-CSF rescues neuronal cells from apoptosis and improves neurological functions in a spinal cord injury model[13]. Results from the present study demonstrated a significantly decreased percentage of apoptotic cells in the BMSCs-GM-CSF group compared with control and BMSCs groups at 8 and 15 days after MCAO. VEGF has been shown to increase the number and volume of cerebral cortical microvessels following ischemia in rats[20], and results from the present study showed significantly increased VEGF mRNA levels at 48 hours after BMSCs-GM-CSF transplantation compared with control and BMSCs groups. In addition, vascular density significantly increased, as indicated by F-8 Rag, in the BMSCs-GM-CSF group compared with control and BMSCs groups at 8 and 15 days after MCAO. Increased vascular density in the ischemic boundary zone of BMSCs-GM-CSF transplanted rats, in association with decreased apoptosis, suggested that neovascularization could rescue apoptotic cells in the ischemic region and subsequently contribute to measure improvements in neurological function. Results from our previous study demonstrated that the GM-CSF receptor is abundantly distributed in BMSCs, and GM-CSF increases neural differentiation of BMSCs by activating the GM-CSF receptor[15]. Together, these results suggested that the mechanisms of recovery following ischemia by BMSCs-GM-CSF are more likely due to GM-CSF release, which is thought to activate the GM-CSF receptor and promote neural differentiation of BMSCs, thereby stimulating angiogenesis and reducing cell death in the ischemic boundary zone.
In conclusion, results suggested that gene-transfected BMSCs provide superior results compared with BMSCs transplantation alone, and the intracerebral transplantation of BMSCs-GM-CSF into the ischemic boundary zone decreased infarction volume and promoted functional recovery in rats.
MATERIALS AND METHODS
Design
A randomized, controlled, animal experiment.
Time and setting
All experiments were performed in Nanjing Medical University, Affiliated Nanjing Brain Hospital, Experimental Animal Center and Department of Pathology, China from January 2006 to December 2009.
Materials
Animals
A total of 105 male, specific pathogen-free, Sprague- Dawley rats, weighing 250–280 g, were purchased from the National Rodent Laboratory Animal Resources of Shanghai, China (license No. SCXK (Hu) 2003-0003). The rats were housed at 19–25°C and 30–35% relative humidity and were exposed to a 12-hour light/dark cycle, with free access to food and water. All procedures were performed in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[21].
Plasmid
The pUMVC1-mGM-CSF[22] plasmids were kindly provided by HaiGui Biosciences, Shanghai, China.
Methods
Preparation of rat BMSCs and transfection of the mGM-CSF gene into BMSCs
BMSCs were isolated and cultured as previously described[5]. Briefly, bone marrow was aspirated from the tibia of anesthetized rats using a syringe containing 100 IU heparin with an 18-gauge needle. Marrow cells were seeded into 60-mm tissue culture flasks with α-MEM (Sigma, St Louis, MO, USA) + 20% fetal bovine serum (Gibco, Grand Island, NY, USA) + 100 U/mL penicillin + 100 mg/mL streptomycin. Cells were incubated at 37°C in 95% humidity and 5% CO2. Media was replaced every 2–3 days accompanied by vigorous shaking. Antibiotics were removed after two media changes. BMSCs were passaged following a 10-minute incubation in 0.25% trypsin/ethylenediaminetetraacetic acid, followed by gentle scraping. Cells from passages 3–6 were used for the following experiments. Plasmid-mediated gene transfection was performed using the Lipofectamine™ 2000 kit (Invitrogen, Carlsbad, CA, USA) according to manufacture instructions. Briefly, cells from the third passage and at 80% confluency were infected by pUMVC1-mGM-CSF. At 6 hours post-transfection, the medium was changed to normal BMSC culture medium, and the cells were continuously cultured for 24 hours prior to transplantation. GM-CSF gene transfection efficiency was determined by measuring GM-CSF protein levels after plasmid infection.
Preparation of the ischemic stroke model
Focal cerebral ischemia was induced by intraluminal MCAO as previously described[23]. In brief, rats were anesthetized with ketamine (100 mg/kg, i.p.), and a 4-0 nylon monofilament was inserted into the right internal carotid artery via the external carotid stump. The filamine was then advanced 20–21 mm past the carotid bifurcation until slight resistance was felt. The filament was left in place for 2 hours and then withdrawn.
Intracerebral transplantation of BMSCs and BMSCs-GM-CSF
Intracerebral transplantation of BMSCs was performed according to previously described methods[24]. At 24 hours after MCAO onset, the rats were anesthetized with a ketamine injection (3 mg/100 g, i.p.) and positioned in a stereotaxic frame (KOPF, Tujunga, California, CA, USA). In the BMSCs and BMSCs-GM- CSF groups, a 26-gauge needle with a Hamilton syringe was utilized to inject 1 × 106 BMSCs and BMSCs-GM- CSF in 10 μL of serum-free α-MEM into the right dorsolateral striatum, 4 mm beneath the skull surface and 3 mm lateral to bregma, over a 2.5-minute period[25]. This position approximated the ischemic boundary zone. Rats were administered cyclosporin A (10 mg/kg per day, i.p.) to prevent rejection of the BMSC transplantations. Control group rats were injected with serum-free α-MEM.
Western blot detection of GM-CSF protein levels after GM-CSF transfection in ischemic boundary zone before and after transplantation
BMSCs transfected with the GMSF gene were rinsed in ice-cold PBS prior to collection in lysis buffer as previously described[26]. After lysis for 15 minutes on ice, whole lysates were centrifuged at 20 000 × g for 15 minutes. The equivalent amount of protein sample was then applied to 12% acrylamide denaturing gels. After electrophoresis, proteins were transferred to nitrocellulose membranes (Amersham, Uppsala, Sweden) using a Bio-Rad mini-protein-III wet transfer unit overnight at 4°C. Blotting membranes were incubated in 5% non-fat milk in Tris-buffered saline Tween-20 (including 10 mM Tris, pH 7.6; 150 mM NaCl; 0.01% Tween-20) for 1 hour at room temperature. The membranes were then washed three times and incubated with rabbit anti-GM-CSF polycolonal antibody (1:2 000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and mouse anti-rat β-actin antibody (1:10 000; ABCam, Cambridge, UK) in Tris-buffered saline Tween-20 overnight at 4°C. After several washes with Tris-buffered saline Tween-20 buffer, the membranes were incubated for 1 hour with horseradish peroxidase-linked goat anti-rabbit IgG secondary antibody (Boster, Wuhan, China; 1:5 000) at 37°C, followed by four washes. The membranes were then processed with enhanced chemiluminescence western blot detection reagents (Pierce, Rockford, IL, USA). The films were scanned and band absorbance values were measured using ‘Quantity One’ image software (Bio-Rad, Hercules, CA, USA). Western blots were quantified according to GM-CS/β-actin ratio at each time point. To detect in vivo levels of GM-CSF before and after BMSC transplantation, the rats were anesthetized with ketamine (100 mg/kg, i.p.), the brains were removed, and tissue samples from the ischemic boundary zone of the ischemic hemisphere were dissected, placed on ice, and then stored at −80°C until further use. The tissue samples were then suspended in lysis buffer and GM-CSF levels were measured in vivo using the same procedure.
Evaluation of neurological function
Neurological functions were tested before MCAO, at 1 day (pre-injection) and at 8 and 15 days after MCAO. Rats were subjected to the mNSS[27] and limb placement score[28] tests to evaluate neurological function. Briefly, mNSS is a composite of motor, sensory (visual, tactile and proprioceptive), and reflex tests. According to severity scores of injury, one score point is awarded for the inability to perform the test or lack of a tested reflex. Therefore, a higher score represents a more severe injury. A score of 18 is the maximum. The limb placement score included eight subtests, as previously described[28]. Briefly, all four limbs were evaluated using the top and edges of a counter top. For each subtest, animals received a score of 0 if they were unable to place their limbs, 1 if they displayed partial and/or delayed (more than 2 seconds) placement of their limbs, or 2 if they exhibited immediate and correct limb placement. A lower score represented a more severe injury.
Determination of infarct volume
The rat brains were sectioned into 2-mm thick slices and stained with 2,3,5-triphenyl-tetrazolium chloride (Sigma St. Louis, MO, USA) to measure infarct volume. Disposition of the ischemic area was evaluated by calculating hemispheric lesion volumes using imaging software (Scion Image, version Beta 4.0.2; Scion, Frederick, MD, USA). For each slice, the ischemic tissue was marked and infarct volume was calculated by taking slice thickness (2 mm/slice) into account. To avoid overestimation of the infarct volume, a corrected infarct volume was calculated as previously described[29]. Relative infarct volumes were expressed as a percentage of the right hemispheric volume.
TUNEL staining for apoptosis
Rats were anesthetized and transcardially perfused with saline, followed by 4% paraformaldehyde in PBS. The brain tissues were embedded in paraffin, and tissue sections (2-μm thick) were cut on a microtome and subsequently stained using the TUNEL method[30] for in situ apoptosis detection (In Situ Cell Apoptosis Detection Kit I; Boster, Wuhan, China). The total number of TUNEL-positive cells was individually quantified from three sections of each brain, with each slide containing five random fields from the ischemic boundary zone.
Vascular density in the ischemic region
Briefly, following pepsin treatment of brain sections, endogenous peroxidase and biotin activities were blocked for 10 minutes with 3% hydrogen peroxidase and an avidin/biotin blocking kit. The brain sections were incubated with rabbit anti-rat factor VIII-related antigen (F-8 RAg) at 1:2 000 for 1 hour (Dako-Cytomation, Mississauga, ON, USA). After washing, the sections were incubated with secondary antibodies conjugated with peroxidase for 30 minutes, followed by horseradish peroxidase-conjugated goat anti-rabbit ultrastreptavidin label reagent (Boster; 1:5 000). The sections were developed with freshly prepared Nova Red solution for 5–10 minutes and counterstained with hematoxylin. Three 40 × fields from ischemic boundary zone per section were selected for analysis. The number of blood vessels in each field was quantified by a blinded observer using an image analysis system (Carl Zeiss, Dublin, CA, USA). Counts per field were averaged to reflect mean vascular density/0.2 mm2 in the ischemic region. Positive expression of factor VIII antibodies in vascular endothelial cells indirectly indicated proliferation of blood vessels.
Reverse transcription-PCR detection of vascular endothelial growth factor mRNA
To measure vascular endothelial growth factor mRNA expression, total RNA samples were extracted from the ischemic region at baseline (before MCAO; normal tissue), 24 hours after MCAO (before implantation), and 48 hours after MCAO in all groups using Trizol reagent (Invitrogen, Carlsbad, CA, USA). Vascular endothelial growth factor primers are as follows: forward: 5’-GTC CAA TTG AGA CCC TGG TG-3’; reverse: 5’-CTA TGT GCT GGC TTT GGT GA-3’. GAPDH was used as an internal standard gene. GAPDH primers were as follows: forward 5’-GTT CCA GTA TGA CTC TAC CC-3’, reverse 5’-AGT CTT CTG AGT GGC AGT GAT GGC-3’. Products were 306 bp and 424 bp, respectively. The PCR conditions comprised 32 cycles of denaturation at 94°C for 45 seconds, annealing at 55°C (for vascular endothelial growth factor) or 56°C (for GAPDH) for 40 seconds, and extension at 72°C for 45 seconds. PCR products were then separated by electrophoresis on a 1.5% agarose gel containing 0.5% ethidium bromide and were imaged using a BioDoc-IT imaging system (Bio-Rad, Hercules, CA, USA); absorbance value of the bands were determined using a GS-710 calibrated imaging Densitometer (Bio-Rad).
Statistical analysis
All data were expressed as mean ± SD. Statistical significance was determined using two-way analysis of variance followed by the post-hoc Tukey test. P < 0.05 was considered statistically significant. The statistical analysis software used in this study was SPSS (Version 12.0; SPSS, Chicago, IL, USA).
Acknowledgments:
We would like to acknowledge the contribution of colleagues, who provided technical support for cell culture and western blot analysis, from Nanjing Medical University, Affiliated Nanjing Brain Hospital, Department of Neurology and Nanjing Medical University in Nanjing, China.
Footnotes
Funding: This research was supported by a grant from “135 Project” Foundation of the Public Health Department of Jiangsu Province, China and Nanjing Medical Science and Technique Development Foundation.
Conflicts of interest: None declared.
Ethical approval: All experimental procedures were approved by the Committee for Experimental Animals of Nanjing Medical University in China.
Supplementary information: Supplementary data associated with this article can be found, in the online version, by visiting www.nrronline.org.
(Edited by Cao H, Bu XY/Yang Y/Song LP)
REFERENCES
- [1].Liu TM, Martina M, Hutmacher DW, et al. Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells. 2007;25(3):750–760. doi: 10.1634/stemcells.2006-0394. [DOI] [PubMed] [Google Scholar]
- [2].Lu J, Moochhala S, Moore XL, et al. Adult bone marrow cells differentiate into neural phenotypes and improve functional recovery in rats following traumatic brain injury. Neurosci Lett. 2006;398(1-2):12–17. doi: 10.1016/j.neulet.2005.12.053. [DOI] [PubMed] [Google Scholar]
- [3].Ding Y, Yan Q, Ruan JW, et al. Electro-acupuncture promotes survival, differentiation of the bone marrow mesenchymal stem cells as well as functional recovery in the spinal cord-transected rats. BMC Neurosci. 2009;10:35. doi: 10.1186/1471-2202-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Liu W, Jiang X, Fu X, et al. Bone marrow stromal cells can be delivered to the site of traumatic brain injury via intrathecal transplantation in rabbits. Neurosci Lett. 2008;434(2):160–164. doi: 10.1016/j.neulet.2007.12.067. [DOI] [PubMed] [Google Scholar]
- [5].Ban DX, Ning GZ, Feng SQ, et al. Combination of activated Schwann cells with bone mesenchymal stem cells: the best cell strategy for repair after spinal cord injury in rats. Regen Med. 2011;6(6):707–720. doi: 10.2217/rme.11.32. [DOI] [PubMed] [Google Scholar]
- [6].Suzuki H, Taguchi T, Kato Y, et al. Transplantation of neurospheres derived from bone marrow stromal cells promotes neurological recovery in rats with spinal cord injury. Med Mol Morphol. 2011;44(3):131–138. doi: 10.1007/s00795-010-0519-y. [DOI] [PubMed] [Google Scholar]
- [7].Chen J, Li Y, Wang L, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32(4):1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- [8].Chiba Y, Kuroda S, Maruichi K, et al. Transplanted bone marrow stromal cells promote axonal regeneration and improve motor function in a rat spinal cord injury model. Neurosurgery. 2009;64(5):991–1000. doi: 10.1227/01.NEU.0000341905.57162.1D. [DOI] [PubMed] [Google Scholar]
- [9].Rismanchi N, Floyd CL, Berman RF, et al. Cell death and long-term maintenance of neuron-like state after differentiation of rat bone marrow stromal cells: a comparison of protocols. Brain Res. 2003;991(1-2):46–55. doi: 10.1016/j.brainres.2003.07.004. [DOI] [PubMed] [Google Scholar]
- [10].Brugger W, Möcklin W, Heimfeld S, et al. Ex vivo expansion of enriched peripheral blood CD34+ progenitor cells by stem cell factor, interleukin-1 beta (IL-1 beta), IL-6, IL-3, interferon-gamma, and erythropoietin. Blood. 1993;81(10):2579–2584. [PubMed] [Google Scholar]
- [11].Oka M, Hirose K, Iizuka N, et al. Cytokine mRNA expression patterns in human esophageal cancer cell lines. J Interferon Cytokine Res. 1995;15(11):1005–1009. doi: 10.1089/jir.1995.15.1005. [DOI] [PubMed] [Google Scholar]
- [12].Lehners N, Goldschmidt H, Raab MS. Immunostimulating drugs and cytokines. Ther Umsch. 2011;68(11):655–658. doi: 10.1024/0040-5930/a000226. [DOI] [PubMed] [Google Scholar]
- [13].Ha Y, Kim YS, Cho JM, et al. Role of granulocyte- macrophage colony-stimulating factor in preventing apoptosis and improving functional outcome in experimental spinal cord contusion injury. J Neurosurg Spine. 2005;2(1):55–61. doi: 10.3171/spi.2005.2.1.0055. [DOI] [PubMed] [Google Scholar]
- [14].Kong T, Choi JK, Park H, et al. Reduction in programmed cell death and improvement in functional outcome of transient focal cerebral ischemia after administration of granulocyte-macrophage colony-stimulating factor in rats. Laboratory investigation. J Neurosurg. 2009;111(1):155–163. doi: 10.3171/2008.12.JNS08172. [DOI] [PubMed] [Google Scholar]
- [15].Lin X, Zhang Y, Dong J, et al. GM-CSF enhances neural differentiation of bone marrow stromal cells. Neuroreport. 2007;18(11):1113–1117. doi: 10.1097/WNR.0b013e3282010aff. [DOI] [PubMed] [Google Scholar]
- [16].Yamamoto S, Nakajima K, Kohsaka S. Macrophage- colony stimulating factor as an inducer of microglial proliferation in axotomized rat facial nucleus. J Neurochem. 2010;115(4):1057–1067. doi: 10.1111/j.1471-4159.2010.06996.x. [DOI] [PubMed] [Google Scholar]
- [17].Huang X, Choi JK, Park SR, et al. GM-CSF inhibits apoptosis of neural cells via regulating the expression of apoptosis-related proteins. Neurosci Res. 2007;58(1):50–57. doi: 10.1016/j.neures.2007.01.015. [DOI] [PubMed] [Google Scholar]
- [18].Fortin CF, Larbi A, Dupuis G, et al. GM-CSF activates the Jak/STAT pathway to rescue polymorphonuclear neutrophils from spontaneous apoptosis in young but not elderly individuals. Biogerontology. 2007;8(2):173–187. doi: 10.1007/s10522-006-9067-1. [DOI] [PubMed] [Google Scholar]
- [19].Derouet M, Thomas L, Cross A, et al. Granulocyte macrophage colony-stimulating factor signaling and proteasome inhibition delay neutrophil apoptosis by increasing the stability of Mcl-1. J Biol Chem. 2004;279(26):26915–26921. doi: 10.1074/jbc.M313875200. [DOI] [PubMed] [Google Scholar]
- [20].Zhang ZG, Zhang L, Jiang Q, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106(7):829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]
- [22].Li J, Bouton-Verville H, Holmes LM, et al. Inhibition or promotion of tumor growth by granulocyte-macrophage colony stimulating factor derived from engineered tumor cells is dose-dependent. Anticancer Res. 2004;24(5A):2717–2721. [PubMed] [Google Scholar]
- [23].Chauhan A, Sharma U, Jagannathan NR, et al. Rapamycin protects against middle cerebral artery occlusion induced focal cerebral ischemia in rats. Behav Brain Res. 2011;225(2):603–609. doi: 10.1016/j.bbr.2011.08.035. [DOI] [PubMed] [Google Scholar]
- [24].Goto S, Yamada K, Yoshikawa M, et al. GABA receptor agonist promotes reformation of the striatonigral pathway by transplant derived from fetal striatal primordia in the lesioned striatum. Exp Neurol. 1997;147(2):503–509. doi: 10.1006/exnr.1997.6628. [DOI] [PubMed] [Google Scholar]
- [25].Jang KS, Lee KS, Yang SH, et al. In vivo tracking of transplanted bone marrow-derived mesenchymal stem cells in a murine model of stroke by bioluminescence imaging. J Korean Neurosurg Soc. 2010;48(5):391–398. doi: 10.3340/jkns.2010.48.5.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Frangogiannis NG, Mendoza LH, Ren G, et al. MCSF expression is induced in healing myocardial infarcts and may regulate monocyte and endothelial cell phenotype. Am J Physiol Heart Circ Physiol. 2003;285(2):H483–492. doi: 10.1152/ajpheart.01016.2002. [DOI] [PubMed] [Google Scholar]
- [27].Morris DC, Chopp M, Zhang L, et al. Thymosin beta4 improves functional neurological outcome in a rat model of embolic stroke. Neuroscience. 2010;169(2):674–682. doi: 10.1016/j.neuroscience.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ohlsson AL, Johansson BB. Environment influences functional outcome of cerebral infarction in rats. Stroke. 1995;26(4):644–649. doi: 10.1161/01.str.26.4.644. [DOI] [PubMed] [Google Scholar]
- [29].Neumann-Haefelin T, Kastrup A, de Crespigny A, et al. Serial MRI after transient focal cerebral ischemia in rats: dynamics of tissue injury, blood-brain barrier damage, and edema formation. Stroke. 2000;31(8):1965–1973. doi: 10.1161/01.str.31.8.1965. [DOI] [PubMed] [Google Scholar]
- [30].Charriaut-Marlangue C, Ben-Ari Y. A cautionary note on the use of the TUNEL stain to determine apoptosis. Neuroreport. 1995;7(1):61–64. [PubMed] [Google Scholar]


