Abstract
A rat model of acute high intraocular pressure was established by injecting saline into the anterior chamber of the left eye. Synaptophysin expression was increased in the inner plexiform layer at 2 hours following injury, and was widely distributed in the outer plexiform layer at 3–7 days, and then decreased to the normal level at 14 days. This suggests that expression of this presynaptic functional protein experienced spatiotemporal alterations after elevation of intraocular pressure. There was no significant change in the fluorescence intensity and distribution pattern for synapse-associated protein 102 following elevated intraocular pressure. Synapse-associated protein 102 immunoreactivity was confined to the outer plexiform layer, while synaptophysin immunoreactivity spread into the outer plexiform layer and the outer nuclear layer at 3 and 7 days following injury. These alterations in presynaptic elements were not accompanied by changes in postsynaptic components.
Keywords: synaptophysin, synapse-associated protein 102, synaptic plasticity, elevated intraocular pressure, retina, neural regeneration
Abbreviations:
SYN, synaptophysin; SAP102, synapse-associated protein 102; IPL, inner plexiform layer; OPL, outer plexiform layer; HIOP, high intraocular pressure
INTRODUCTION
The morphologic changes in synapses and the modulation of the strength or efficacy of synaptic signaling are commonly known as synaptic plasticity. Synaptic plasticity, which plays an important role in the development of synaptic connections and in the functioning of the mature nervous system, is dependent on presynaptic as well as postsynaptic changes. Synaptophysin (SYN), also known as P38, is an acidic calcium binding glycoprotein closely associated with synaptic structure and function, and is an integral membrane protein of synaptic vesicles[1]. SYN is widely used as a marker of synaptogenesis and presynaptic terminals[2,3]. Synapse-associated protein 102 (SAP102) is a member of the membrane-associated guanylate kinase protein family, and is enriched in postsynaptic densities and is involved in receptor-mediated synaptic transmission. SAP102 is crucial for the regulation of synaptic signaling and plasticity[4,5].
In the rat retina, synapses in the outer and inner plexiform layers (OPL and IPL) play an important role in visual signal transmission[6]. Studies on synaptic changes in these regions following injury may provide significant insight into pathogenetic and protective mechanisms in eye diseases such as glaucoma. Elevation of intraocular pressure (IOP) is a risk factor for glaucoma. Previous studies have shown that acute high intraocular pressure (HIOP) causes thinning of the inner part of the retina and selective loss of cells in the ganglion cell layer; thus visual function suffers irreversible damage[7].
Does elevated IOP impact on synapses in the retina? We previously investigated the expression of SYN between 1 and 14 days after acute HIOP, and found that changes in protein expression mainly occurred within the OPL and outer nuclear layer. SYN expression was transiently up-regulated, with broadened distribution, returning to a normal pattern by day 14[8]. Unfortunately, we were unable to investigate alterations in synaptic structure and function because of significant damage in the IPL after 1 day following acute HIOP.
The formation and maintenance of normal synaptic structure and function needs the mutual participation of presynaptic and postsynaptic elements. However, the changes in postsynaptic elements in the retina after elevated IOP are still unclear. In the present study, we investigate the expression and relationship of presynaptic and postsynaptic markers SYN and SAP102 in the OPL and IPL within 1 day (at which stage there is no obvious structural loss in the IPL) and after 1 day following acute elevated IOP to understand synaptic changes in the rat retina.
RESULTS
Quantitative analysis of experimental animals
A total of 108 healthy adult Sprague-Dawley rats were included in this experiment. Two animals died after anesthesia, four died after induction of HIOP, and six rats were deemed invalid due to unsuccessful induction of HIOP. The remaining 96 rats were equally and randomly divided into sham surgery, HIOP 2 hours, 6 hours, 12 hours, 1 day, 3 days, 7 days and 14 days groups. Six rats from each group were used for immunofluorescence detection, and the remaining six rats for immunoblotting. Acute HIOP model was established by injecting saline in the anterior chamber of the left eye in animals in the HIOP groups. The right eye served as the normal control. A total of 96 rats were included in the final analysis.
SYN expression in the retina of rats with acute HIOP
The expression of SYN protein in the retina of normal and acute HIOP rats was examined using immunofluorescence staining (Figure 1).
Figure 1.
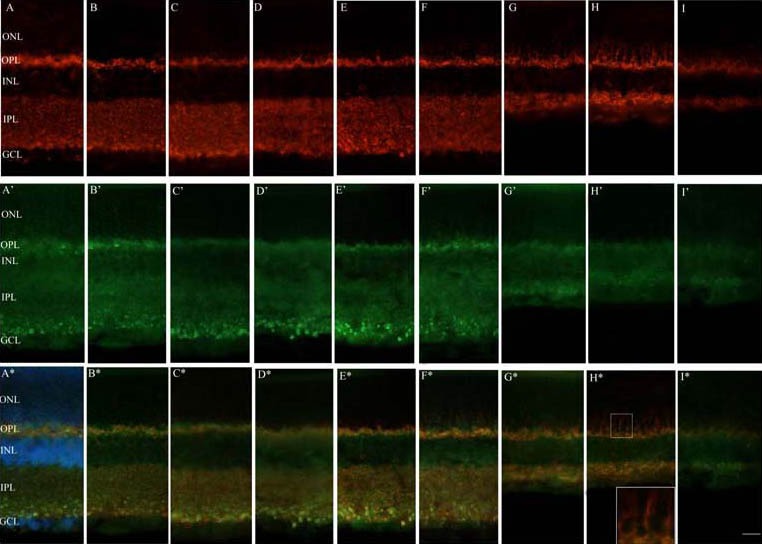
Fluorescence immunoreactivity of synaptophysin (SYN) and synapse-associated protein 102 (SAP102) following acute high intraocular pressure (HIOP) in the rat retina under a fluorescence microscope.
Panels A–I depict SYN (red) antibody labeling in the normal control, sham surgery, and HIOP 2 hours, 6 hours, 12 hours, 1 day, 3 days, 7 days and 14 days groups, respectively. Panels A’–I’ indicate SAP102 (green) antibody labeling in the normal control, sham surgery, and HIOP 2 hours, 6 hours, 12 hours, 1 day, 3 days, 7 days and 14 days groups, respectively. Panels A*–I* show double-labeling of SYN (red) and SAP102 (green) antibody in the normal control, sham surgery, and HIOP 2 hours, 6 hours, 12 hours, 1 day, 3 days, 7 days and 14 days groups, respectively. Nuclei of the cells were marked with Hoechst in A* (blue). The rectangle below in H* is a higher magnification of the upper selected region.
ONL: Outer nuclear layer; OPL: outer plexiform layer; INL: inner nuclear layer; IPL: inner plexiform layer; GCL: ganglion cell layer. Scale bar represents 25 μm.
Immunofluorescence for SYN was most prominent in the OPL and IPL in the normal retina, with a punctate appearance (Figure 1A). The expression and distribution of SYN were stable in the OPL within 1 day of HIOP induction. However, in the IPL within 1 day, SYN immunofluoresence appeared slightly more intense than in the normal eye (Figures 1C–F). After 1 day, SYN immunoreactivity gradually broadened in the OPL and extended into the inner part of the outer nuclear layer where there was no SYN immunostaining normally, reaching maximum distribution in the OPL and outer nuclear layer on day 7 post injury. On day 14, the distribution of SYN in the OPL and outer nuclear layer narrowed, gradually returning to normal. In this period, SYN immunoreactivity in the IPL was still present in the entire layer, but IPL structure was markedly lost and the IPL became progressively thinner, as reported previously[7,9,10] (Figures 1G–I). SYN immunoreactivity in the sham surgery group was similar to the normal control group (Figure 1B).
Western blot analysis was employed to examine the expression of SYN protein in the retina following acute HIOP. SYN expression was easily detectable in the normal retina as a single 38 kDa band. Expression began to increase and the band became black and thick in the acute HIOP rats at 2 hours compared with the normal control group, and then decreased gradually after 6 hours (Figure 2).
Figure 2.
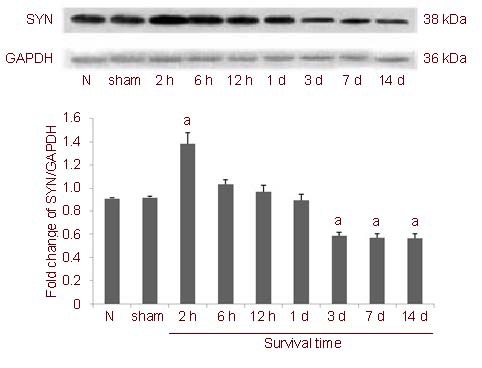
Western blot assay for expression of synaptophysin (SYN) protein in rats with acute high intraocular pressure (HIOP).
Data are presented as mean ± SD. aP < 0.05, vs. normal control group (one-way analysis of variance). To quantify the temporal change in SYN protein, six eyes were evaluated at each survival time point, and all experiments were performed three times.
N: normal control group; sham: sham surgery group; 2 h, 6 h, 12 h, 1 d, 3 d, 7 d, 14 d: survival time of 2, 6, 12 hours, 1, 3, 7 and 14 days after operation.
Statistical analysis showed that expression was significantly upregulated at 2 hours compared with the normal control group, and its relative integrated absorbance value was higher than in the normal control group (P < 0.01). Subsequently, SYN expression gradually diminished. Between 6 hours and 1 day, there was no significant difference in SYN expression (P > 0.05, vs. normal control group; Figure 2). From 3 to 14 days, the SYN expression reached the lowest level, which was significantly lower than in the normal control group (P < 0.01; Figure 2).
Double labeling for SYN and SAP102 in the retina of rats with acute HIOP
In the normal retina, immunofluorescence for SAP102 was distinctly located in the IPL and OPL, and was similar to the distribution of SYN, with a punctate appearance in the inner part of the IPL (Figure 1A’). In the OPL, SAP102 immunoreactivity mainly overlapped with that of SYN, although the distribution of SAP102 in the OPL was closer to the inner nuclear layer, while SYN was closer to the outer nuclear layer (Figure 1A*). In the IPL, SAP102 immunoreactivity mostly overlapped with that for SYN. Furthermore, weak labeling was also present in the inner nuclear layer. There was no significant change in the fluorescence intensity or distribution pattern for SAP102 within 1 day of elevated IOP (Figures 1A’–I’). Both SAP102 and SYN expression in the IPL was decreased, accompanied with a marked reduction in the thickness of the IPL, after 1 day, but their distribution pattern did not change remarkably. It is worth noting that while SAP102 immunoreactivity did not extend beyond the OPL, SYN labeling gradually broadened in the OPL from the third day after injury and spread to the inner part of the outer nuclear layer, where there was no SYN immunostaining under normal conditions. Only SYN labeling was detected in the outer nuclear layer, and there was no double labeling of SAP102 and SYN. Immunofluorescence for SYN and SAP102 in the sham surgery group was similar to the normal control group (Figures 1A*–I*).
DISCUSSION
SYN participates in synaptic vesicle formation and exocytosis, playing an important role in neurotransmitter release[2,11]. SYN participates in multiple crucial aspects of synaptic vesicle trafficking, including the initiation of Ca2+-dependent neurotransmitter release, recycling of synaptic vesicles, synaptogenesis, as well as in synaptic plasticity associated with short-term depression and long-term potentiation[12,13,14]. SYN immunoreactivity has been used to label synaptic densities in many studies, and its expression is a reliable indicator of synaptogenesis[15,16].
In the present study, we found that the expression of SYN in the rat retina following acute elevated IOP had a distinct spatiotemporal pattern. The results of SYN expression in the OPL after 1 day were in accordance with our preliminary work[8], suggesting that synapses in the retinal OPL might undergo functional enhancement or synaptogenesis following elevated IOP determined by western blot method. The reduction in SYN expression after 3 days of elevated IOP might be due to extensive loss of SYN protein in the IPL. SYN expression increased in the IPL in the early stage, with a widened distribution in the OPL in the middle stage of injury, and recovered to normal in the later period. The spatiotemporal alterations indicate that synapses in the retina might undergo plastic changes, internally to externally, following acute IOP elevation, which may be due to enhanced synaptic activity or new synapse formation.
Changes in synaptic function or formation and maturation of new synapses require the coordinated participation of both presynaptic and postsynaptic elements. We sought to clarify whether retinal synaptic plasticity was accompanied by changes in the expression of the presynaptic protein SYN, and if this was linked to alterations of postsynaptic elements as well.
We also examined expression of the postsynaptic marker SAP102 in rats with elevated IOP. SAP102 is a member of the membrane-associated guanylate kinase protein family, which are essential proteins at the postsynaptic density, clustering and anchoring glutamate receptors and other proteins at synapses, including postsynaptic density 95 (SAP90), postsynaptic density 93 (Chapsyn-110), SAP102 and SAP97[17,18]. These proteins are required for the proper localization and function of glutamate receptors and K1 channels, and are involved in neurotransmitter release and nerve growth and development and also play crucial roles in synaptic organization and plasticity[19,20,21,22]. SAP102 is highly expressed at postsynaptic sites of excitatory synapses in both young and mature neurons as well as in dendrites and axons, and mediates receptor trafficking during synaptogenesis[4,5,18]. SAP102 is concentrated in the IPL with a punctate appearance in processes, which are postsynaptic at bipolar cell ribbon synapses (dyads). Furthermore, distinct SAP102 labeling was also present in horizontal cell processes in the OPL, which is inserted as lateral elements into photoreceptor ribbon synapses (triads)[23]. We detected SAP102 immunoreactivity in the entire IPL and OPL. After elevated IOP, the fluorescence intensity and distribution pattern for SAP102 did not show substantial changes. In the IPL, SAP102 expression mostly overlapped with SYN, and their expression gradually decreased with time of injury and with the thinning of the IPL. However, SAP102 immunoreactivity remained confined to the OPL, whereas SYN distribution broadened and extended into the outer nuclear layer. These results indicate that presynaptic changes might not be accompanied by changes in postsynaptic components.
Studies on retinal damage and repair by elevated IOP have mainly concentrated on the protection of retinal ganglion cells (RGCs), such as promoting RGC survival and axon regeneration[24,25,26,27]. However, these protective measures have had limited efficacy, suggesting that not only RGCs, but also other retinal neurons and their connections on the visual pathway upstream of RGCs impact on recovery following injury.
Our experiments confirmed the presence of synaptic plasticity in the rat retina after elevated IOP, and that SYN protein expression exhibited a spatiotemporal pattern. Expression was increased and distribution was widened in the early and middle stages, and returned to normal in the later period. The changes appeared first in the IPL and then spread into the OPL. However, these presynaptic changes were not accompanied by changes in postsynaptic components or the formation of new synapses. Furthermore, these alterations were transient. We speculate that RGCs are the cells most susceptible to elevated IOP injury and are the first to suffer damage. However, visual signaling between the first and second order retinal neurons was likely to have remained intact. Thus, when visual signaling transmitted by RGCs is interrupted, bipolar cells and photoreceptor cells (upstream of RGCs) might make reactive changes, such as increasing protein production or synapse number or enhancing synaptic function to compensate for the damaged visual transmission. Rudzinski et al[28] reported that early after ocular hypertension, the retina might maintain levels of neurotrophins through autocrine or paracrine mechanisms, which provide support for protein synthesis in synapses. However, without any intervention, synaptogenesis was temporary and transient and did not result in the formation of new functional synapses.
Synaptogenesis is a consumptive process, which leads to nutrient (such as neurotrophins) exhaustion. Elevation of IOP would cause reduction of retinal cell function, cell death, collapse of tissue structure, more production of harmful substances, and disrupted homeostasis. These factors might underlie the fact that new functional synapses cannot form effectively and synaptogenesis cannot continue under this experimental condition. Therefore, if appropriate interventions to promote synaptogenesis are conducted in the proper time frame, normal synaptic function might be retained in the retina, and we would gain more time for RGC recovery following injury.
MATERIALS AND METHODS
Design
A randomized controlled animal experiment.
Time and setting
The experiment was performed at the Laboratory of the Department of Anatomy and Neurobiology, Xiangya School of Medicine, Central South University, China, between September 2010 and July 2011.
Materials
Healthy adult Sprague-Dawley rats, weighing 200–250 g, of both genders, were acquired in-house from the Animal Center of Central South University, China (Permission No. SCXK (Xiang) 2009-0004). They were given tap water and food in an environmentally controlled room at a temperature of 25°C and a relatively humidity of 50–60% with a 12-hour light-dark cycle (light on 7:00–19:00). All protocols for animal use were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals, 1985 revision (NIH Publication. No. 86-23).
Methods
Establishment of acute HIOP model in rats
Following the procedure described by Adachi et al[29], the animal model of acute HIOP was prepared as follows. Animals were anesthetized by an intraperitoneal injection of a 1:1 solution (0.5 mL/100 g) of 10% chloral hydrate and 25% urethane. A drop of chloramphenicol eye drop was administered to the conjunctival sac. A sterile disposable intravenous infusion needle, connected to an instillation instrument filled with normal saline, was inserted into the anterior chamber through the lateral border of the left eye. The intraocular pressure (1.83 ± 0.13 kPa) of all left eyes was elevated until the b wave of the flash electroretinogram (Chengdu, China) disappeared, and was maintained for 60 minutes, then slowly decreased to the normal level. Animals were sacrificed after 2 hours, 6 hours, 12 hours, 1 day, 3 days, 7 days or 14 days. For the sham surgery group, the needle was inserted into the anterior chamber without elevating the pressure (2.66 kPa), and animals were sacrificed after 14 days. Each group was composed of 12 rats (6 for immunofluorescence, 6 for western blot assay). The unoperated right eyes served as normal controls.
Retinal tissue preparation
All rats were sacrificed at specific time points by intraperitoneal injection of an overdose of 10% chloral hydrate (0.5 mL/100 g). For immunofluorescence staining, rats were perfused transcardially with 0.9% sodium chloride, followed by 4% paraformaldehyde in PB (0.1 M, pH 7.4). After perfusion, the eyeballs were dissected out, the cornea and lens removed and the eyecups post-fixed in the same fixative for 2 hours at room temperature, and then dehydrated by immersion in sequential sucrose solution gradients (15% and 30% in 0.1 M PB for 4 hours and overnight, respectively) at 4°C. Eye cups were subsequently embedded in optimal cutting temperature medium. Next, 20-μm-thick cryosections were cut with a microtome (Thermo Electron Corporation, Cheshire, UK), and sections containing the optic nerve were transferred to gelatin- coated slides and stored at −20°C until use. To minimize methodological variation, control and experimental sections for each time point were placed on the same slide. For western blot assay, using deep anesthesia with excessive 10% chloral hydrate (0.5 mL/ 100 g), the eyeballs were dissected out, and the cornea, lens and sclera were removed on an ice-cold plate and the retina was cryopreserved at −80°C after weighing for western blot analysis to measure synaptophysin protein levels.
Immunofluorescence for SYN and SAP102
The retinal sections were rinsed three times for 10 minutes with 0.01 M PBS containing 0.1% Tween-20, subsequently immersed in pre-warmed 1:1 solution of 10 mM citrate buffer (pH 6.0) and formamide for 1 hour for antigen retrieval at 65°C in the thermostatic water bath. After cooling, slides were washed three times again as before. Then, retinal sections were incubated in 5% normal bovine serum in 0.1 M PB/0.3% Triton X-100 for 1 hour at room temperature to improve tissue permeability and block any nonspecific binding of the antibodies. The sections were then incubated with a mouse anti-rat SYN monoclonal antibody (1:2 000; Sigma-Aldrich, St. Louis, MO, USA) or a rabbit anti-SAP102 polyclonal antibody (1:150; Abcam, Hong Kong, China) overnight at 4°C. Subsequently, retinae were incubated for 2 hours at room temperature in the dark with Cy™3-conjugated AffiniPure Donkey Anti-Mouse IgG (1:400; Jackson Immuno Research, West Grove, PA, USA) or Alexa Fluor 488-conjugated AffiniPure donkey anti-rabbit IgG (1:400; Jackson Immuno Research). Cell nuclei were stained for 8 minutes in the dark with Hoechst 33258 staining solution (Beyotime Institute of Biotechnology, Haimen, China). Finally, the sections were mounted in 1:1 PBS and glycerol (by volume) and stored at 4°C in the dark. Negative control sections were processed in exactly the same manner except for the replacement of the primary antibody with PBS. All images were captured using a laser scanning fluorescence microscope (Nikon, Tokyo, Japan). For consistency, photographs were always taken at the same distance from both sides of the optic nerve head.
Western blot assay for SYN
Western blot analyses were performed on retinal extracts that had been homogenized by ultrasound homogenizer in 10 volumes of lysis buffer. Protein quantification was conducted using bicinchoninic acid protein assay kit (CWBIO Technology, Beijing, China) in duplicate and the results were averaged. Protein bands were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and subsequently electrotransferred onto a nitrocellulose membrane in Tris-glycine-methanol buffer. The membranes were blocked for 1 hour at room temperature in a blocking solution containing 5% nonfat dry milk, 0.3% Triton X-100 and PB (pH 7.4). The membrane was subsequently incubated with mouse anti-SYN monoclonal antibody (1:2 000; Sigma-Aldrich) in blocking solution overnight at 4°C. The membrane was rinsed three times with 0.1% Tween-20 in PBS for 30 minutes each, followed by incubation for 2 hours at room temperature in the appropriate horseradish peroxidase-conjugated donkey anti-mouse IgG (1:15 000; Zhongshan Goldenbridge Biotechnology, Beijing, China). The blot was washed four times as described above for 30 minutes each, and the resulting bands were visualized by a high sensitivity chemiluminescence detection kit (CWBIO Technology). GAPDH was used as an internal reference control. SYN protein bands and GAPDH bands were scanned (BenQ 8550T, Taiwan, China). Integrated density value of SYN bands in different time points was calculated by Fluor Chem 8900 software (Alpha Innotech Corporation, San Leandro, USA) and normalized against the corresponding GAPDH band.
Statistical analysis
Measurement data were presented as mean ± SD. One-way analysis of variance was used to compare differences among groups in the measurement of SYN by SPSS 13.0 statistical software (SPSS, Chicago, IL, USA). A level of P < 0.05 was considered statistically significant.
Footnotes
Funding: This study was financially sponsored by the Ph.D. Programs Foundation of the Ministry of Education of China, No 20090162110019; the Natural Science Foundation of Hunan Province, No. 10JJ4023; the Fundamental Research Funds for the Central Universities of China, No. 2011QNZT128; Graduate Scientific Research Innovation Projects of Hunan Province in 2011, No. CX2011B047.
Conflicts of interest: None declared.
Ethical approval: The experimental protocol was approved by the Animal Ethics Committee of Central South University, China.
(Edited by Luo XG, Luan XP/Qiu Y/Song LP)
REFERENCES
- [1].Wiedenmann B, Franke WW. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985;41(3):1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- [2].Valtorta F, Pennuto M, Bonanoi D, et al. Synaptophysin: leading actor or walk-on role in synaptic vesicle exocytosis? Bioessays. 2004;26(4):445–453. doi: 10.1002/bies.20012. [DOI] [PubMed] [Google Scholar]
- [3].Pereno GL, Beltramino CA. Timed changes of synaptic zinc, synaptophysin and MAP2 in medial extended amygdala of epileptic animals are suggestive of reactive neuroplasticity. Brain Res. 2010;1328:130–138. doi: 10.1016/j.brainres.2010.01.087. [DOI] [PubMed] [Google Scholar]
- [4].Zheng CY, Petralia RS, Wang YX, et al. SAP102 is a highly mobile MAGUK in spines. J Neurosci. 2010;30(13):4757–4766. doi: 10.1523/JNEUROSCI.6108-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chen BS, Thomas EV, Sanz-Clemente A, et al. NMDA receptor-dependent regulation of dendritic spine morphology by SAP102 splice variants. J Neurosci. 2011;31(1):89–96. doi: 10.1523/JNEUROSCI.1034-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nag TC, Wadhwa S. Differential expression of syntaxin-1 and synaptophysin in the developing and adult human retina. J Biosci. 2001;26(2):179–191. doi: 10.1007/BF02703642. [DOI] [PubMed] [Google Scholar]
- [7].Xiong K, Huang JF, Tong JB, et al. Changes of inner retina under different ischemic reperfusion induced by acute intraocular hypertension in rats. Jiepou Xue Zazhi. 2005;28(1):46–49. [Google Scholar]
- [8].Chen D, Tong JB, Wang H, et al. Synaptophysin expression in rat retina following acute high intraocular pressure. Acta Histochem Cytochem. 2008;41(6):173–178. doi: 10.1267/ahc.08034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cheon EW, Park CH, Kang SS, et al. Betaxolol attenuates retinal ischemia/reperfusion damage in the rat. Neuroreport. 2003;14(15):1913–1917. doi: 10.1097/00001756-200310270-00006. [DOI] [PubMed] [Google Scholar]
- [10].Fernandez DC, Bordone MP, Chianelli MS, et al. Retinal neuroprotection against ischemia-reperfusion damage induced by postconditioning. Invest Ophthalmol Vis Sci. 2009;50(8):3922–3930. doi: 10.1167/iovs.08-3344. [DOI] [PubMed] [Google Scholar]
- [11].Dhingra NK, Ramamohan Y, Raju TR. Developmental expression of synaptophysin, synapsin I and syntaxin in the rat retina. Brain Res Dev Brain Res. 1997;102(2):267–273. doi: 10.1016/s0165-3806(97)00085-0. [DOI] [PubMed] [Google Scholar]
- [12].Ding JY, Kreipke CW, Schafer P, et al. Synapse loss regulated by matrix metalloproteinases in traumatic brain injury is associated with hypoxia inducible factor-1alpha expression. Brain Res. 2009;1268:125–134. doi: 10.1016/j.brainres.2009.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [13].Clare R, King VG, Wirenfeldt M, et al. Synapse loss in dementias. J Neurosci Res. 2010;88(10):2083–2090. doi: 10.1002/jnr.22392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kwon SE, Chapman ER. Synaptophysin regulates the kinetics of synaptic vesicle endocytosis in central neurons. Neuron. 2011;70(5):847–854. doi: 10.1016/j.neuron.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Glantz LA, Gilmore JH, Hamer RM, et al. Synaptophysin and postsynaptic density protein 95 in the human prefrontal cortex from mid-gestation into early adulthood. Neuroscience. 2007;149(3):582–591. doi: 10.1016/j.neuroscience.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jung SH, Lee ST, Chu K, et al. Cell proliferation and synaptogenesis in the cerebellum after focal cerebral ischemia. Brain Res. 2009;1284:180–190. doi: 10.1016/j.brainres.2009.05.051. [DOI] [PubMed] [Google Scholar]
- [17].Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5(10):771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- [18].Fujita A, Kurachi Y. SAP family proteins. Biochem Biophys Res Commun. 2000;269(1):1–6. doi: 10.1006/bbrc.1999.1893. [DOI] [PubMed] [Google Scholar]
- [19].Gardoni F, Marcello E, Di Luca M. Postsynaptic density-membrane associated guanylate kinase proteins (PSD-MAGUKs) and their role in CNS disorders. Neuroscience. 2009;158(1):324–333. doi: 10.1016/j.neuroscience.2008.07.068. [DOI] [PubMed] [Google Scholar]
- [20].Zheng CY, Seabold GK, Horak M, et al. MAGUKs, synaptic development, and synaptic plasticity. Neuroscientist. 2011;17(5):493–512. doi: 10.1177/1073858410386384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Akgul G, Wollmuth LP. Expression pattern of membrane-associated guanylate kinases in interneurons of the visual cortex. J Comp Neurol. 2010;518(24):4842–4854. doi: 10.1002/cne.22491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zheng CY, Wang YX, Kachar B, et al. Differential localization of SAP102 and PSD-95 is revealed in hippocampal spines using super-resolution light microscopy. Commun Integr Biol. 2011;4(1):104–105. doi: 10.4161/cib.4.1.14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Koulen P, Garner CC, Wässle H. Immunocytochemical localization of the synapse-associated protein SAP102 in the rat retina. J Comp Neurol. 1998;397(3):326–336. [PubMed] [Google Scholar]
- [24].Fischer D. Stimulating axonal regeneration of mature retinal ganglion cells and overcoming inhibitory signaling. Cell Tissue Res. doi: 10.1007/s00441-011-1302-7. in press. [DOI] [PubMed] [Google Scholar]
- [25].Almasieh M, Wilson AM, Morquette B, et al. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012;31(2):152–181. doi: 10.1016/j.preteyeres.2011.11.002. [DOI] [PubMed] [Google Scholar]
- [26].Wilson AM, Di Polo A. Gene therapy for retinal ganglion cell neuroprotection in glaucoma. Gene Ther. 2012;19(2):127–136. doi: 10.1038/gt.2011.142. [DOI] [PubMed] [Google Scholar]
- [27].Fu QL, Liao XX, Li X, et al. Soluble Nogo-66 receptor prevents synaptic dysfunction and rescues retinal ganglion cell loss in chronic glaucoma. Invest Ophthalmol Vis Sci. 2011;52(11):8374–8380. doi: 10.1167/iovs.11-7667. [DOI] [PubMed] [Google Scholar]
- [28].Rudzinski M, Wong TP, Saragovi HU. Changes in retinal expression of neurotrophins and neurotrophin receptors induced by ocular hypertension. J Neumbiol. 2004;58(3):341–354. doi: 10.1002/neu.10293. [DOI] [PubMed] [Google Scholar]
- [29].Adachi M, Takahashi K, Nishikawa M, et al. High intraocular pressure-induced ischemia and reperfusion injury in the optic nerve and retina in rats. Graefes Arch Clin Exp Ophthalmol. 1996;234(7):445–451. doi: 10.1007/BF02539411. [DOI] [PubMed] [Google Scholar]


