Abstract
This study aimed to explore the pathological change to hippocampal neurons and the expression of growth associated protein 43 in 21-day-old young rats following chronic intermittent hypoxia. Hematoxylin-eosin staining results showed varying degrees of degeneration and necrosis in hippocampal neurons depending on the modeling time. Immunohistochemistry revealed that growth associated protein 43 expression in young rats following chronic intermittent hypoxia decreased, but that levels were still higher than those of normal rats at each time point, especially 4 weeks after modeling. During 1–5 weeks after modeling, a slow growth in rat weight was observed. Experimental findings indicate that chronic intermittent hypoxia may induce growth dysfunction and necrosis of hippocampal neurons, as well as increase the expression of growth associated protein 43 in young rats.
Keywords: chronic intermittent hypoxia, brain injury, growth associated protein 43, obstructive sleep apnea hypopnea syndrome, hippocampus, young rats, neural regeneration
Abbreviations:
OSAHS, obstructive sleep apnea hypopnea syndrome; CIH, chronic intermittent hypoxia; GAP-43, growth associated protein 43
INTRODUCTION
Obstructive sleep apnea hypopnea syndrome (OSAHS) has a great impact on multiple systems, especially the nervous system, and can induce cognitive dysfunction[1,2,3,4,5,6]. Existing studies regarding OSAHS have mainly focused on adult rat chronic intermittent hypoxia (CIH) models. However, there is a substantial difference between child and adult OSAHS[7,8,9,10], with child OSAHS classified as an independent clinical syndrome[11]. Currently, studies on children with OSAHS are few, and many questions remain unanswered.
Growth associated protein 43 (GAP-43) mainly exists in differentiated neurons that present with axon outgrowth. GAP-43 content is abundant in the neuronal cytoplasm at the early development stage of the nervous system; the expression of GAP-43 may gradually decrease as the brain becomes mature and sharply increases when the brain is injured[12,13,14,15]. Therefore, GAP-43 is an important marker for studying neural regeneration and repair[16,17]. At present, various models of anoxic/ischemic brain damage have been produced in adult or neonatal rats to observe changes in GAP-43 expression[18,19,20,21]. However, it is unknown if changes in GAP-43 expression occur after anoxic brain injury in young rats.
The present study aimed to establish a CIH model in 21-day-old young rats to simulate typical pathophysiological characteristics of OSAHS. Body weight gain of CIH young rats at each week was measured, and neuronal pathology in the hippocampus was observed using hematoxylin-eosin staining. In addition, hippocampal GAP-43 expression was determined by immunohistochemistry. We aim to identify (1) the effect of CIH on body weight growth of young rats; (2) GAP-43 expression changes in hippocampal neurons of CIH young rats at the growth and development period; (3) whether expression changes are consistent with that in adult rats.
RESULTS
Quantitative analysis of experimental animals
A total of 60 21-day-old Sprague-Dawley male rats were randomly divided into a control group (normal feeding) and a CIH group (CIH modeling). Ten rats in each group were selected for experiments at 2, 4, and 6 weeks after modeling. In total, 60 rats were involved in the final result analysis.
Body weight gain of young rats
Increased body weight of CIH young rats at each week was significantly decreased compared with the control group during 1–5 weeks after modeling (P < 0.05 or P < 0.01), and there was no significant difference in body mass gain between CIH young rats and control rats at 6 weeks (P > 0.05; Figure 1).
Figure 1.
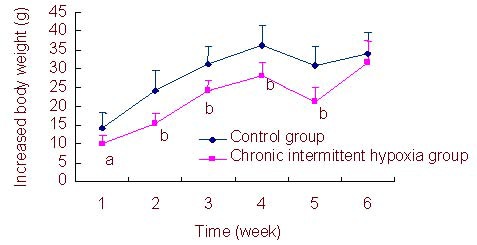
Comparison of increased body weight per week in each group for 6 weeks.
Data are expressed as mean ± SD of ten rats in each group per week. aP < 0.05, bP < 0.01, vs. control group (independent-samples t-test). Body weight gain = the measured body weight on the tested week – the measured body weight on the previous week.
Pathological changes of hippocampal neurons in CIH rats
Hematoxylin-eosin staining and light microscopic observation results showed no degeneration and necrosis of hippocampal neurons in the control group. At 2 and 4 weeks after modeling, hippocampal neurons in the control group were smaller in size, increased in number, had an ordered arrangement, and had unclear intracellular constituents. At 6 weeks, hippocampal neurons in the control group increased in volume, reduced in number, and cell nuclei were visible.
The hippocampal neurons in the CIH group were different at each time point. At 2 weeks after modeling, no apparent degeneration or necrosis of hippocampal neurons was found, and cells had a slightly disordered arrangement. At 4 and 6 weeks, hippocampal neurons showed varying degrees of degeneration and necrosis, which was accompanied by cell swelling, cytoplasmic vacuoles, nuclear shrinkage, and disappearance of intracellular constituents (Figure 2, supplementary Figure 1 online).
Figure 2.
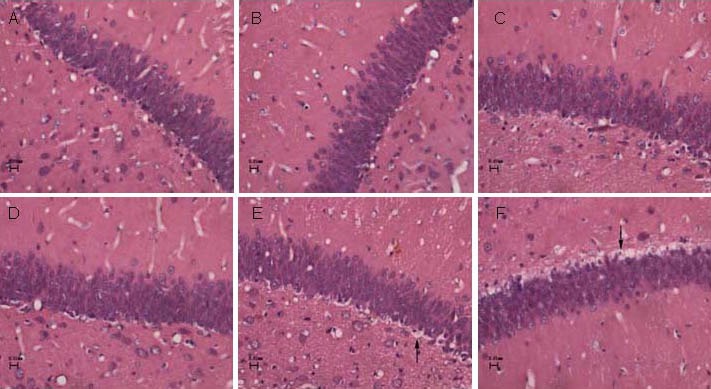
Histological changes in the hippocampal dentate gyrus in the control group and chronic intermittent hypoxia (CIH) group (hematoxylin-eosin staining, light microscope, × 400).
There was no degeneration or necrosis of neurons in the control group at 2 (A), 4 (B), and 6 weeks (C), and the CIH group at 2 weeks (D). At 4 (E) and 6 weeks (F) in the CIH group, necrotic neurons (arrows) were visible.
Compared with surrounding normal cells, cytoplasmic vacuoles appeared and intracellular components disappeared.
GAP-43 expression in the CIH rat hippocampus
Immunohistochemical staining showed that GAP-43-positive immunoreactive products were brown punctate or granular deposits, which mainly distributed in the cytoplasm of hippocampal neurons (Figure 3).
Figure 3.
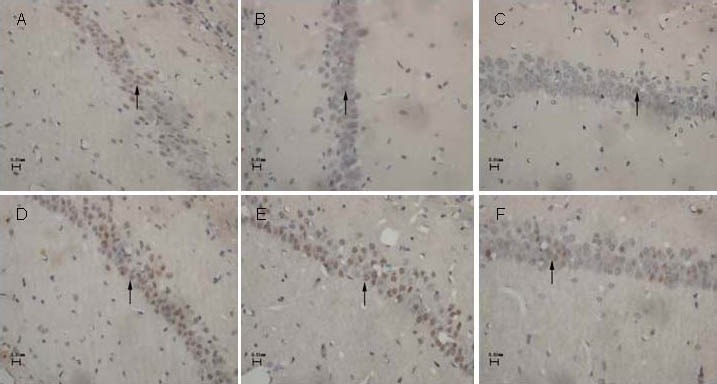
Expression of growth associated protein 43 (GAP-43) in the hippocampal CA1 region of control rats and chronic intermittent hypoxia (CIH) rats (immunohistochemistry staining, light microscope, × 400).
In the control group (A–C) and CIH group (D–F), GAP-43 was expressed as brown/yellow color in the neuronal cytoplasm. GAP-43 expression in the CIH group was stronger than that in the control group.
At 2 (A, D), 4 (B, E), and 6 weeks (C, F) after modeling, GAP-43-positive neurons in the hippocampal CA1 region gradually decreased (arrows).
With the modeling time, GAP-43 expression (absorbance) in the CIH group and control group gradually decreased. In the control group, GAP-43 absorbance values were significantly reduced at 4 and 6 weeks compared with 2 weeks (P < 0.01), and no significant difference was observed between 4 weeks and 6 weeks (P > 0.05). In the CIH group, the absorbance values were similar at 2 and 4 weeks (P > 0.05), while they were significantly reduced at 6 weeks (P < 0.01). The CIH group showed significantly higher GAP-43 absorbance values than the control group at the same time point (P < 0.01; Table 1).
Table 1.
Expression of growth associated protein 43 (absorbance, ×10-2) in the rat hippocampus

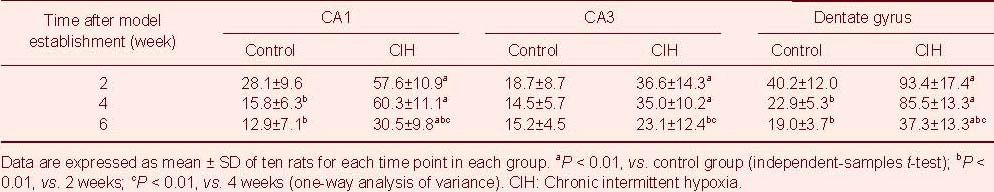
Counting results revealed that the number of GAP-43-positive neurons in the hippocampal CA1 and dentate gyrus region of control rats at 4 and 6 weeks was significantly decreased compared with that at 2 weeks (P < 0.01). In the CIH group, there were significantly less positive neurons in the hippocampal CA1, CA3 and dentate gyrus region at 6 weeks, compared with at 2 and 4 weeks (P < 0.01). The number of GAP-43-positive neurons in the hippocampal CA1, CA3 and dentate gyrus of CIH young rats was significantly higher than that of control rats at each time point (P < 0.01), except the number in the hippocampal CA3 region at 6 weeks (P > 0.05; Table 2).
Table 2.
Comparison of growth associated protein 43-positive cells (n/200 μm × 100 μm) in the rat hippocampus
DISCUSSION
OSAHS is a common sleep problem among children, which can cause growth retardation, mental retardation, hypertension and impairment of learning and memory[22,23]. OSAHS can occur in infancy and adolescence, especially in 2–5 years old children[2]. Twenty-two days after birth is the weaning period of rats, which is equal to the childhood period of human cognitive development[24]. Rats aged 3–12 months are generally classified as adults, and those less than 3 months of age are known as early developing rats[25]. The present study adopted 21-day-old Sprague-Dawley rats, to observe the effect of CIH on body weight gain in early developing rats and GAP-43 expression in the hippocampus.
Our results showed an increase in body weight in both the control group and CIH group. However, growth in CIH rats was significantly slower than that in the control group, which was consistent with the studies of Xia et al[26]. The majority of scholars believe that the reason may be due to the fact that long-term chronic hypoxia affects growth hormone secretion in the pituitary gland, thus leading to growth retardation[27,28]. In addition, the increased weight was not significant at 6 weeks. This could be due to enhanced hypoxia resistance, which reduces the impact on growth. However, the underlying mechanism remains unclear.
GAP-43 plays an important role in nerve fiber growth, development, axonal regeneration and synaptic function maintenance[29]. An increasing number of studies regarding brain damage models have demonstrated that, GAP-43 expression was transiently increased after injury, and then decreased to normal levels, which indicates that GAP-43 can promote the regeneration and repair of injured cerebral neurons, as well as protect brains against injury[14,15]. GAP-43 expression in hippocampal neurons of CIH young rats was significantly increased compared with the control group at each time point, and peak expression levels were longer (for 4 weeks). This result was inconsistent with previous findings[15], which showed a transient increase (3–14 days) of GAP-43 expression in adult rats following brain injury. After brain injury in early developing rats, hippocampal tissue has an active self-repair capacity, suggesting that the nervous system may initiate protective mechanisms and repair damaged neurons immediately after hypoxic brain damage. One of the important repair pathways that promote synaptic growth involves an increase in GAP-43 expression[30]. Increasing GAP-43 expression in the brain of CIH young rats may occur as a result of compensatory mechanisms for hypoxia stimulating non-damaged neurons, thus increasing the synthesis of GAP-43. Also, it is believed to be associated with the release of GAP-43 from the dead nerve cells[13]. In this study, only a small number of hippocampal neurons appeared to degenerate and undergo necrosis, although varying degrees of pathological changes were visible in CIH young rats. This result is evidence that increased GAP-43 expression in the hippocampus of CIH young rats was not completely dependent on the release of necrotic neurons. Regulation of GAP-43 is a very complex process that is affected by many factors, and mechanisms underlying the increase in GAP-43 in CIH rats remain unclear.
In summary, CIH-induced slow body weight gain, different degrees of pathological injury of hippocampal neurons, and enhanced expression of GAP-43 in CIH young rats all support that hypothesis that CIH greatly affects the growth and development of neonatal rats, but has a certain compensatory restorative ability for CIH-induced brain injury in young rats. Further studies are required to conclusively determine the mechanism underlying hypoxic injury stimulation on GAP-43 synthesis and changes in GAP-43 expression.
MATERIALS AND METHODS
Design
A randomized controlled animal experiment.
Time and setting
Experiments were performed from July 2009 to March 2011 in the Physiology and Pathology Laboratory of Luzhou Medical College, China.
Materials
Sixty healthy, male, 21-day-old Sprague-Dawley young rats, weighing 50 ± 5 g, were provided by the Experimental Animal Center of Luzhou Medical College, China (license No. SYXK (Chuan) 2004–065). All experimental procedures adhered to the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[31].
Methods
Establishment of CIH models
The CIH rat model was prepared according to the method of Yang et al[8]. Rats in the CIH group were placed daily in a hypoxic cabin, which was filled with soda lime and silica gel to absorb carbon dioxide and water vapor, the bulkhead was present with small gaps to provide a balance between the cabin pressure and the atmospheric pressure. During experimentation, the cabin was filled with nitrogen at 9:00 a.m. to 5:00 p.m. and discharged with mixing air, for 6 minutes each. The air transport and exhaust unit was programmed and controlled based on monitored oxygen concentrations within the cabin, to ensure 8% minimum oxygen concentration within the hypoxia cabin. Hypoxia gas was expelled and fresh air was added to recover oxygen concentrations to 21%. The carbon dioxide concentration in the cabin was maintained at < 3%, and the temperature was 22 ± 2°C. During hypoxia intervention, rats were allowed normal feeding and drinking. Control rats were placed in the same hypoxia cabin, which was filled with fresh air, and other treatments were performed in the same as that of the CIH group.
Body weight determination
The body weight of experimental rats was measured at the beginning of the experiment, and then once per week. Body weight gain = body weight measured on the tested week – body weight measured on the previous week.
Pathological changes of hippocampal neurons detected by hematoxylin-eosin staining
Ten rats in each group were anesthetized with ether and perfused with 4% (w/v) paraformaldehyde at 2, 4, and 6 weeks after CIH modeling. The brain was harvested and tissues were cut into three pieces at the optic chiasm posterior border and the junction between the cerebral peduncle and the pons[32], fixed in 4% (w/v) paraformaldehyde for more than 4 hours, followed by routine dehydration, xylene transparency and embedded in paraffin. Sections were cut at 4 μm, stained with hematoxylin-eosin and observed under light microscopy (Nikon, Tokyo, Japan).
Hippocampal GAP-43 expression detected by immunohistochemical staining
Hippocampal tissue was cut into slices. Specimens were conventionally dewaxed to water and incubated with 3% (v/v) H2O2 at room temperature for 10 minutes, to block endogenous peroxidase. Sections subjected to heat-induced antigen retrieval, were incubated with normal goat serum at 37°C for 30 minutes, and incubated with rabbit anti-GAP-43 polyclonal antibody (Bioworld Technology Inc., St. Louis, MO, USA; 50 μL; 1:200) at 4°C overnight. Sections were washed, and incubated with reagents using a two-step non-biotin detection kit (polymer auxiliary agent and horseradish enzyme-conjugated anti-rabbit IgG poly-antibody; Beijing Zhongshan Golden Bridge Biological Technology Co., Ltd, Beijing, China; 50 μL; 1:1). Diaminobenzidine (Beijing Zhongshan Golden Bridge Biological Technology Co., Ltd) was used for coloration for 5–10 minutes. Cells stained brown/yellow in the cytoplasm were considered positive. A series of procedures were performed, including hematoxylin mild counterstain, dehydration, transparency and mounting. A negative control was included, which was treated with PBS instead of the primary antibody. Specimens were viewed by light microscopy.
Image analysis
Determination of hippocampal GAP-43 absorbance value: GAP-43 absorbance in hippocampal neurons was measured using the ECLIPSE88i photomicrography system (Nikon, Tokyo, Japan) and Image-Pro Plus 6.0 image analysis software (Media Cybernetics Inc., Silver Spring, MD, USA). Three high-power fields (400 ×) in the hippocampal dentate gyrus, CA1, CA2, CA3 and CA4 were randomly selected from three sections in each rat, to determine the absorbance value of positive staining. The average value was calculated, serving as the absorbance value of the rat hippocampus. At the same time, the absorbance of the optic beam on the same section was taken as the background, GAP-43 absorbance value – background absorbance = corrected absorbance, which is the actual absorbance value of GAP-43 immunoreactive products.
Counting of hippocampal GAP-43 neurons: Three non-overlapping high-power fields (400 ×) in the hippocampal dentate gyrus, CA1 and CA3 were randomly selected from three sections in each rat, to count the number of positive neurons within an area of 200 μm × 100 μm. The average value was considered as the number of GAP-43-positive cells at each time point.
Statistical analysis
Measurement data were expressed as mean ± SD and data were statistically analyzed with SPSS 13.0 statistical software (SPSS, Chicago, IL, USA). The data at different time points within the same group were compared with one-way analysis of variance, and data among groups were compared using the independent-samples t-test. A P < 0.05 was considered a significant difference.
Acknowledgments:
We would like to thank Xuanshi Chen and Xingwang Sun from the Department of Pathology in Luzhou Medical College in China for guidance with immunohistochemistry procedures, and thank Bin Luo, Qinghua Hou, Chao Lu, Min Ye, and Xiaofen Ye who are students in Luzhou Medical College for providing great help in the animal modeling.
Footnotes
Funding: This study was supported by a grant from Luzhou Medical College, China.
Conflicts of interest: None declared.
Ethical approval: The experiment was approved by the Experimental Animal Ethics Committee of Luzhou Medical College in China.
Supplementary information: Supplementary data associated with this article can be found, in the online version, by visiting www.nrronline.org.
(Edited by Zhu DL, Liu ZX/Yang Y/Song LP))
REFERENCES
- [1].Li RC, Row BW, Kheirandish L, et al. Nitric oxide synthase and intermittent hypoxia-induced spatial learning deficits in the rat. Neurobio Dis. 2004;17(1):44–53. doi: 10.1016/j.nbd.2004.05.006. [DOI] [PubMed] [Google Scholar]
- [2].Liner LH, Marcus CI. Ventilatory management of sleep-disordered breathing in children. Curr Opin Pediatr. 2006;18(3):272–276. doi: 10.1097/01.mop.0000193301.63259.84. [DOI] [PubMed] [Google Scholar]
- [3].Sans-Capdevila O, Gozal D. Neurobiological consequences of sleep apnea syndrome in children. Rev Neurol. 2008;47(12):659–664. [PubMed] [Google Scholar]
- [4].Torelli F, Moscufo N, Garreffa G, et al. Cognitive profile and brain morphological changes in obstructive sleep apnea. Neuroimage. 2011;54(2):787–793. doi: 10.1016/j.neuroimage.2010.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lurie A. Obstructive sleep apnea in adults: epidemiology, clinical presentation, and treatment options. Adv Cardiol. 2011;46:1–42. doi: 10.1159/000327660. [DOI] [PubMed] [Google Scholar]
- [6].Rajagopalan N. Obstructive sleep apnea: not just a sleep disorder. J Postgrad Med. 2011;57(2):168–175. doi: 10.4103/0022-3859.81866. [DOI] [PubMed] [Google Scholar]
- [7].Xue QF, Xie JM, Hu CG, et al. A model of isobaric hypoxic pulmonary hypertension in rats. (381-382).Zhonghua Jiehe he Huxi Zazhi. 1989;12(6):350–352. [PubMed] [Google Scholar]
- [8].Yang Y, Tan SY, Zhang XM, et al. Effects of intermittent hypoxia on cognition and the ultrastructure in CA1 region of the hippocampus in rats. Zhongguo Shenjing Mianyi Xue he Shenjingbing Xue Zazhi. 2007;3(14):157–163. [Google Scholar]
- [9].Iturriaga R, Moya EA, Del Rio R. Cardiorespiratory alterations induced by intermittent hypoxia in a rat model of sleep apnea. Adv Exp Med Biol. 2010;669(9):271–274. doi: 10.1007/978-1-4419-5692-7_55. [DOI] [PubMed] [Google Scholar]
- [10].Ward CP, McCoy JG, McKenna JT. Spatial learning and memory deficits following exposure to 24 h of sleep fragmentation or intermittent hypoxia in a rat model of obstructive sleep apnea. Brain Res. 2009;1294(6):128–137. doi: 10.1016/j.brainres.2009.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hoban TF. Sleep disorders in children. Ann N Y Acad Sci. 2010;1184:1–14. doi: 10.1111/j.1749-6632.2009.05112.x. [DOI] [PubMed] [Google Scholar]
- [12].Hsu JY, Stein SA, Xu XM. Temporal and spatial distribution of growth-associated molecules and astroglial cells in the rat corticospinal tract during development. J Neurosci Res. 2005;80(3):330–340. doi: 10.1002/jnr.20472. [DOI] [PubMed] [Google Scholar]
- [13].Farina V, Gadau S, Lepore G, et al. Growth-associated protein expression in the frontal and occipital cortices of callosotomized rats. Funct Neurol. 2004;19(3):181–184. [PubMed] [Google Scholar]
- [14].Guo YL, Sun GL, Gong WW, et al. Relationship between growth-associated protein and Nogo protein gene expression after cerebral ischemia reperfusion and nervous behavioral function. Zhongguo Linchuang Kangfu. 2004;8(25):5424–5425. [Google Scholar]
- [15].Granziera C, D’Arceuil H, Zai L, et al. Long-term monitoring of post-stroke plasticity after transient cerebral ischemia in mice using in vivo and ex vivo diffusion tensor MRI. Open Neuroimag J. 2007;1:10–17. doi: 10.2174/1874440000701010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kawasaki T, Nishio T, Kawaguchi S, et al. Spatiotemporal distribution of GAP-43 in the developing rat spinal cord: a histological and quantitative immunofluorescence study. Neurosci Res. 2001;39(3):347–358. doi: 10.1016/s0168-0102(00)00234-0. [DOI] [PubMed] [Google Scholar]
- [17].Crestini A, Piscopo P, Malvezzi Campeggi L, et al. Proteic marker of hypoxic -ischemic damage. Ann Ist Super Sanita. 2001;37(4):581–591. [PubMed] [Google Scholar]
- [18].Zhou Y, Xiong Y, Yuan SY. Effect of tacrolimus on growth-associated protein-43 expression in the hippocampus of neonatal rats with hypoxic-ischemic brain damage. Zhongguo Dang Dai Er Ke Za Zhi. 2009;11(1):65–68. [PubMed] [Google Scholar]
- [19].Valdez SR, Patterson SI, Ezquer ME, et al. Acute sublethal global hypoxia induces transient increase of GAP-43 immunoreactivity in the striatum of neonatal rats. Synapse. 2007;61(3):124–137. doi: 10.1002/syn.20353. [DOI] [PubMed] [Google Scholar]
- [20].Miyake K, Yamamoto W, Tadokoro M, et al. Alterations in hippocampal GAP-43, BDNF, and L1 following sustained cerebral ischemia. Brain Res. 2002;935(1-2):24–31. doi: 10.1016/s0006-8993(02)02420-4. [DOI] [PubMed] [Google Scholar]
- [21].Hai J, Su SH, Lin Q, et al. Cognitive impairment and changes of neuronal plasticity in rats of chronic cerebral hypoperfusion associated with cerebral arteriovenous malformations. Acta Neurol Belg. 2010;110(2):180–185. [PubMed] [Google Scholar]
- [22].Tatlipinar A, Duman D, Uslu C, et al. The effects of obstructive sleep apnea syndrome due to adenotonsillar hypertrophy on the cardiovascular system in children. Turk J Pediatr. 2011;53(4):359–363. [PubMed] [Google Scholar]
- [23].Sinha D, Guilleminault C. Sleep disordered breathing in children. Indian J Med Res. 2010;131:311–320. [PubMed] [Google Scholar]
- [24].Jiang W, Duong TM, de Lanerolle NC. The neuropathology of hyperthermic seizures in the rat. Epilepsia. 1999;40(1):5–19. doi: 10.1111/j.1528-1157.1999.tb01982.x. [DOI] [PubMed] [Google Scholar]
- [25].Hoffmann H, Hunt P, Spear NE. Ontogenetic differences in the association of gustatory and tactile cues with lithium chloride and footshock. Behav Neural Biol. 1990;53(3):441–450. doi: 10.1016/0163-1047(90)90324-y. [DOI] [PubMed] [Google Scholar]
- [26].Xia HF, Lv LY, Zhang DY, et al. The influence of chronic episodic hypoxia to the development of juvenile Sprague-Dawley rats. Sichuan Yixue. 2010;31(6):695–696. [Google Scholar]
- [27].Nieminen P, Löppönen T, Tolonen U, et al. Growth and biochemical markers of growth in children with snoring and obstructive sleep apnea. Pediatrics. 2002;109(4):e55. doi: 10.1542/peds.109.4.e55. [DOI] [PubMed] [Google Scholar]
- [28].Lanfranco F, Motta G, Minetto MA, et al. Growth hormone/insulin-like growth factor-I axis in obstructive sleep apnea syndrome: an update. J Endocrinol Invest. 2010;33(3):192–196. doi: 10.1007/BF03346580. [DOI] [PubMed] [Google Scholar]
- [29].Strata P, Buffo A, Rossi F. Mechanisms of axonal plasticity. Arch Ital Biol. 1999;137(2-3):181–192. [PubMed] [Google Scholar]
- [30].Lu LQ, Zhao CR, Pu ZX. Expression of growth-associated protein in hippocampus of neonatal rats with hypoxic-ischemic brain damage. Disan Junyi Daxue Xuebao. 2006;28(21):2160–2162. [Google Scholar]
- [31].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]
- [32].Paxions G, Watson C. Beijing: People's Medical Publishing House; 2005. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]


