Abstract
In this study, we investigated the effects of mobile phone radiation on spatial learning, reference memory, and morphology in related brain regions. After the near-field radiation (0.52–1.08 W/kg) was delivered to 8-week-old Wistar rats 2 hours per day for 1 month, behavioral changes were examined using the Morris water maze. Compared with the sham-irradiated rats, the irradiated rats exhibited impaired performance. Morphological changes were investigated by examining synaptic ultrastructural changes in the hippocampus. Using the physical dissector technique, the number of pyramidal neurons, the synaptic profiles, and the length of postsynaptic densities in the CA1 region were quantified stereologically. The morphological changes included mitochondrial degenerations, fewer synapses, and shorter postsynaptic densities in the radiated rats. These findings indicate that mobile phone radiation can significantly impair spatial learning and reference memory and induce morphological changes in the hippocampal CA1 region.
Keywords: mobile phone, memory, Morris water maze, electron microscopy, synapse, postsynaptic density, quantitative analysis, ultrastructure, neural regeneration
Abbreviations:
EMR, electromagnetic radiation; MWM, Morris water maze; PSD, postsynaptic density
INTRODUCTION
A number of epidemiological investigations have indicated that excessive electromagnetic radiation (EMR) exposure can impair cognitive functions[1]. Animal models focusing on histopathological and functional alterations have indicated that EMR causes progressive defects in hippocampal-dependent learning and memory[2,3]. Radiation-induced brain injuries may be a widespread problem of which there is increasing concern.
In rodents, performance on hippocampal-dependent spatial learning and memory tasks is compromised after whole-brain radiation. Specifically, radiation of young adult rodents leads to impaired performance on the Morris water maze (MWM)[3,4], Barnes maze[5], passive avoidance[4,6] and T-maze tasks[7]. However, cognitive changes after brain radiation have not been studied adequately. With the dramatic increase in mobile phone use, establishing the long-term cognitive consequences of brain radiation exposure is important.
Previous studies have shown that performance on the MWM task is dependent on hippocampal integrity[8]. Lesion studies have indicated that partial or complete hippocampal ablations lead to impaired performance in spatial learning and memory[8,9]. The underlying mechanisms for such radiation-related cognitive deficits have not been defined clearly, but they likely involve changes in multiple neural processes, such as synapse formation, pruning, and synaptic plasticity[10,11,12,13,14]. Synaptic changes may be reflected either by a loss of synapses in the dentate gyrus[10,11] or by changes in synaptic function, such as the impaired synaptic transmission and long-term potentiation observed in the hippocampus of old rats[12,13,14]. Synaptic function changes are also reflected in alterations in the morphology of synaptic configurations, permitting an indirect assessment of synaptic function in which synaptic profiles can be quantified[15].
The present study aimed to test the hypotheses that 1 month of exposure to mobile phone radiation leads to impaired spatial learning and reference memory, and these impairments are related to changes in the number of synapses and length of postsynaptic density (PSD). Consistent with these hypotheses, we found that mobile phone radiation induced impairments in MWM performance, fewer synapses, mitochondrial degeneration, and shorter PSDs.
RESULTS
Quantitative analysis of experimental animals
A total of 60 Wistar rats were included in this study. They were randomly assigned to an irradiated group and a sham-irradiated group, with 30 rats in each group. The rats in the irradiated group were exposed to the radiation at a specific absorption rate of 0.52–1.08 W/kg, whereas the rats in the sham-irradiated group were exposed at a specific absorption rate of 0 W/kg. All 60 rats were included in the final analysis.
EMR impairs MWM performance
Training trial: The path length to the platform for the irradiated rats did not differ from that for sham-irradiated rats during the first day. However, the irradiated rats showed less improvement during the last 3 days of the test (Figure 1B). A significant effect was observed for both the escape latency (P < 0.05; Figure 1A) and the escape path length (P < 0.05; Figure 1B), with irradiated animals exhibiting both larger distance moved and longer escape latency during the training trails compared with the sham-irradiated animals. Although both groups showed improved location of the platform across the training trials, the irradiated rats were impaired in spatial learning after 1 month of radiation exposure compared with the sham-irradiated rats.
Figure 1.
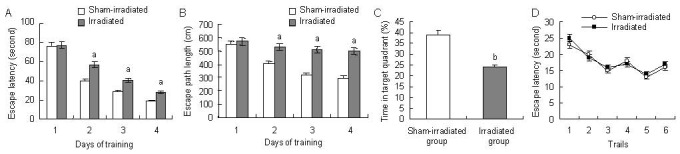
Performance of the irradiated and age-matched sham-irradiated control Wistar rats in the training and probe trials of the Morris water maze.
(A) Escape latency to the platform as a function of the training trial.
(B) Escape path length to the platform as a function of the training trial.
(C) Percentage of time spent in the target quadrant during the probe trial.
(D) Escape latency to the platform in the visible platform trial.
Data are expressed as mean ± SEM, and there were 30 rats in each group. aP < 0.05, bP < 0.01, vs. sham-irradiated group (one-way analysis of variance with Bonferroni's post hoc test).
Probe trial: The frequency of crossings where the platform had been placed during training and the percentage of time spent in the quadrant where the platform had been placed during training, were lower in the irradiated rats compared with sham-irradiated rats (P < 0.01; Figure 1C). Video recordings revealed that the irradiated animals exhibited robust swimming but did not use the spatial information learned during the 4 days of training regarding the submerged platform. In contrast, sham-irradiated rats exhibited a clear preference for the quadrant in which the platform was located during training, indicating that they effectively consolidated and retrieved the learned spatial information.
In visible platform learning test, there were no significant differences in escape latencies (P > 0.05; Figure 1D) and swimming speeds (data not shown) between the irradiated and sham-irradiated rats. Thus, the effects of radiation exposure on spatial learning and memory do not appear to be due to altered motor activities or visual abilities in our experimental conditions.
EMR impairs synaptic structure in the hippocampal CA1 region
Through examination of pyramidal neurons and synapses in the hippocampal CA1 region, we found differences in the gross appearance of the neuropil between the sham-irradiated and irradiated animals. In the sham-irradiated rats, the neuropil was continuous with abundant, tightly packed neuroglial profiles. The neuropil was rich in synaptic contacts (Figure 2A; black arrows). In the irradiated animals, the neuropil showed numerous scant and empty areas with multiple “blank” spaces containing profiles that appeared damaged and sparse synapses (Figure 2B).
Figure 2.
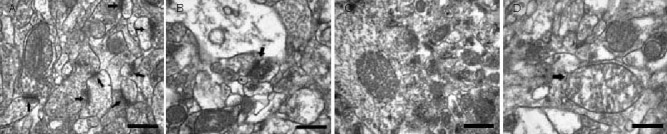
Radiation-induced ultrastructural disarray in the hippocampal CA1 neuropil of Wistar rats (transmission electron microscopy, scale bars: 0.5 μm).
(A) The stratum radiatum of the hippocampal CA1 region from a sham-irradiated rat shows an abundance of tightly packed neuro-glial profiles with numerous synaptic contacts (arrows).
(B) The stratum radiatum of the hippocampal CA1 region from an irradiated rat from a different litter shows a striking scarcity of synaptic contacts (synaptic contacts are indicated by arrow) and a gross loss of neuro-glial profiles.
(C) Mitochondria in the hippocampal CA1 region of sham-irradiated rats appear healthy with clear double membranes and tight, orderly cristae.
(D) Mitochondria in the hippocampal CA1 region of irradiated rats are swollen with balloon-like cristae (arrow).
In addition to the changes in the neuropil, we observed ultrastructural changes in the mitochondria in the hippocampal CA1 region. In the sham-irradiated animals, the mitochondria appeared to have healthy membranes and tight orderly cristae (Figure 2C). In the irradiated animals, the mitochondria appeared swollen with balloon-like cristae (Figure 2D).
The numerical density of CA1 pyramidal neurons was compared between the sham-irradiated and irradiated groups. No significant effects of radiation on neuronal density were detected (P > 0.05; Figure 3A). The synaptic densities in the CA1 stratum radiatum were determined in the two groups (Figure 3B) and normalized to the numerical density of CA1 pyramidal neurons by deriving the ratios of total synapses per neuron. Analysis of the ratio of total synapses per neuron between the sham-irradiated and irradiated groups revealed a main effect of radiation exposure, with a significant decrease in synapses per neuron in the irradiated group compared with sham-irradiated group (P < 0.05; Figure 3C).
Figure 3.
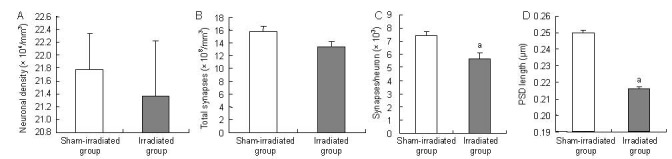
The number of neurons and synapses, ratio of total synapses per neuron, postsynaptic density (PSD) length in the stratum radiatum of the hippocampal CA1 of Wistar rats.
(A) Number of CA1 pyramidal neurons.
(B) Number of synapses in the CA1 stratum radiatum.
(C) The ratio of total synapses per neuron in the CA1 stratum radiatum.
(D) The PSD length in the CA1 stratum radiatum.
Data are expressed as mean ± SEM, with 30 rats in each group. aP < 0.05, vs. sham-irradiated group (Student's t-tests).
We also evaluated whether radiation exposure induced changes in the length of the PSDs. In the CA1 stratum radiatum, the irradiated rats showed shorter PSDs than the sham-irradiated rats (P < 0.05; Figure 3D).
Thus, although 1 month of radiation exposure did not decrease the number of pyramidal neurons in the hippocampal CA1 region, there was a significant loss of synapses in the stratum radiatum in irradiated compared with sham-irradiated rats. Moreover, the PSD length, which is the parameter that is correlated with synaptic function, was smaller in the irradiated rats.
DISCUSSION
There is a growing concern regarding the potential deleterious effects of exposure to radio-frequency EMR. With the worldwide increase in mobile phone use, there is a need for research concerning the effects of EMR on the brain. The present results suggest that EMR in middle-aged Wistar rats leads to impaired spatial learning and reference memory. This demonstration of cognitive deficits in a hippocampal-dependent task supports the use of this model system to address the underlying synaptic changes induced by EMR. In addition, to our knowledge, the present study demonstrates for the first time that radiation-induced cognitive impairment may be associated with changes in synaptic organization.
Animal studies have addressed EMR-induced cognitive impairments after single-dose radiation schedules. For example, radiation at a specific absorption rate of 0.02–4.0 W/kg produced cognitive deficits when applied to fetal, early postnatal, or young adult rodents from 4 weeks to 12 months of age[16,17,18]. However, some studies indicated that the effects of such specific absorption rate of whole-brain radiation do not produce learning and memory deficits under the same conditions[19,20].
Spatial navigation is an important and complex cognitive skill that is often engaged in conjunction with mobile phone use[21]. Behavioral studies of radiation-associated changes in a hippocampal-dependent task in 10–12 weeks old rats that received a GSM (900/1 800 MHz) mobile phone radiation, found cognitive deficits at 4 weeks post-irradiation compared with the un-irradiated controls using the MWM task[6,22]. Using the same test, the present study revealed a significant cognitive impairment in irradiated rats. Interestingly, another recent behavioral study did not reveal any significant differences in MWM performance[23]. However, in that study, the rats were exposed to radiation during early development and studied later in adulthood. The extended time lapse between the period of exposure and the behavioral assessments may therefore have allowed for compensatory responses or repair in the areas affected by the EMR exposure.
In investigations of the effects of EMR in humans, Mann et al[24] found changes in the electroencephalography pattern in sleeping subjects during an 8-hour exposure to GSM radiofrequency radiation. Increased responsiveness in choice reaction time during analogue simulation has been reported, and GSM-like exposure at 915 MHz produced a trend towards increased responsiveness[25]. Luria et al[26] reported that the average frequency of right-hand responses following left-side radiation exposure was significantly longer than following right-side exposure or sham-exposure averaged together during the first two trial blocks. That study demonstrates that the effects of radiofrequency radiation exposure may be time-dependent. Based on these studies, further investigations of EMR effects on cognitive functions may contribute to our understanding of learning and memory impairments in humans and rats.
As described above, the effects of EMR include compromised spatial learning and rapid loss of newly acquired information[21,22]. The structural and electrophysiological changes associated with these radiation-related behavioral deficits have not been defined clearly. It is possible that a loss of hippocampal neurons, a loss of synaptic connections, or both contribute to the observed impairments in rats. However, the present study indicates that CA1 neurons are not lost following radiation exposure. This finding, together with previous reports of synaptic loss, is probably the best current pathologic correlate of cognitive decline. That synaptic dysfunction is evident long before neurons are lost[27,28] indicates that the radiation-related learning and memory impairments may be due to subtle changes in synaptic organization.
Synapses are the places where information is transferred between neurons, and nervous system function is based on their activity. Synaptic loss and synaptic structural changes may be associated with reduced synaptic function and concomitantly impaired cognitive abilities[29]. Geinisman et al[30] have demonstrated that aged animals with memory impairments show a loss of perforated axospinous synapses in the dentate gyrus of the hippocampal formation in comparison with either young adults or aged rats with intact memory functions. This finding suggests that the loss of perforated axospinous synapses in the hippocampal formation underlies age-related deficits in spatial memory. In our experiments, we found decreased synaptic densities in the hippocampus following EMR. These decreases in synaptic densities may result in decreased synaptic function and impaired cognitive abilities in irradiated rats.
In addition, we showed that the PSD length of hippocampal CA1 synapses was smaller following 1 month of radiation exposure. The PSD includes cytoskeletal elements and scaffolding molecules that are critical for signal transduction, and both the NMDA and AMPA subtypes of glutamate receptors are clustered in PSDs[15,31]. Change in these receptors and enzymes may cause ultrastructural changes in the PSD, such as changes in PSD height that may be closely associated with cognitive impairments. Shortening of the PSD by radiation is a morphological correlate of weaker synaptic transmission and reduced synaptic complexity, membrane recycling, and synaptic function[32,33].
Moreover, the functions of mitochondria, including ATP production, are essential for maintaining normal synaptic physiology[34,35]. They can potentiate inhibitory drives by heightening inhibitory synaptic activity mediated by GABAA receptors or silence excitatory (NMDA-mediated) synaptic transmission[36]. Thus, it is possible that radiation-induced degenerative changes in mitochondria and concomitant decreases in ATP production preferentially impairs activated inhibitory synaptic functions because of an inadequate metabolic ratio of supply to demand. However, the precise mechanisms for the selective homeostatic changes in neuronal function following EMR exposure remain to be examined.
We conclude that radiation induces marked morphological disturbances in the hippocampal CA1 region. This may, at least in part, contribute to learning and memory impairments that occur after exposure to EMR.
MATERIALS AND METHODS
Design
A completely randomized controlled animal experiment.
Time and setting
These experiments were conducted at the Institute of Basic Medical Sciences, Chengde Medical University, China between July 2009 and February 2011.
Materials
Sixty inbred Wistar rats, with an equal gender ratio, aged 8 weeks and weighting 270–300 g, were provided by the Chinese Academy of Science (permission No. SCXK (Jing) 2009-0003). The animals were housed according to standard regulations of the National Institutes of Health in regular laboratory cages, three rats per cage, under a standard 22°C room temperature with artificial daylight illumination. Rodent chow and tap water were provided ad libitum. The experimental procedure was approved by the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[37].
Methods
Exposure conditions
Thirty male and thirty female rats were divided into two groups with an equal number of male and female rats in each group. Each animal was given a code and the rats were randomly divided into two groups with reference to the code. Before each set of experiments, careful dosimetry was performed by measuring the mean power density of the radiation emitted by a Mobile Communication-900 MHz (GSM-900) phone (Hewlett-Packard Corporation, Palo Alto, CA, USA) at 900 MHz with a field meter using a probe placed inside the cage with the animals. In addition, we measured in the same way the mean electric and magnetic field intensities at the extremely low frequency range. The exposure values measured using the field meter were in general below the established exposure limits. The animals in the irradiated group were exposed to radiation for 2 hours every day over 1 month, with a specific absorption rate ranging from 0.52 to 1.08 W/kg[38], depending on rat position. The control rats were sham irradiated. In this study, the rats were exposed to EMR within their Plexiglas cages, and the probe from the mobile phone test was placed in the middle of and underneath the cage. Six rats were exposed concurrently in each session using “variable whole body exposure conditions”. To simulate human voices and activate the mobile phone electric and magnetic field emissions, a radio station played at 60 dB throughout the exposure.
Learning and memory assessed by the MWM test
Spatial reference memory was assessed using a MWM (120 cm in diameter; Academy of Medical Sciences Institute of Medicine, Beijing, China) filled with water maintained at 23 ± 1°C in a suitably equipped room with a constant temperature and humidity[39]. The surface of a clear Plexiglas movable escape platform (8 cm × 28 cm) was submerged 1 cm below the water surface. The spatial cues included the consistent position of the researchers and the yellow curtain, which was about 50 cm from the periphery of the pool. All rats were allowed 120 seconds of free swimming to habituate to the environment. Then, the animals were trained to climb on the submerged escape platform. For the spatial learning component of the MWM test, the tank was divided into four quadrants of equal sizes (designated as quadrants I, II, III, and IV relative to the spatial cues) with the escape platform located in quadrant IV for all training trials. The animals were always faced towards the wall when they were carefully placed into the maze at the four different starting positions, equally spaced in the center of a quadrant. The starting location for each trial was chosen randomly. In each trial, rats that did not find the platform within 120 seconds were placed on the platform. At the end of each trial, the animals were allowed to remain on the platform for 20 seconds. They were then returned to their home cage and left there for 15 minutes before the beginning of the next trial. Each rat performed four trials daily for 4 days. On day 5, 24 hours after the last training trial, a 90-second retrieval test without the platform was used to examine long-term memory. Finally, visible platform trials were conducted on day 6 to assess visual acuity.
Transmission electron microscopy of hippocampal structures
After the MWM test, the rats were anesthetized with ketamine 100 mg/mL and xylazine 20 mg/mL at a 1:1 ratio according to 0.1 mL/kg of body weight. They were then perfused transcardially with phosphate buffered saline (pH 7.4) followed by fixative (2% paraformaldehyde/2.5% glutaraldehyde). Brains were sectioned coronally at 200 μm on a vibratome. The CA1 region containing the dorsal hippocampus was localized and removed based on anatomical maps[40]. The blocks were rinsed in 0.1 M PBS and fixed again with 1% OsO4 in 0.1 M of cacodylate buffer for 1 hour. Then, the blocks were dehydrated with a series of ethanol dilutions and embedded in Epon 812 and cut on an ultramicrotome at either 1 μm (semithin sections) or 70 nm (ultrathin sections) thickness. The brain analysis was limited to the dorsal hippocampus because this area is more closely associated with spatial learning and memory performance than the ventral hippocampus in rats[41,42].
We took 12 dissector pairs per animal from semithin sections for stereological quantification of neurons and 30 physical dissectors from three blocks per animal for stereological quantification of synaptic profiles. Pairs of serial sections were chosen randomly and each dissector contained a pair of photomicrographs (× 5 000; Hitachi 7500 electron microscope, Tokyo, Japan) from the serial ultrathin sections through each CA1 layer. From these pairs of sections, we derived the average number of synapses/layer for each individual. The pairs of serial sections were chosen randomly[43], consistent with the requirements for stereological analysis. To provide sampling of synaptic profiles and neurons through coincident anatomical space, semithin sections were collected from alternating sectors in individual blocks. Since our focus was on synapses, our electron micrographs mainly depicted neuropil. The investigator taking electronmicrographs was blind to the experimental conditions.
Quantitative analysis of pyramidal neurons and synaptic profiles in hippocampal CA1 region
Neurons were identified by the presence of a definitive neuronal nucleus with a clear nuclear membrane. The numerical density of pyramidal neurons in the CA1 stratum pyramidale was quantified with the physical dissector technique[44]. For each pair of ultrathin sections, a ‘reference’ and an ‘empirical’ section were designated, a counting frame was superimposed and the neurons were counted. The number of nuclei per counting frame was determined as from those that were present in the ‘empirical’ section but not in the ‘reference’ section.
Synapses were identified by the presence of presynaptic components, with at least three synaptic vesicles[45] and a clearly defined PSD. The numerical density of synaptic profiles in the stratum radiatum of hippocampal CA1 was determined using StereoInvestigator software (MicroBrightField Inc., Colchester, VT, USA). For each serial pair of micrographs (’reference’ and ‘look-up’), a counting frame of known area was superimposed and synaptic profiles were quantified[44]. If the synapses were present in the empirical but not in the reference section, they were counted and the overlapping synapses were excluded from the study.
The mathematical formula[45] used for calculating the numerical density of synaptic profiles and neurons is as follows:
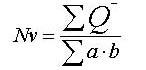
where Q− is the number of counting objects (PSDs or neuronal nuclei) per counting frame that were present in the empirical but not in the reference section, a is the area of the counting frame, and b is the height of the physical dissector.
For all synaptic profiles identified in the CA1 stratum radiatum in the present study, the PSD length was determined using Image-Pro Plus 6.1 computer software (MediaCybernetics, Bethesda, MD, USA). The PSD lengths of all identified synaptic profiles in the six randomly selected electron micrographs from each animal were measured along parallel, aligned plasma membranes. Counting was done by an experienced histopathologist who was unaware of the treatment condition.
Statistical analysis
The data are expressed as mean ± SEM. One-way analyses of variance with Bonferroni's post-hoc test were used to compare escape latencies, escape path lengths, and time in the target quadrant. Student's t-tests were used for analysis of the histological studies. Significance was set at a P < 0.05. All tests were performed with SPSS 13.0 software (SPSS, Chicago, IL, USA).
Footnotes
Funding: This study was supported by the Natural Science Foundation of Hebei Province, No. C2007000921.
Conflicts of interest: None declared.
Ethical approval: This study received permission from the Animal Care and Research Committee of Chengde Medical University in China.
(Edited by Zhang H, Yu DW/Yang Y/Song LP)
REFERENCES
- [1].Habash RW, Elwood JM, Krewski D, et al. Recent advances in research on radiofrequency fields and health: 2004-2007. Toxicol Environ Health B Crit Rev. 2009;12(4):250–288. doi: 10.1080/10937400903094125. [DOI] [PubMed] [Google Scholar]
- [2].Koivisto M, Krause CM, Rev onsuo A, et al. The effects of electromagnetic field emitted by GSM phones on working memory. Neuroreport. 2000;11(8):1641–1643. doi: 10.1097/00001756-200006050-00009. [DOI] [PubMed] [Google Scholar]
- [3].Rola R, Raber J, Rizk A, et al. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188(6):316–330. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- [4].Akiyama K, Tanaka R, Sato M, et al. Cognitive dysfunction and histological findings in adult rats one year after whole brain irradiation. Neurol Med Chir (Tokyo) 2001;41(12):590–598. doi: 10.2176/nmc.41.590. [DOI] [PubMed] [Google Scholar]
- [5].Raber J, Rola R, LeFevour A, et al. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162(1):39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- [6].Yoneoka Y, Satoh M, Akiyama K, et al. An experimental study of radiation-induced cognitive dysfunction in an adult rat model. Br J Radiol. 1999;72(864):1196–1201. doi: 10.1259/bjr.72.864.10703477. [DOI] [PubMed] [Google Scholar]
- [7].Madsen TM, Kristjansen PE, Bolwig TG, et al. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119(3):635–642. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- [8].Moser E, Moser MB, Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci. 1993;13(9):3916–3925. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Morris RG, Schenk F, Tweedie F, et al. Ibotenate lesions of hippocampus and/or subiculum: Dissociating components of allocentric spatial learning. Eur J Neurosci. 1990;2(12):1016–1028. doi: 10.1111/j.1460-9568.1990.tb00014.x. [DOI] [PubMed] [Google Scholar]
- [10].Geinisman Y, Toledo-Morrell L, Morrell F, et al. Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus. 1992;2(4):437–444. doi: 10.1002/hipo.450020411. [DOI] [PubMed] [Google Scholar]
- [11].Martin SJ, Morris RG. New life in an old idea: The synaptic plasticity and memory hypothesis revisited. Hippocampus. 2002;12(5):609–636. doi: 10.1002/hipo.10107. [DOI] [PubMed] [Google Scholar]
- [12].Auerbach JM, Segal M. Peroxide modulation of slow onset potentiation in rat hippocampus. J Neurosci. 1997;17(22):8695–8701. doi: 10.1523/JNEUROSCI.17-22-08695.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Foster TC. Involvement of hippocampal synaptic plasticity in age-related memory decline. Brain Res Brain Res Rev. 1999;30(3):236–249. doi: 10.1016/s0165-0173(99)00017-x. [DOI] [PubMed] [Google Scholar]
- [14].Tombaugh GC, Rowe WB, Chow AR, et al. Theta-frequency synaptic potentiation in CA1 in vitro distinguishes cognitively impaired from unimpaired aged Fischer 344 rats. J Neurosci. 2002;22(22):9932–9940. doi: 10.1523/JNEUROSCI.22-22-09932.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Luscher C, Nicoll RA, Malenka RC, et al. Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat Neurosci. 2000;3(6):545–550. doi: 10.1038/75714. [DOI] [PubMed] [Google Scholar]
- [16].Yan JG, Agresti M, Zhang LL, et al. Upregulation of specific mRNA levels in rat brain after cell phone exposure. Electromagn Biol Med. 2008;27(2):147–154. doi: 10.1080/15368370802072208. [DOI] [PubMed] [Google Scholar]
- [17].D’Andrea JA, Ziriax JM, Adair ER. Radio frequency electromagnetic fields: mild hyperthermia and safety standards. Proq Brain Res. 2007;162:107–135. doi: 10.1016/S0079-6123(06)62007-4. [DOI] [PubMed] [Google Scholar]
- [18].Fragopoulou AF, Miltiadous P, Stamatakis A, et al. Whole body exposure with GSM 900 MHz affects spatial memory in mice. Pathophysiology. 2010;17(3):179–187. doi: 10.1016/j.pathophys.2009.11.002. [DOI] [PubMed] [Google Scholar]
- [19].Cassel JC, Cosquer B, Galani R, et al. Whole-body exposure to 2.45GHz electromagnetic fields does not alter radial-maze performance in rats. Behav Brain Res. 2004;155(1):37–43. doi: 10.1016/j.bbr.2004.03.031. [DOI] [PubMed] [Google Scholar]
- [20].Cosquer B, Kuster N, Cassel JC. Whole-body exposure to 2.45GHz electromagnetic fields does not alter 12-arm radial-maze with reduced access to spatial cues in rats. Behav Brain Res. 2005;161(2):331–334. doi: 10.1016/j.bbr.2005.02.026. [DOI] [PubMed] [Google Scholar]
- [21].Wiholm C, Lowden A, Kuster N, et al. Mobile phone exposure and spatial memory. Bioelectromagnetics. 2009;30(1):59–65. doi: 10.1002/bem.20443. [DOI] [PubMed] [Google Scholar]
- [22].Narayanan SN, Kumar RS, Potu BK, et al. Spatial memory perfomance of wistar rats exposed to mobile phone. Clinics. 2009;64(3):231–234. doi: 10.1590/S1807-59322009000300014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Daniels WM, Pitout IL, Afullo TJ, et al. The effect of electromagnetic radiation in the mobile phone range on the behaviour of the rat. Metab Brain Dis. 2009;24(4):629–641. doi: 10.1007/s11011-009-9164-3. [DOI] [PubMed] [Google Scholar]
- [24].Mann K, Röschke J. Effects of pulsed high frequency electromagnetic fields on human sleep. Neuropsychobiology. 1996;33(1):41–47. doi: 10.1159/000119247. [DOI] [PubMed] [Google Scholar]
- [25].Preece AW, Goodfellow S, Wright MG, et al. Effect of 902 MHz mobile phone transmission on cognitive function in children. Bioelectromagnetics. 2005;(Suppl 7):S138–143. doi: 10.1002/bem.20128. [DOI] [PubMed] [Google Scholar]
- [26].Luria R, Eliyahu I, Hareuveny R, et al. Cognitive effects of radiation emitted by cellular phones: the influence of exposure side and time. Bioelectromagnetics. 2009;30(3):198–204. doi: 10.1002/bem.20458. [DOI] [PubMed] [Google Scholar]
- [27].Wong TP, Marchese G, Casu MA, et al. Loss of presynaptic and postsynaptic structures is accompanied by compensatory increase in action potential-dependent synaptic input to layer V neocortical pyramidal neurons in aged rats. J Neurosci. 2000;20(22):8596–8606. doi: 10.1523/JNEUROSCI.20-22-08596.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Coleman P, Federoff H, Kurlan R. A focus on the synapse for neuroprotection in Alzheimer disease and other dementias. Neurology. 2004;63(7):1155–1162. doi: 10.1212/01.wnl.0000140626.48118.0a. [DOI] [PubMed] [Google Scholar]
- [29].Cai F, Wang F, Lin FK, et al. Redox modulation of long-term potentiation in the hippocampus via regulation of the glycogen synthase kinase-3beta pathway. Free Radic Biol Med. 2008;45(7):964–970. doi: 10.1016/j.freeradbiomed.2008.06.014. [DOI] [PubMed] [Google Scholar]
- [30].Geinisman Y, de Toledo-Morrell L, Morrell F. Loss of perforated synapses in the dentate gyrus: morphological substrate of memory deficit in aged rats. Proc Natl Acad Sci U S A. 1986;83(9):3027–3031. doi: 10.1073/pnas.83.9.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ziff EB. Enlightening the postsynaptic density. Neuron. 1997;19(6):1163–1174. doi: 10.1016/s0896-6273(00)80409-2. [DOI] [PubMed] [Google Scholar]
- [32].Nikonenko I, Jourdain P, Alberi S, et al. Activity-induced changes of spine morphology. Hippocampus. 2002;12(5):585–591. doi: 10.1002/hipo.10095. [DOI] [PubMed] [Google Scholar]
- [33].Sekino Y, Kojima N, Shirao T. Role of actin cytoskeleton in dendritic spine morphogenesis. Neurochem Int. 2007;51(2-4):92–104. doi: 10.1016/j.neuint.2007.04.029. [DOI] [PubMed] [Google Scholar]
- [34].Li Z, Okamoto K, Hayashi Y, et al. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119(6):873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- [35].Inquimbert P, Rodeau JL, Schlichter R. Regional differences in the decay kinetics of GABA(A) receptor-mediated miniature IPSCs in the dorsal horn of the rat spinal cord are determined by mitochondrial transport of cholesterol. J Neurosci. 2008;28(13):3427–3437. doi: 10.1523/JNEUROSCI.5076-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9(5):370–386. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- [37].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]
- [38].ICNIRP. Guidelines for limiting exposure to time-varying electric, magnetic and electromagnetic fields (up to 300GHz) Health Phys. 1998;74(4):494–522. [PubMed] [Google Scholar]
- [39].Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11(1):47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- [40].Paxinos G, Watson C. 2nd ed. San Diego: Academic Press; 1986. The Rat Brain in Stereotaxic Coordinates. [DOI] [PubMed] [Google Scholar]
- [41].Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8(6):608–619. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- [42].Moser MB, Moser EI, Forrest E, et al. Spatial learning with a minislab in the dorsal hippocampus. Proc Natl Acad Sci U S A. 1995;92(21):9697–9701. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Geinisman Y, Gundersen HJ, van der ZE, et al. Unbiased stereological estimation of the total number of synapses in a brain region. J Neurocytol. 1996;25(12):805–819. doi: 10.1007/BF02284843. [DOI] [PubMed] [Google Scholar]
- [44].Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984;134(2):127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- [45].Shi L, Linville MC, Tucker EW, et al. Differential effects of aging and insulin-like grouth factor-1 on synapses in CA1 of rat hippocampus. Cerebral Cortex. 2005;15(5):571–577. doi: 10.1093/cercor/bhh158. [DOI] [PubMed] [Google Scholar]


