Abstract
OBJECTIVE:
To identify global research trends in the application of MRI for monitoring stem cell transplantation using a bibliometric analysis of Web of Science.
DATA RETRIEVAL:
We performed a bibliometric analysis of studies relating to the application of MRI for detecting stem cell transplantation for the treatment of cerebral ischemia using papers in Web of Science published from 2002 to 2011.
SELECTION CRITERIA:
The inclusion criteria were: (a) peer-reviewed articles on the application of MRI for detecting transplanted stem cells published and indexed in Web of Science; (b) year of publication between 2002 and 2011. Exclusion criteria were: (a) articles that required manual searching or telephone access; (b) some corrected papers.
MAIN OUTCOME MEASURES:
(1) Annual publication output; (2) distribution according to journals; (3) distribution according to institution; (4) distribution according to country; (5) top cited authors over the last 10 years.
RESULTS:
A total of 1 498 studies related to the application of MRI for monitoring stem cell transplantation appeared in Web of Science from 2002 to 2011, almost half of which were derived from American authors and institutes. The number of studies on the application of MRI for detecting stem cell transplantation has gradually increased over the past 10 years. Most papers on this topic appeared in Magnetic Resonance in Medicine.
CONCLUSION:
This analysis suggests that few experimental studies have been investigated the use of MRI for tracking SPIO-labeled human umbilical cord blood-derived mesenchymal stem cells during the treatment of cerebral ischemia.
Keywords: neural stem cells, embryonic stem cells, bone marrow mesenchymal stem cells, cell transplantation, cerebral ischemia, Web of Science, neural regeneration
Abbreviations:
MSCs, mesenchymal stem cells; UCB-MSCs, umbilical cord blood-derived MSCs; SPIO, superparamagnetic iron oxide
INTRODUCTION
Cerebral ischemia is a common acute cerebrovascular disease. The key to its treatment involves the rescue of dying neurons in the ischemic penumbra and the promotion of injury recovery. Cerebral ischemia is associated with high levels of mortality and disability, and neural cell replacement therapy is the only effective treatment. Neural cell transplantation can rebuild nerve conduction loops and restore some neurological function, possibly via the differentiation of transplanted stem cells into functional glial cells or neurons, which can thus substitute for some of the affected neurons. This replacement therapy also activates endogenous neural stem cells. The transplanted stem cells secrete cytokines to improve the local microenvironment of ischemic necrosis in terms of inflammation, tissue necrosis and glial scarring[1,2]. However, cerebral ischemic damage can affect large areas and many kinds of nerve cells. Ischemic lesions may involve a number of sites in the hypothalamus, striatum, hippocampus and cortex, and reconstruction of this complex system presents a challenge for cell transplantation.
The sources of stem cells to treat cerebral ischemia include embryonic stem cells, bone marrow mesenchymal stem cells (MSCs), human umbilical cord blood (UCB) cells, human adipose mesenchymal cells, and fetal brain central nervous system cells. MSCs differ from hematopoietic stromal cells in that they are pluripotent precursor cells. UCB-derived MSCs (UCB-MSCs) have some advantages, such as strong amplification, convenience and availability, and lack of immune rejection, and they have proven a feasible and effective means of neural cell replacement therapy for cerebral ischemia[3].
Transplanted stem cells show different migration and differentiation patterns under different pathological and physiological conditions in the central nervous system. These characteristics of stem cells have been investigated by sacrificing experimental animals at certain time points after transplantation. However, this invasive method does not allow the dynamic observation of the stem cells in an individual animal. Non-invasive methods are therefore required to monitor transplanted stem cells in vivo.
In 1999, Weissleder[4] first proposed the concept of molecular imaging, i.e., using modern imaging techniques to carry out real-time microscopic imaging of physiological or pathological processes in vivo at a molecular level. MRI demonstrates high resolution at the molecular level and has powerful functional imaging capabilities. MRI is currently widely used for the dynamic monitoring of stem-cell migration, homing, proliferation and differentiation, and to analyze the efficacy and prognosis of stem cell transplantation in damaged zones. There is thus growing emphasis on the prospects of MRI for live-cell tracking[5,6]. The contrast agent superparamagnetic iron oxide (SPIO) nanomaterial forms a core-shell structure with a dextran biopolymer coating. SPIO mainly produces a strong T2-negative contrast effect. It is characterized by small particle size and strong penetrating power, with a relaxation rate of approximately 7–10 times that of gadolinium under the same conditions. SPIO can produce signal contrast in MRI at very low concentrations. It is also biodegradable with no toxic accumulation; after metabolism, it enters the normal plasma iron pool and combines with red blood cell hemoglobin, or is used in other metabolic processes[7]. Some forms (such as cross-linked iron oxide particles) can also be combined with fluorescent markers to double-label stem cells, or with membrane proteins for imaging of apoptosis[8]. Studies have shown that staining of SPIO magnetically-labeled cells with Prussian blue allowed the SPIO in the cells to be displayed, and the cytoplasm was shown to contain a large number of iron-containing vesicles or endosomes. Investigation of the biological activity of the labeled stem cells confirmed that the labeling had no short- or long-term toxic effects. The viability, differentiation and apoptosis rates of the labeled stem cells were unaffected compared to the unlabeled cells, and did not change over time[9]. A combination of green fluorescent protein and SPIO could effectively mark neural stem cells cultured in vitro, and the proliferation and differentiation abilities of double-labeled neural stem cells were similar to those of unlabeled cells[10]. Stoll et al[11] used MRI to track the migration of transplanted neural stem cells labeled with ultra-magnetic iron oxide in the host brain in vivo. Chen et al[12] tracked rat bone marrow-derived neural stem cells labeled with ultra-small SPIO Sinerem and transfection reagent poly-lysine complexes using MRI in vivo. Nevertheless, few experimental studies have investigated the use of MRI for tracking SPIO-labeled human UCB-MSCs during the treatment of cerebral ischemia[13].
DATA SOURCES AND METHODOLOGY
Data retrieval
Bibliometric methods were used to quantitatively and qualitatively investigate research trends in the application of MRI for monitoring stem cell transplantation for the treatment of cerebral ischemia. SCI-E is a searchable database of publications maintained by the Institute for Scientific Information in Philadelphia, PA, USA, and was used for this study. SCI-E was searched using the keywords “neural stem cells” “embryonic stem cells” “bone marrow mesenchymal stem cells” “cell transplantation” and “cerebral ischemia”. A bibliography of all articles related to the application of MRI for monitoring stem cell transplantation was compiled for the publication period 2002 to 2011.
Inclusion criteria
Articles closely related to the application of MRI for monitoring stem cell transplantation for the treatment of cerebral ischemia and published between 2002 and 2011 were included.
Exclusion criteria
Articles included in Social Sciences Citation Index, Arts & Humanities Citation Index, Conference Proceedings Citation Index – Science, Conference Proceedings Citation Index – Social Science & Humanities, Current Chemical Reactions, as well as Index Chemicus were excluded.
Web of Science data were performed statistical analysis using Excel. All articles referring to the application of MRI for monitoring stem cells for the treatment of cerebral ischemia that met the inclusion criteria were analyzed regarding output distribution in subject categories and journals, publication outputs of countries and institutes, and citations.
RESULTS
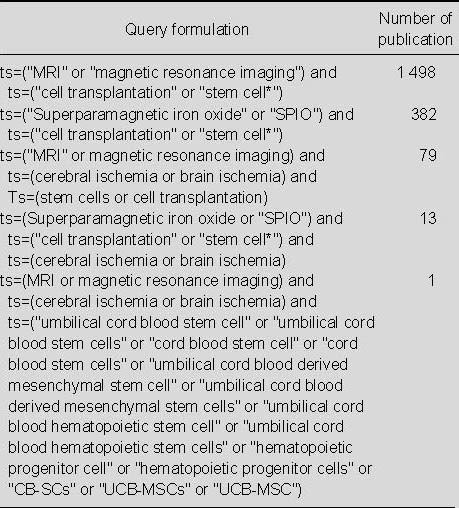
Search results for publications relating to the application of MRI for monitoring stem cell transplantation from 2002 to 2011 (Table 1)
Table 1.
Search results for application of MRI for detecting transplanted stem cells in the Web of Science from 2002 to 2011
Annual publication output relating to the application of MRI for detecting transplanted stem cells from 2002 to 2011 (Figure 1)
Figure 1.
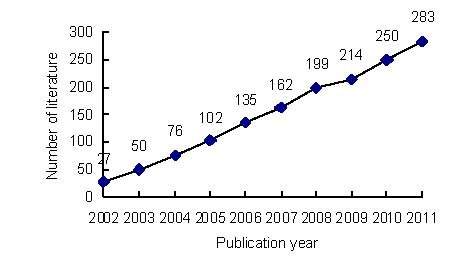
Annual number of publications relating to the application of MRI for detecting transplanted stem cells in Web of Science from 2002 to 2011.
A total of 1 498 publications relating to the application of MRI for detecting transplanted stem cells were identified in Web of Science from 2002 to 2011. The number of publications gradually increased over the past 10 years; only 27 papers were published and included in Web of Science in 2002, which was much fewer than in 2011.
Distribution of published papers relating to the application of MRI for detecting transplanted stem cells according to journal (Table 2)
Table 2.
Top 15 journals that published studies related to the application of MRI for detecting transplanted stem cells included in Web of Science from 2002 to 2011
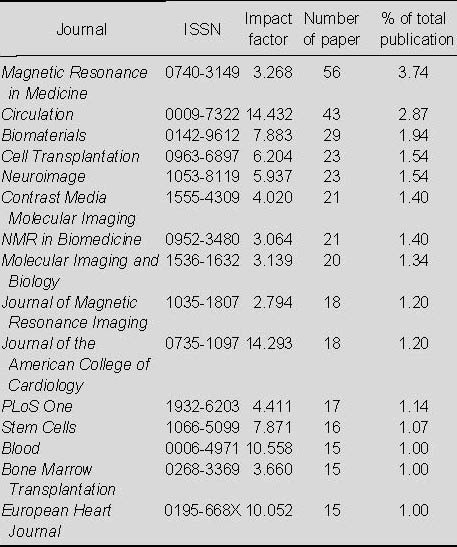
From Table 2, it is evident that most papers relating to the application of MRI for detecting transplanted stem cells appeared in Magnetic Resonance in Medicine, which published 56 papers, accounting for 3.74% of the total number of publications; this was followed by Circulation, which published 43 papers (2.87%).
Distribution of numbers of publications relating to the application of MRI for detecting transplanted stem cells according to country (Table 3)
Table 3.
Top 10 countries in terms of numbers of publications relating to the application of MRI for detecting transplanted stem cells in Web of Science from 2002 to 2011
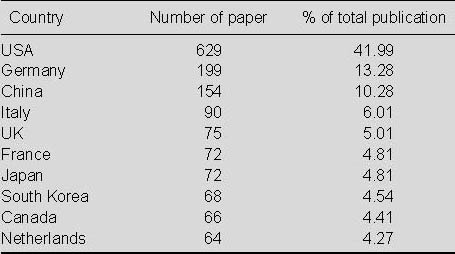
As seen in Table 3, most papers relating to the application of MRI for detecting transplanted stem cells were published in the USA, with 629 papers, accounting for 41.99% of the total. This output was much higher than the numbers of publications from other countries; Germany ranked second with 199 papers (13.28%) and China ranked third with 154 papers (10.28%).
Numbers of publications relating to the application of MRI for detecting transplanted stem cells according to institution (Table 4)
Table 4.
Top 12 institutions in terms of numbers of publications relating to the application of MRI for detecting transplanted stem cells in Web of Science from 2002 to 2011
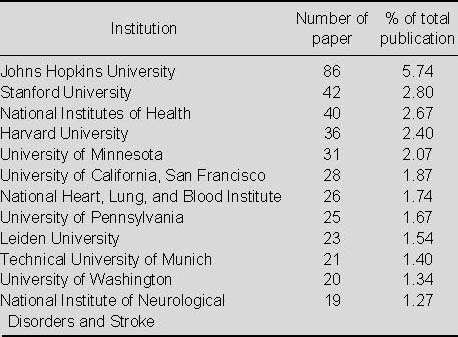
In Table 4 shows that the top institution for publishing studies on the application of MRI for detecting transplanted stem cells during the period analyzed was Johns Hopkins University, followed by Stanford University.
Distribution of publications relating to the application of MRI for detecting transplanted stem cells according to document type
Seven document types were found among the 1 498 publications from 2002 to 2011. Articles (1 182) were the most frequently used document type comprising 78.91%, followed by reviews (195; 13.02%), meeting abstracts (99; 6.61%), and proceedings papers (63; 4.21%). The remaining types of articles were editorial material (17), letters (5), and book chapters (1).
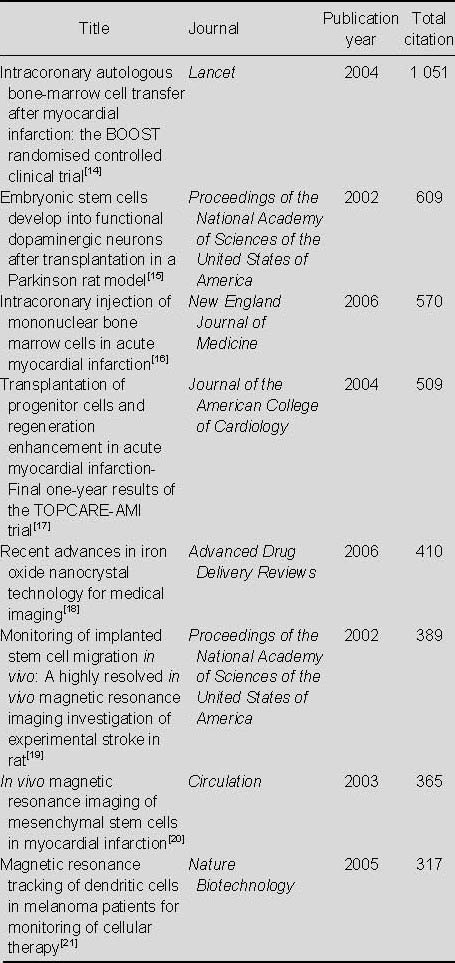
Most-cited articles relating to the application of MRI for detecting transplanted stem cells (Table 5)
Table 5.
Most-cited articles relating to the application of MRI for detecting transplanted stem cells in the Web of Sciencefrom 2002 to 2011
Annual publication output relating to the application of MRI for detecting transplanted SPIO-labeled stem cells from 2002 to 2011 (Figure 2)
Figure 2.
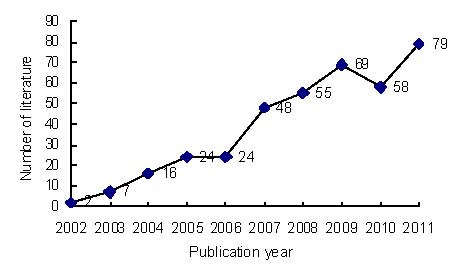
Annual number of publications relating to the application of MRI for detecting transplanted superparamagnetic iron oxide-labeled stem cells in Web of Science from 2002 to 2011.
A total of 382 publications relating to the application of MRI for the detection of transplanted SPIO-labeled stem cells were identified in Web of Science from 2002 to 2011. The numbers of publications gradually increased over the past 10 years, apart from a slight decrease in 2010.
Distribution of published studies relating to the application of MRI for detecting transplanted SPIO-labeled stem cells according to journal (Table 6)
Table 6.
Top 10 journals that published studies relating to the application of MRI for detecting transplanted superparamagnetic iron oxide-labeled stem cells in Web of Science from 2002 to 2011
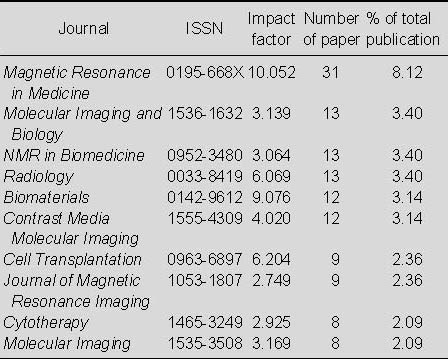
From Table 6 shows that most papers relating to the application of MRI for detecting transplanted SPIO-labeled stem cells appeared in Magnetic Resonance in Medicine, which published 31 papers, accounting for 8.12% of the total number of publications; this was followed by Molecular Imaging and Biology and NMR in Biomedicine and Radiology, which published 13 papers (3.40%) each.
Distribution of publications relating to the application of MRI for detecting transplanted SPIO-labeled stem cells according to country (Figure 3)
Figure 3.
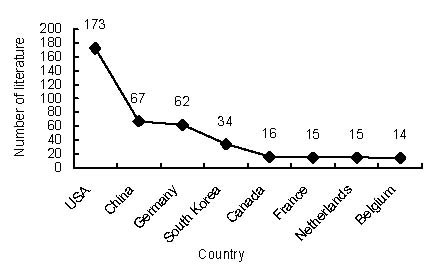
Top eight countries in terms of numbers of studies relating to the application of MRI for detecting transplanted superparamagnetic iron oxide-labeled stem cells included in Web of Science from 2002 to 2011.
As seen in Figure 3, the USA published the highest number of papers (173; 45.29%), followed by China (67; 17.54%) and Germany (62; 16.23%).
Distribution of published studies relating to the application of MRI for detecting transplanted SPIO-labeled stem cells according to institution (Table 7)
Table 7.
Top 14 institutions publishing studies relating to the application of MRI for detecting transplanted superparamagnetic iron oxide-labeled stem cells in Web of Science from 2002 to 2011
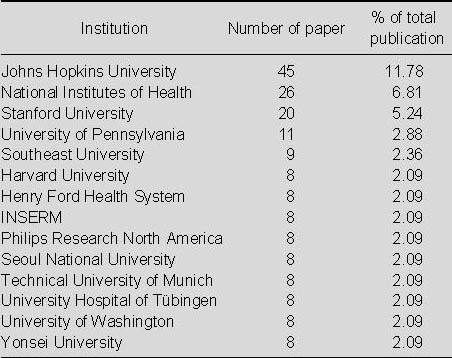
As seen in Table 7, Johns Hopkins University was the most productive institution in terms of studies related to the application of MRI for detecting transplanted SPIO-labeled stem cells.
Most-cited articles relating to the application of MRI for detecting transplanted SPIO-labeled stem cells (Table 8)
Table 8.
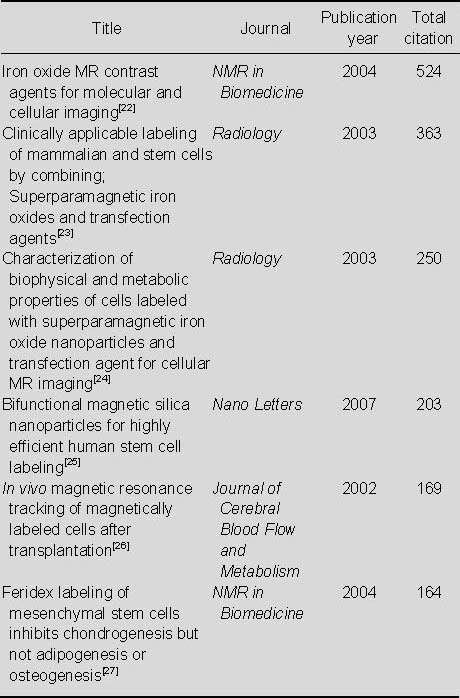
Most-cited articles relating to the application of MRI for detecting transplanted superparamagnetic iron oxide-labeled stem cells in Web of Science from 2002 to 2011
Annual publication output relating to the application of MRI for detecting transplanted stem cells in the treatment of cerebral ischemia from 2002 to 2011 (Figure 4)
Figure 4.
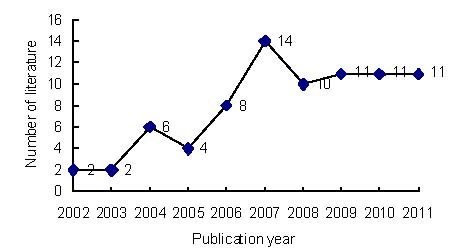
Annual numbers of publications related to the application of MRI for detecting transplanted stem cells in the treatment of cerebral ischemia in Web of Science from 2002 to 2011.
A total of 79 publications relating to the application of MRI for detecting transplanted stem cells in the treatment of cerebral ischemia were identified in Web of Science from 2002 to 2011. The numbers of publications showed an increasing tendency from 2002 to 2007, reached a peak in 2007, (apart from a slight decrease in 2005), then remained steady after 2007.
Distribution of published papers relating to the application of MRI for detecting transplanted stem cells in the treatment of cerebral ischemia according to journal (Table 9)
Table 9.
Top 10 journals that published papers relating to the application of MRI for detecting transplanted stem cells in the treatment of cerebral ischemia included in Web of Science from 2002 to 2011
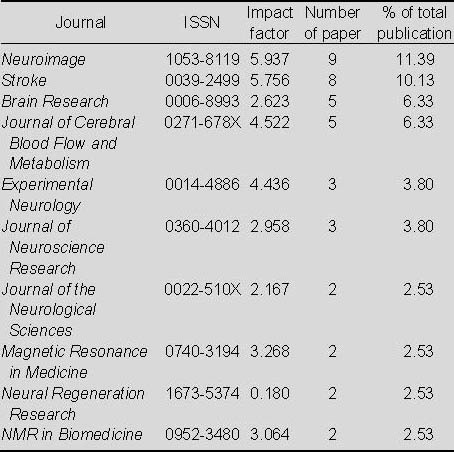
As seen in Table 9, most of the papers relating to the application of MRI for detecting transplanted stem cells in the treatment of cerebral ischemia appeared in Neuroimage, followed by Stroke and Brain Research.
Distribution of publications relating to the application of MRI for detecting transplanted stem cells in the treatment of cerebral ischemia according to country and institution
The USA published the highest number of papers (40, 50.63%). Japan ranked second (13, 16.46%), Germany ranked third (11). Sapporo Medical University, Oakland University, and Yale University were the three most productive institutions in terms of publishing papers relating to the application of MRI for detecting transplanted stems cells in the treatment of cerebral ischemia.
The most-cited papers from researchers at Sapporo Medical University from 2002 to 2011 were:
BDNF gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model[28], by Kurozumi K, et al., published in Molecular Therapy in 2004, with 125 citations.
Intravenous infusion of immortalized human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat[29], by Honma T, et al., published in Experimental Neurology in 2006, with 80 citations.
Intravenous administration of glial cell line-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in the adult rat[30], by Horita Y, et al., published in Journal of Neuroscience Research in 2006, with 72 citations.
Therapeutic benefits by human mesenchymal stem cells (hMSCs) and Ang-1 gene-modified hMSCs after cerebral ischemia[31], by Onda T, et al., published in Journal of Cerebral Blood Flow and Metabolism in 2008, with 52 citations.
The most cited relevant papers from Oakland University were:
In vivo magnetic resonance imaging tracks adult neural progenitor cell targeting of brain tumor[32], by Zhang Z, et al., published in Neuroimage in 200, with citations.
Neurogenesis, angiogenesis, and MRI indices of functional recovery from stroke[33], by Chopp M, published in Stroke in 2007, with 58 citations.
Effects of administration route on migration and distribution of neural progenitor cells transplanted into rats with focal cerebral ischemia, an MRI study[34], by Li L, et al., published in Journal of Cerebral Blood Flow and Metabolism in 2010, with 10 citations.
The most cited relevant papers from Yale University were:
Therapeutic benefits of angiogenetic gene-modified human mesenchymal stem cells after cerebral ischemia[35], by Toyama K, et al., published in Experimental Neurology in 2009, with 31 citations.
Optimization of a therapeutic protocol for intravenous injection of human mesenchymal stem cells after cerebral ischemia in adult rats[36], by Omori Y, et al., published in Brain Research in 2008, with 15 citations.
DISCUSSION
The results of the current bibliometric study showed several research trends in terms of the application of MRI for monitoring stem cells transplantation. First, a total of 1 498 publications relating to the application of MRI for detecting transplanted stem cells were identified in Web of Science from 2002 to 2011, 382 of which regarding MRI for detecting transplanted SPIO-labeled stem cells and only 13 publications focus on application of MRI for detecting transplanted SPIO-labeled stem cells in the treatment of cerebral ischemia. The number of papers published annually has increased since 2002, indicating an increasing global interest in this topic over the last 10 years. Second, most relevant articles were published in Magnetic Resonance in Medicine. Finally, most articles on this topic were published in the USA.
In conclusion, the USA is the most productive country in terms of research into the application of MRI for tracking the progress of stem cell transplantation, while China ranked second for publications on the use of MRI for detecting SPIO-labeled stem cells, and fourth in relation to papers on MRI for detecting transplanted stem cells in the treatment of cerebral ischemia. The findings of this study demonstrated that few experimental studies have been investigated the use of MRI for tracking SPIO-labeled human UCB-MSCs during the treatment of cerebral ischemia.
Footnotes
Conflicts of interest: None declared.
(Edited by Zhao LJ/Song LP)
REFERENCES
- [1].Chang YC, Shyu WC, Lin SZ, et al. Regenerative therapy for stroke. Cell Transplant. 2007;16(2):171–181. [PubMed] [Google Scholar]
- [2].Bang OY, Lee JS, Lee PH, et al. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57(6):874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- [3].Kraitchman DL, Gilson WD, Lorenz CH. Stem cell therapy: MRI guidance and monitoring. J Magn Reson Imaging. 2008;27(2):299–310. doi: 10.1002/jmri.21263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Weissleder R. Molecular imaging: exploring the next frontier. Radiology. 1999;212(3):609–614. doi: 10.1148/radiology.212.3.r99se18609. [DOI] [PubMed] [Google Scholar]
- [5].Bulte JW, Douglas T, Witwer B, et al. Monitoring stem cell therapy in vivo using magnetodendrimers as a new class of cellular MR contrast agents. Acad Radiol. 2002;9(2):S332–335. doi: 10.1016/s1076-6332(03)80221-0. [DOI] [PubMed] [Google Scholar]
- [6].Sehoefer R, KeMbach R, Wiskirchen J, et al. Transferrin respector upregulation: in vitro labeling of rat mesenchymal stem cells with superparamagnetic iron oxide. Radiology. 2007;244(2):514–523. doi: 10.1148/radiol.2442060599. [DOI] [PubMed] [Google Scholar]
- [7].Frank JA, Zywicke H, Jordan EK, et al. Magnetic intracellular labeling of mammalian cells by combining (FDA-approved) superparamagnetic iron oxide MR contrast agents and commonly used transfection agents. Acad Radiol. 2002;9(2):S484–487. doi: 10.1016/s1076-6332(03)80271-4. [DOI] [PubMed] [Google Scholar]
- [8].Arbab AS, Bashaw LA, Miller BR, et al. Characterization of biophysical and metabolic properties of cells labeled with superparamagnetie iron oxide nanoparticles and transfection agent for cellular MR imaging. Radiology. 2003;229(3):838–846. doi: 10.1148/radiol.2293021215. [DOI] [PubMed] [Google Scholar]
- [9].Arbab AS, Yoeum GT, Wilson LB, et al. Comparison of transfection agents in forming complexes with ferumoxides, cell labeling efficiency, and cellular viability. Mol Imaging. 2004;3(1):24–32. doi: 10.1162/15353500200403190. [DOI] [PubMed] [Google Scholar]
- [10].Norman AB, Thomas SR, Pratt RG, et al. Magnetic resonance imaging of neural transplants in rat brain using a superparamagnetic contrast agent. Brain Res. 1992;594(2):279–283. doi: 10.1016/0006-8993(92)91135-2. [DOI] [PubMed] [Google Scholar]
- [11].Stoll G, Wesemeier C, Gold R, et al. In vivo monitoring of macro-phage infiltration in experimental autoimmune neuritis by magneticresonance imaging. J Neuroimmunol. 2004;149(1-2):142–146. doi: 10.1016/j.jneuroim.2003.12.024. [DOI] [PubMed] [Google Scholar]
- [12].Chen ZC, Xu RX, Yang ZJ, et al. Sinerem labeling and MRI tracking of neural stem cells in vivo and in vitro. Nanfang Yike Daxue Xuebao. 2007;27(5):611–615. [PubMed] [Google Scholar]
- [13].Chung DJ, Choi CB, Lee SH, et al. Intraarterially delivered human umbilical cord blood-derived mesenchymal stem cells in canine cerebral ischemia. J Neurosci Res. 2009;87(16):3554–3567. doi: 10.1002/jnr.22162. [DOI] [PubMed] [Google Scholar]
- [14].Wollert KC, Meyer GP, Lotz J, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364(9429):141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- [15].Bjorklund LM, Sánchez-Pernaute R, Chung S, et al. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci U S A. 2002;99(4):2344–2349. doi: 10.1073/pnas.022438099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lunde K, Solheim S, Aakhus S, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355(12):1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- [17].Schächinger V, Assmus B, Britten MB, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI Trial. J Am Coll Cardiol. 2004;44(8):1690–1699. doi: 10.1016/j.jacc.2004.08.014. [DOI] [PubMed] [Google Scholar]
- [18].Corot C, Robert P, Idée JM, et al. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv Drug Deliv Rev. 2006;58(14):1471–1504. doi: 10.1016/j.addr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- [19].Hoehn M, Küstermann E, Blunk J, et al. Monitoring of implanted stem cell migration in vivo: a highly resolved in vivo magnetic resonance imaging investigation of experimental stroke in rat. Proc Natl Acad Sci U S A. 2002;99(25):16267–16272. doi: 10.1073/pnas.242435499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kraitchman DL, Heldman AW, Atalar E, et al. In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation. 2003;107(18):2290–2293. doi: 10.1161/01.CIR.0000070931.62772.4E. [DOI] [PubMed] [Google Scholar]
- [21].de Vries IJ, Lesterhuis WJ, Barentsz JO, et al. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat Biotechnol. 2005;23(11):1407–1413. doi: 10.1038/nbt1154. [DOI] [PubMed] [Google Scholar]
- [22].Bulte JW, Kraitchman DL. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 2004;17(7):484–499. doi: 10.1002/nbm.924. [DOI] [PubMed] [Google Scholar]
- [23].Frank JA, Miller BR, Arbab AS, et al. Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology. 2003;228(2):480–487. doi: 10.1148/radiol.2281020638. [DOI] [PubMed] [Google Scholar]
- [24].Arbab AS, Bashaw LA, Miller BR, et al. Characterization of biophysical and metabolic properties of cells labeled with superparamagnetic iron oxide nanoparticles and transfection agent for cellular MR imaging. Radiology. 2003;229(3):838–846. doi: 10.1148/radiol.2293021215. [DOI] [PubMed] [Google Scholar]
- [25].Lu CW, Hung Y, Hsiao JK, et al. Bifunctional magnetic silica nanoparticles for highly efficient human stem cell labeling. Nano Lett. 2007;7(1):149–154. doi: 10.1021/nl0624263. [DOI] [PubMed] [Google Scholar]
- [26].Bulte JW, Duncan ID, Frank JA. In vivo magnetic resonance tracking of magnetically labeled cells after transplantation. J Cereb Blood Flow Metab. 2002;22(8):899–907. doi: 10.1097/00004647-200208000-00001. [DOI] [PubMed] [Google Scholar]
- [27].Kostura L, Kraitchman DL, Mackay AM, et al. Feridex labeling of mesenchymal stem cells inhibits chondrogenesis but not adipogenesis or osteogenesis. NMR Biomed. 2004;17(7):513–517. doi: 10.1002/nbm.925. [DOI] [PubMed] [Google Scholar]
- [28].Kurozumi K, Nakamura K, Tamiya T, et al. BDNF gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model. Mol Ther. 2004;9(2):189–197. doi: 10.1016/j.ymthe.2003.10.012. [DOI] [PubMed] [Google Scholar]
- [29].Honma T, Honmou O, Iihoshi S, et al. Intravenous infusion of immortalized human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Exp Neurol. 2006;199(1):56–66. doi: 10.1016/j.expneurol.2005.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Horita Y, Honmou O, Harada K, et al. Intravenous administration of glial cell line-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in the adult rat. J Neurosci Res. 2006;84(7):1495–1504. doi: 10.1002/jnr.21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Onda T, Honmou O, Harada K, et al. Therapeutic benefits by human mesenchymal stem cells (hMSCs) and Ang-1 gene-modified hMSCs after cerebral ischemia. J Cereb Blood Flow Metab. 2008;28(2):329–340. doi: 10.1038/sj.jcbfm.9600527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhang Z, Jiang Q, Jiang F, et al. In vivo magnetic resonance imaging tracks adult neural progenitor cell targeting of brain tumor. Neuroimage. 2004;23(1):281–287. doi: 10.1016/j.neuroimage.2004.05.019. [DOI] [PubMed] [Google Scholar]
- [33].Chopp M, Zhang ZG, Jiang Q. Neurogenesis, angiogenesis, and MRI indices of functional recovery from stroke. Stroke. 2007;38(2 Suppl):827–831. doi: 10.1161/01.STR.0000250235.80253.e9. [DOI] [PubMed] [Google Scholar]
- [34].Li L, Jiang Q, Ding G, et al. Effects of administration route on migration and distribution of neural progenitor cells transplanted into rats with focal cerebral ischemia, an MRI study. J Cereb Blood Flow Metab. 2010;30(3):653–662. doi: 10.1038/jcbfm.2009.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Toyama K, Honmou O, Harada K, et al. Therapeutic benefits of angiogenetic gene-modified human mesenchymal stem cells after cerebral ischemia. Exp Neurol. 2009;216(1):47–55. doi: 10.1016/j.expneurol.2008.11.010. [DOI] [PubMed] [Google Scholar]
- [36].Omori Y, Honmou O, Harada K, et al. Optimization of a therapeutic protocol for intravenous injection of human mesenchymal stem cells after cerebral ischemia in adult rats. Brain Res. 2008;1236:30–38. doi: 10.1016/j.brainres.2008.07.116. [DOI] [PMC free article] [PubMed] [Google Scholar]


