Abstract
Background:
There have been numerous researches on ozone application in dentistry; yet the data regarding its whitening effect is very limited. The present study compares the bleaching effect of ozone with office bleaching.
Materials and Methods:
In this experimental study, 15 maxillary premolar teeth were selected and sectioned mesio-distally and bucco-lingually. The sections were then placed in tea for 1 week according to the Sulieman method and were divided into three groups each comprised of 15 sections. The samples were bleached as followed; Group I: Bleached with 35% hydrogen peroxide in three intervals of 8 min each, Group II: Underwent ozone treatment using Ozotop unite for 4 min and Group III: Bleached with a combination of both methods. The color indices of the samples, i.e., (a) green-red pigment, (b) blue-yellow pigment, (L) brightness, (ΔE) overall color change, were evaluated pre- and post-bleaching utilizing a digital camera, Photoshop software and CIE lab index. The color changes of specimens then were calculated and analyzed through randomized analysis of variance and Tukey tests. P < 0.001 was considered to be significant.
Results:
The color change (ΔE) in Group II was significantly lower than those in the two other groups (P < 0.001). There was no significant difference between the color change of Groups I and III (P = 0.639). In addition, the results of L, a and b brought forth a similar pattern to the findings obtained from ΔE.
Conclusion:
The hydrogen peroxide gel has a more powerful whitening effect than ozone; in addition, ozone has no synergistic effect when is used simultaneously with hydrogen peroxide.
Keywords: Hydrogen peroxide, ozone, tooth bleaching
INTRODUCTION
Tooth whitening was first introduced in 1848 employing oxalic acid; then it was progressed by the usage of peroxide products. In 1989, the Night Guard bleaching method was introduced.[1]
The color of tooth is highly based on the light reflection and absorption characteristics of the enamel and dentin, as well as their chemical composition.[2,3] Numerous methods have been introduced for brightening of tooth color including scaling and polishing, whitening tooth pastes, internal and external bleaching, micro-abrasion with acids or abrasive materials and veneering of teeth.[4,5] The procedures such as veneering and full coverage restorations are not only invasive with a high risk of pulpal injury and technical sensitivity but also costly; therefore, bleaching seems to be more convenient and conservatively priced.[6]
Until date, various bleaching materials have been introduced to the market that among them peroxide products have been well-established in clinic due to their high efficiency in bleaching discolored teeth. This substance is a strong oxidant agent, which is able to bleach discolored teeth. However, some studies indicated that bleaching with peroxide products can cause calcium loss, changes in morphology and in chemical components of tooth, which are able to decrease the fracture resistance of tooth.[7]
Another product which has recently been suggested for whitening of discolored teeth is ozone.[8] This material is used for different dental procedures, such as sterilizing incipient caries which culminates in remineralization of tooth structure; therefore, it's usage results in non-invasive dental treatment and can be considered an effective method in geriatric and pediatric dentistry. Bleaching with ozone was proposed due to its oxidizing ability; thus, it can be used as an alternative bleaching substance to brighten discolored teeth.[9] In this regard, a recent study was carried out to investigate the concurrent application of ozone with hydrogen peroxide in order to establish the effect of ozone as an activating agent on bleaching of teeth with hydrogen peroxide.[10] Accordingly and to the best of our knowledge, there are limited data investigating the bleaching effect of ozone alone or in combination with hydrogen peroxide; therefore, this study was conducted to evaluate the effectiveness of ozone in bleaching of discolored teeth compared with hydrogen peroxide gel.
MATERIALS AND METHODS
In this experimental study, 15 newly extracted sound maxillary premolar teeth were selected under approval of the ethical committee and were kept in 0.5% chloramine solution for 24 h, the teeth were then cleaned with pumice powder and rubber cup and stored in distilled water.
Prior to grouping, crowns of teeth were separated from their roots and were sectioned mesio-distally and bucco-lingually, so each crown was divided into four sections which three of them were distributed equally among the three test groups and the remaining section from each tooth was excluded in order to homogenize the groups. The specimens were dyed using the Sulieman et al. method. For this purpose, 2 g of black tea (Golestan black tea, Golestan, Tehran, Iran) were boiled in 100 mL of distilled water for 5 min, then filtered and left to cool. The specimens were preserved in the prepared solution which was changed daily for 1 week and were rinsed and placed in distilled water until the bleaching process.[11]
Photographs of samples were taken prior to the test using a digital camera (Finepix S9600, Fuji film, Japan). In this method, the samples were placed on a black background and the camera was positioned with 25 cm distance right above the samples in a completely darken room while the samples were lighted by two 6500 k lamp from right to left. The color change of specimens was assessed using CS3 Photoshop program by CIE lab index, which calculate the color change (ΔE) by the following equation:[12]
ΔE = ([L2−L1]2 + [a2−a1]2 + [b2−b1]2)½
The specimens were divided into three groups each comprising 15 and the following procedures were conducted on each group:
Group I: Hydrogen peroxide gel (35%) (Polo office, SDI, Australia) was applied according to the manufacturer's instruction (1-2 mm thick in three 8-min sessions without light/heat curing).
Group II: The specimens were placed in a sealed container in which a wet cotton pellet was placed to prevent dehydration. The container was exposed to ozone gas for 4 min using Ozotop unit (Mectron, Switzerland) then kept in the same container for 24 h at 37 ± 1°C.
Group III: Hydrogen Peroxide gel (35%) (Polo office, SDI, Victoria, Australia) was applied 1-2 mm thick followed by 80s ozone treatment, then the gel remained for 8-min over tooth surface in a sealed container. The whole procedure was repeated 3 times, each time with a new mixed gel. Subsequently, the photographs of samples were taken again and the color changes were assessed compared with the photographs before bleaching.
The statistical analysis of collected ΔE for each group was done by randomized block analysis of variance (ANOVA) and Tukey honestly significant difference (HSD) tests using SPSS 15 (SPSS Inc., Chicago, IL, USA). P < 0.001 was considered as significant.
RESULTS
The normal distribution of data was monitored by one-sample Kolmogrov-Smirnof and Levene tests. Table 1 indicates the mean ΔE and the standard deviation of the three groups. The existence of difference among the groups was assessed by randomized block ANOVA and the differences between the groups were analyzed by Tukey HSD test using SPSS 15 (SPSS Inc., Chicago, USA). ANOVA indicated a significant difference in mean ΔE of all test groups (P < 0.001).
Table 1.
ΔE values for test groups
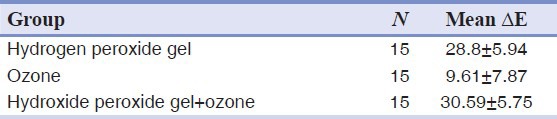
Tukey test was used for paired comparison of the groups. The results revealed no significant difference in the mean ΔE of Groups I and III (P = 0.639) while a significant difference was indicated between Group II and the other two groups (P < 0.001).
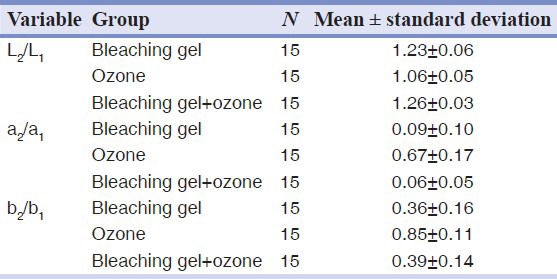
In order to confirm ΔE variation in each test group, a, b and L variables (a: Green-red pigment, b: Blue-yellow pigment, L: Brightness) were measured before and after bleaching and to determine their variation level the post/pre-bleaching ratios were calculated and statistically analyzed. The equivalency of the variance for b2/b1, a2/a1, L2/L1, in test groups was verified with P = 0.284, P = 0.096 and P = 0.800 respectively. Moreover, P < 0.001 revealed the significant difference among the mean of all test groups.
The increase of L2/L1 and the decrease of b2/b1and a2/a1 were obviously revealed in three groups. The paired comparison of the results in the test groups indicated the presence of two homogeneous subgroups that confirm the results of the subgroups of the mean ΔE. The first homogeneous subgroup (ozone-GroupII) showed a lower mean for L2/L1 and a higher mean for b2/b1, a2/a1 compared with the second homogeneous subgroup (hydrogen peroxide gel and hydrogen peroxide gel ± ozone- Groups I and III) [Table 2].
Table 2.
b2/b1, a2/a1, L2/L1 indices in test groups
DISCUSSION
In the present study, the application of hydrogen peroxide gel and its combination with ozone have noticeable whitening effect which was specified with ΔE of 28.8 and 30.59 respectively. This variation in ΔE demonstrates an extremely considerable bleaching that is in accordance with the study of Sulieman et al. who investigated the effectiveness of office bleaching with 35% hydrogen peroxide on specimens dyed by Sulieman's method (ΔE = 23.49).[11] The described method for dyeing samples was also applied by Bengel.[12] In addition Griffiths et al. who evaluated the degree of color change of stained teeth by spectrophotometer, reported similar results to that of ours (ΔE = 25).[13] Moreover, the increase of L2/L1 and decreasing of b2/b1 in all groups indicated the obvious bleaching which is confirmed by Gerlach et al.[14]
Comparison of the whitening ability of Groups I and II indicated a significant higher bleaching efficiency for hydrogen peroxide than ozone (P < 0.001). In addition, the non-significant difference among Groups I and III support the fact that hydrogen peroxide has a higher bleaching potential compared with ozone. The higher whitening ability of hydrogen peroxide is also confirmed by the significant variation in L value obtained pre- and post-bleaching, which confirms the results of ΔE in test groups [Table 2]. This is may be due to the ability of hydrogen peroxide to produce hydroxyl radicals, thus increasing pH. Since it is indicated that in a higher pH teeth are bleached more effectively, hydroxyl radicals produced by hydrogen peroxide are capable to create superior bleaching power.[15]
These results reveal the whitening ability of ozone, even though the ΔE values between Groups I and III are not significantly different; however, the lower ΔE in ozone group compared to other two groups may be related to the lower concentration and inadequate application time which can affect its penetration efficiency.[16] It is noteworthy to say that ozone is able to bleach teeth due to its ability of decomposition and oxygen radical production. This phenomenon could occur more efficiently in the water than in the air.[16] Consequently, the lower bleaching power of ozone in the present study may be attributed to the use of ozone in the air as well as the constant level of pH which may reduce the decomposition of ozone, thus, decreasing its whitening efficiency.[17] In contrast, an animal study performed by Tessier et al. on rat incisors showed that the ozone gas can be successfully used for bleaching of tetracycline-stained rat incisors; however, these contradictory results with the present study may be due to the difference in size and chemical composition of rat teeth compared to that of the human, the nature of pigments and also the method in which ozone was applied for tooth bleaching.[18]
Another fact that can be drawn by comparison of the test groups is that although ozone seems to produce observable bleaching effect when used alone, its simultaneous application with hydrogen peroxide does not significantly intensify the whitening ability of hydrogen peroxide. This is in accordance with a recent study which investigated the concurrent application of hydrogen peroxide gel with ozone in which the results indicated that the application of ozone prior to or concurrent with hydrogen peroxide does not significantly increase the bleaching effectiveness.[19]
In the present study, ozone was applied with the concentration of 140 ppm using Ozotop unit (the machine that was used in the present study), in which the lack of suctioning ability limits its utilization to a higher concentration of ozone; nevertheless, due to the suctioning ability of the Heal Ozone Machine -which is used in studies investigating other applications of ozone- the concentration of 2100 ppm is achievable;[20] yet, to the best of our knowledge, the bleaching efficiency of ozone in higher concentrations is poorly investigated which should be noted and considered as possible area for future researches.
Noteworthy is also the duration of ozone application in operative dentistry and endodontic treatment which is varied between 40 and 60 s, but in some cases with caries extended to the pulp chamber it is expanded to 2-3 min.[20] With regards to the aforementioned time intervals, in the present study, ozone was applied for 4 min which is much lower than the application period for hydrogen peroxide gel (three intervals of 8 min each). Accordingly, it could be suggested that if the duration of ozone application is expanded, it may be capable to induce a higher bleaching effect.
CONCLUSION
Based on the above study it can be concluded that although ozone produced a significant color change, the hydrogen peroxide gel showed a more prominent whitening effect. In addition, ozone has no synergistic effect in combination with hydrogen peroxide.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Bartold PM. Dentinal hypersensitivity: A review. Aust Dent J. 2006;51:212–8. [PubMed] [Google Scholar]
- 2.Joiner A. Tooth colour: A review of the literature. J Dent. 2004;32(Suppl 1):3–12. doi: 10.1016/j.jdent.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Jarad FD, Griffiths CE, Jaffri M, Adeyemi AA, Youngson CC. The effect of bleaching, varying the shade or thickness of composite veneers on final colour: An in vitro study. J Dent. 2008;36:554–9. doi: 10.1016/j.jdent.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Joiner A. The bleaching of teeth: A review of the literature. J Dent. 2006;34:412–9. doi: 10.1016/j.jdent.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Joiner A, Hopkinson I, Deng Y, Westland S. A review of tooth colour and whiteness. J Dent. 2008;36(Suppl 1):S2–7. doi: 10.1016/j.jdent.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Lee BS, Huang SH, Chiang YC, Chien YS, Mou CY, Lin CP. Development of in vitro tooth staining model and usage of catalysts to elevate the effectiveness of tooth bleaching. Dent Mater. 2008;24:57–66. doi: 10.1016/j.dental.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Jiang T, Ma X, Wang Z, Tong H, Hu J, Wang Y. Beneficial effects of hydroxyapatite on enamel subjected to 30% hydrogen peroxide. J Dent. 2008;36:907–14. doi: 10.1016/j.jdent.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 8.AbuNaba’a L, Al Shorman H, Holmes J, Petersson LG, Tagami J, Lynch E. Evidence-based research into ozone treatment in dentistry: An overview. In: Lynch E, editor. Ozone: The Revolution in Dentistry. London: Quintessence; 2004. pp. 73–115. [Google Scholar]
- 9.Azarpazhooh A, Limeback H. The application of ozone in dentistry: A systematic review of literature. J Dent. 2008;36:104–16. doi: 10.1016/j.jdent.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Can-Karabulut DC, Karabulut B. Shear bond strength to enamel after power bleaching activated by different sources. Eur J Esthet Dent. 2010;5:382–96. [PubMed] [Google Scholar]
- 11.Sulieman M, Addy M, Rees JS. Development and evaluation of a method in vitro to study the effectiveness of tooth bleaching. J Dent. 2003;31:415–22. doi: 10.1016/s0300-5712(03)00069-1. [DOI] [PubMed] [Google Scholar]
- 12.Bengel WM. Digital photography and the assessment of therapeutic results after bleaching procedures. J Esthet Restor Dent. 2003;15(Suppl 1):S21–32. doi: 10.1111/j.1708-8240.2003.tb00315.x. S32. [DOI] [PubMed] [Google Scholar]
- 13.Griffiths CE, Bailey JR, Jarad FD, Youngson CC. An investigation into most effective method of treating stained teeth: An in vitro study. J Dent. 2008;36:54–62. doi: 10.1016/j.jdent.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Gerlach RW, Barker ML, Sagel PA. Objective and subjective whitening response of two self-directed bleaching systems. Am J Dent. 2002;15(Spec No):7A–12. [PubMed] [Google Scholar]
- 15.Shenberg JE. inventor. Use of ozonated liquids and peroxides to whiten teeth. United States Patent 20090285767A1. 2009 Nov 19; [Google Scholar]
- 16.Sotelo JL, Beltran FJ, Benitez FJ, Beltran-Heredia J. Ozone decomposition in water: Kinetic study. Ind Eng Chem Res. 1987;26:39–43. [Google Scholar]
- 17.Manton DJ, Bhide R, Hopcraft MS, Reynolds EC. Effect of ozone and tooth mousse on the efficacy of peroxide bleaching. Aust Dent J. 2008;53:128–32. doi: 10.1111/j.1834-7819.2008.00021.x. [DOI] [PubMed] [Google Scholar]
- 18.Tessier J, Rodriguez PN, Lifshitz F, Friedman SM, Lanata EJ. The use of ozone to lighten teeth. An experimental study. Acta Odontol Latinoam. 2010;23:84–9. [PubMed] [Google Scholar]
- 19.Millar BJ, Hodson N. Assessment of the safety of two ozone delivery devices. J Dent. 2007;35:195–200. doi: 10.1016/j.jdent.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Loncar B, Mravak Stipetic M, Matosevic D, Tarle Z. Ozone application in dentistry. Arch Med Res. 2009;40:136–7. doi: 10.1016/j.arcmed.2008.11.002. [DOI] [PubMed] [Google Scholar]


