Abstract
Background:
Metal nanoparticles have been recently applied in dentistry because of their antibacterial properties. This study aimed to evaluate antibacterial effects of colloidal solutions containing zinc oxide (ZnO), copper oxide (CuO), titanium dioxide (TiO2) and silver (Ag) nanoparticles on Streptococcus mutans and Streptococcus sangius and compare the results with those of chlorhexidine and sodium fluoride mouthrinses.
Materials and Methods:
After adding nanoparticles to a water-based solution, six groups were prepared. Groups I to IV included colloidal solutions containing nanoZnO, nanoCuO, nanoTiO2 and nanoAg, respectively. Groups V and VI consisted of 2.0% sodium fluoride and 0.2% chlorhexidine mouthwashes, respectively as controls. We used serial dilution method to find minimum inhibitory concentrations (MICs) and with subcultures obtained minimum bactericidal concentrations (MBCs) of the solutions against S. mutans and S. sangius. The data were analyzed by analysis of variance and Duncan test and P < 0.05 was considered as significant.
Results:
The sodium fluoride mouthrinse did not show any antibacterial effect. The nanoTiO2-containing solution had the lowest MIC against both microorganisms and also displayed the lowest MBC against S. mutans (P < 0.05). The colloidal solutions containing nanoTiO2 and nanoZnO showed the lowest MBC against S. sangius (P < 0.05). On the other hand, chlorhexidine showed the highest MIC and MBC against both streptococci (P < 0.05).
Conclusion:
The nanoTiO2-containing mouthwash proved to be an effective antimicrobial agent and thus it can be considered as an alternative to chlorhexidine or sodium fluoride mouthrinses in the oral cavity provided the lack of cytotoxic and genotoxic effects on biologic tissues.
Keywords: Mouthrinse, nanoparticle, Streptococcus mutans, Streptococcus sangius
INTRODUCTION
Dental caries and periodontal problems are among the prevalent oral diseases throughout the world. Acidogenic bacteria such as Streptococcus mutans, Streptococcus sangius and Lactobacilli are considered as the contributory factors of dental caries.[1] S. sangius is also involved in periodontal problems. Mechanical methods such as tooth brushing are effective for plaque removal, but they are directly dependent on personal skills. Furthermore, effective tooth brushing is problematic in disabled or traumatized patients. The use of adjunctive methods such as mouthwashes has been shown to be effective for prevention of plaque accumulation.[2] Routine mouthrinses like chlorhexidine, however, are associated with the disadvantages including enamel staining, taste disturbances and mucosal irritation.[3,4] Therefore, searching for an alternative antimicrobial agent with minimal side effects seems to be quite reasonable.
Metal nanoparticles have long been used in medicine because of their bactericidal and bacteriostatic effects.[5,6,7] Nanotechnology has been introduced to the field of dental materials in recent years and nanoparticles have been inserted into the structure of the dental composites[8,9] and disinfection solutions.[10] The antibacterial properties of metal ions depend on their surface contact area. Decreased size of nanoparticles (<100 nm in diameter) leads to increased surface area and thus increased interaction with organic and inorganic molecules. However, many of the properties of metal nanoparticles are still unknown.[11] For example, cytotoxic properties of nanoparticles still need further research. Moreover, bioavailability and stability of nanoparticles as therapeutic delivery systems should be investigated. In addition, discoloration effects and cosmetic changes of some nanoparticles need further clarification.
Until now, there are only few studies that have determined the antimicrobial effects of nanoparticles against cariogenic and periodontal disease bacteria in simulated oral conditions.[9,12] The present study aimed to investigate the bactericidal and bacteriostatic effects of colloidal solutions containing zinc oxide (ZnO), copper oxide (CuO), titanium dioxide (TiO2) and silver (Ag) nanoparticles on S. mutans and S. sangius and to compare the results with those of chlorhexidine and sodium fluoride mouthrinses.
MATERIALS AND METHODS
Nanoparticles including ZnO (nanoZnO), CuO (nanoCuO), TiO2 (nanoTiO2) and Ag (nanoAg) were purchased from Fanavaran Araz Tajhiz Co., Iran. According to the supplier, nanoparticles were more than 98% pure after ignition. The nanoparticles were added to a water based-solution in pharmaceutics laboratory of Faculty of Pharmacy of Mashhad University of Medical Sciences, Mashhad, Iran. The nanoparticles were characterized by ultraviolet-visible spectroscopy (Shimatzu) and further examined by a particle — size analyzer (Zetasizer [Nano-zs] by Malvern) to find out their size distribution. Mean size of the nanoparticles ranged from 40 to 60 nm for nanoTiO2 and nanoCuO, 50-60 nm for nanoAg and 20 nm for nanoZnO. Colloidal solutions containing nanoparticles were prepared with initial concentration of 25 ppm and were sterilized in gravity autoclave before antimicrobial tests.
The study included six groups of mouthwashes. Groups I to IV included colloidal solutions containing nanoZnO, nanoCuO, nanoTiO2 and nanoAg, respectively. Groups V and VI consisted of 2.0% sodium fluoride and 0.2% chlorhexidine mouthwashes, respectively, which served as controls.
Preparation of bacterial suspensions
Antimicrobial experiments were carried out with S. mutans (PTC 1683) and S. sangius (PTCC 1449) procured from BuAli Research Institute, Mashhad, Iran. They were subculture in 5% sheep's blood agar. At first, 5-6 colonies from an overnight culture were diluted in brain heart infusion broth and were incubated in an aerobic environmental condition for 1-2 h at 35°C to reach the concentration of 1.5 × 108 CFU/ml. The colonies were then diluted with saline solution to a final concentration of 1.5 × 106 CFU/ml.
The lowest concentration of each antimicrobial agent that inhibits the growth of the microorganisms being tested is known as minimum inhibitory concentration (MIC) and is detected by lack of turbidity matching with a negative control. Furthermore, the minimum bactericidal concentration (MBC) is defined as the lowest concentration of an agent killing the majority of bacterial inoculums.[12,13]
The MICs and with subcultures the MBCs of S. mutans and S. sangius were determined from a known concentration of nanoparticles or mouthwashes in micrograms per milliliter, using the liquid microdilution method. In order to mimic the clinical conditions, artificial saliva was used for serial dilution. The cutoff points were compared to those described by the National Committee for Clinical Laboratory Standards, at the Clinical and Laboratory Standards Institutes.
For antibacterial experiments, 0.5 ml of diluted microorganisms was placed in tubes containing different concentrations of each nanoparticle and was incubated overnight at 35-37°C in a closed environment. Determination of MIC was based on the turbidity measured by spectrophotometer (Eppendrof AG, Hamburg, Germany). After determining the MIC, 50 ml of the corresponding bacterial suspension was spread in sheep's blood agar and was incubated at 37°C for 24 h. The numbers of colonies growing from each of the test tubes were counted and the number of colonies corresponding to a thousand-fold reduction was recorded as the MBC. All experiments were conducted in triplicate for each concentration.
To determine the required time before initiating bactericidal effect, 50 ml of each test specimen was mixed with 50 ml of the bacterial suspensions (containing 5 × 103 colonies). After 1 and 5 min, it was cultured on blood agar. Following overnight incubation at 37°C, the remaining colonies were counted. One-way analysis of variance (ANOVA) was run to determine any significant differences in MIC and MBC of the study groups, followed by Duncan multiple range test for pairwise comparisons. The statistical analysis was performed through SPSS (Statistical Package for Social Sciences, version 16, Chicago, IL, USA) and the significance level was determined at P < 0.05.
RESULTS
Table 1 presents the means and standard deviations regarding MICs and MBCs of the study groups against S. mutans and S. sangius. The sodium fluoride mouthrinse did not show antibacterial effects against any of the microorganisms. The colloidal solution containing TiO2 nanoparticles had the lowest MIC against S. mutans and S. sangius and also displayed the lowest MBC against S. mutans. Furthermore, the solutions containing nanoTiO2 and nanoZnO showed the lowest MBC against S. sangius. On the other hand, the highest MIC and MBC against both streptococci pertained to the 0.2% chlorhexidine mouthrinse [Table 1].
Table 1.
Mean MIC and MBC (μg/ml) of the test groups against S. mutans and S. sangius and the results of statistical analysis for comparison between groups
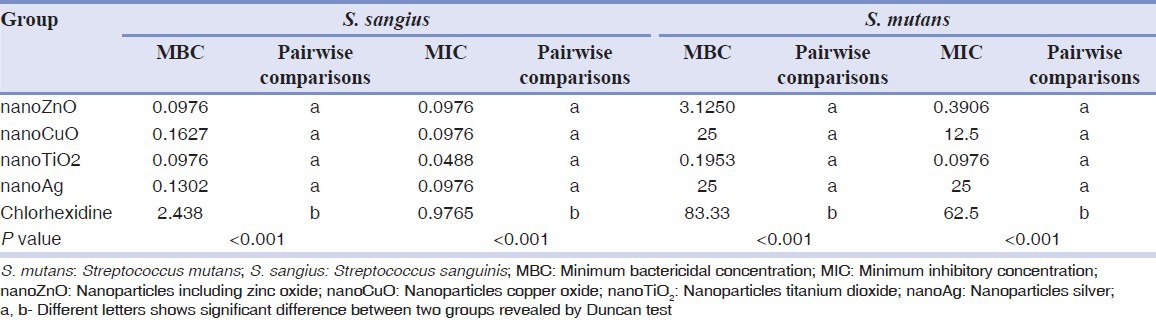
ANOVA revealed a significant difference between MICs and MBCs of the study groups against S. mutans and S. sangius [Table 1]. Between-group comparisons by Duncan test demonstrated that the MICs and MBCs of the colloidal solutions containing nanoparticles were comparable to each other and all were significantly lower than that of the chlorhexidine mouthrinse against both microorganisms [Table 1].
Table 2 demonstrates the number of colonies of S. mutans and S. sangius after 1 and 5 min of bacterial exposure to each colloidal solution or mouthwash. Sodium fluoride mouthrinse showed the largest colony count after both 1 and 5 min of bacterial exposure. In contrast, no S. mutans and S. sangius colonies were observed after 1 and 5 min of exposure to chlorhexidine mouthwash [Table 2]. There was no significant difference in the number of S. mutans colonies between nanoTiO2, nanoZnO and nanoCuO mouthwashes after 1 and 5 min. However, significantly fewer S. mutans colonies were observed in these groups compared to the sodium fluoride mouthrinse and nanoAg colloidal solution [Table 2].
Table 2.
The number of colonies of S. mutans and S. sangius after 1 and 5 min of exposure to each solution and the results of statistical analysis for comparison between groups
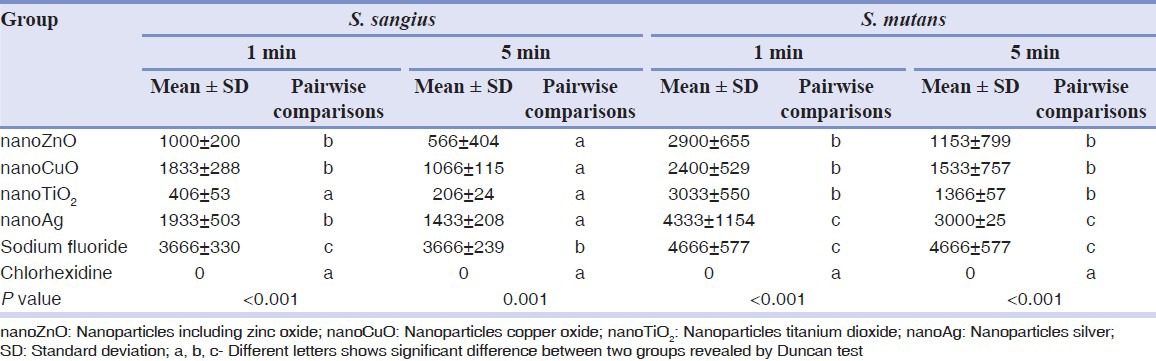
Regarding S. sangius, significantly fewer colonies were counted in chlorhexidine mouthwash and nanoTiO2-containg solution after 1 min compared to the other groups. After 5 min, the number of S. sanguis colonies in all nanoparticles groups was comparable to that of chlorhexidine.
DISCUSSION
S. mutans is known as a main etiological factor in dental caries. Also, S. sangius plays a vital role in dental plaque formation and thus dental caries and periodontal problems.[14] Application of mouthrinses has been proposed as an adjunct to mechanical methods of plaque removal. Sodium fluoride and chlorhexidine are among the routine mouthwashes used for prevention of dental caries or periodontal problems.[2] Discoloration of restorations, unfavorable taste, allergy and xerostomia are some of the disadvantages of chlorhexidine.[3,4] Furthermore, it damages the microflora of the oral cavity due to its long-lasting effects.[15]
In the present study, the serial dilution method was used to determine the MICs of the test groups. This method is more accurate compared with the disc diffusion test and is more easily interpreted.[15] The artificial saliva was used in the test tubes to reveal any probable effect of proteins and other salivary constituents on the antibacterial activity of nanoparticle containing solutions.
Antibacterial properties of some nanoparticles such as silver and gold have been verified in previous studies[12,16] and different mechanisms have been proposed for their effects. Nanosilver inhibits the enzymes of the cell respiratory cycle and damages the deoxyribonucleic acid (DNA) synthesis.[17,18] Hernández-Sierra et al.[12] indicated that nanosilver inhibits the growth of S. mutans at lower concentrations compared to nanoZn and nanoAu and thus it may be more effective against dental caries. Although, the antibacterial effect of Zn nanoparticles against S. mutans has been demonstrated, its mechanism of action is still unknown.[19] It is assumed that the mechanism of action of nanoCu is similar to that of nanosilver. Cu ions adhere to DNA molecules and form cross links within and between nucleic acid chains and thus disrupting the helical structure of the nucleus. Moreover, Cu ions impair the biochemical processes of bacterial cells. Combination of silver and copper nanoparticles may give rise to a more complete bactericidal effect against mixed bacterial populations.[20] TiO2 nanoparticles show photocatalytic characteristics and prevent the accumulation of pathogenic bacteria.[21]
Most of the previous studies investigated the antibacterial properties of nanosilver and there is limited data available on the bactericidal properties of other nanoparticles, especially when they are prepared in colloidal solutions as mouthwashes. Jung et al.[22] obtained an average MIC of 50 μg/ml against S. mutans for silver nanoparticles which were twice that of our finding. This difference can be attributed to the method of disc diffusion test that they used to find the MIC. The contact area of nanoparticles with bacterial microorganisms is higher in serial dilution method compared to the culture media, thus increasing their antibacterial effect. Hernandez-Sierra et al.[12] in their study have reported an average MIC of 4.86 ± 2.71 mg/ml against S. mutans for nanoZnO, which was somewhat higher than that achieved in this study (3.12 ± 0.390 μg/ml). Sadeghi et al.[10] evaluated the antimicrobial effect of chlorhexidine against S. sangius and found an average MIC of 256 μg/ml, which was higher compared to that found in the present study (83.33 μg/ml) possibly due to the different concentration of chlorhexidine (%0.12) they employed.
In this study, the nanoTiO2-containing solution resulted in less number of S. sangius after 1 min of exposure compared to other nanoparticle-containing solutions and its antibacterial effect was comparable to that of chlorhexidine. The solutions containing nanoCuO, nanoZnO and nanoTiO2 resulted in less number of S. mutans colonies after 1 and 5 min of bacterial exposure in comparison to the solution including silver nanoparticles. However, the antibacterial effects of all the nanoparticle groups were significantly lower than that of the 0.2% chlorhexidine mouthrinse against S. mutans. This is in contrary to the results of Sadeghi et al.[10] who showed that nanosilver had bactericidal effects against S. mutans after 30 s, which was comparable to that of chlorhexidine.
In the present study, the antibacterial effect of silver nanoparticles was not desirable against S. mutans. It is possible that nanoAg particles adhere to each other and form micrometer particles at high concentrations, which leads to less antimicrobial activity. The solutions containing TiO2 and ZnO nanoparticles inhibited the development of the S. sangius strain at a lower concentration than other test groups. In general, development of S. sangius was inhibited at lower concentrations of antimicrobial agents compared to S. mutans colonies. The MIC and MBC of the nanoTiO2 colloidal solution against S. mutans was found to be 640 and 427 times lower than that of the chlorhexidine. In other words, nanoTiO2 colloidal solution affected S. mutans at significantly lower concentrations, which may allow achieving clinical effects with reduced side effects. Therefore, TiO2 nanoparticles are promising as antimicrobial agents to be inserted in mouthrinses and be used as an alternative to routine antibacterial mouthwashes. However, detailed research and comparative study of strain specific variability is required to determine its bactericidal efficiency. Furthermore, its biocompatibility should be further investigated before commercialization.
It should be noted that complete simulation of the oral cavity is not possible in the laboratory conditions. The incubator cannot completely resemble the mouth temperature. Furthermore, the antibacterial agents contact constantly with bacterial microorganisms in the culture media or test tubes, but the contents of mouthwashes are diluted and neutralized immediately in the oral cavity.
Further studies are warranted to elucidate the antimicrobial effects of nanoparticle solutions when used as mouthwashes under in vivo conditions and any possible side-effects of these solutions on oral microflora. Future investigations can be designed to reveal the bactericidal properties of combinations of various nanoparticles on different microorganisms. Furthermore, bioavailability of nanoparticle containing mouthwashes compared to commercially available ones need more investigations.
CONCLUSION
The solution containing TiO2 nanoparticles showed the lowest inhibitory concentration against S. mutans and S. sangius compared to those of other nanoparticle containing solutions and chlorhexidine mouthwash and thus it may be further investigated as an alternative to chlorhexidine.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the vice-chancellor for research of Mashhad University of Medical Sciences for the financial support of this project (grant number 900070). The results presented in this paper were taken from a DDS student thesis (thesis number 2589).
Footnotes
Source of Support: Mashhad University of Medical Sciences (grant number 900070).
Conflict of Interest: None declared.
REFERENCES
- 1.Featherstone JD. The science and practice of caries prevention. J Am Dent Assoc. 2000;131:887–99. doi: 10.14219/jada.archive.2000.0307. [DOI] [PubMed] [Google Scholar]
- 2.Wennström JL, Dahlén G, Gröndahl K, Heijl L. Periodic subgingival antimicrobial irrigation of periodontal pockets. II. Microbiological and radiographical observations. J Clin Periodontol. 1987;14:573–80. doi: 10.1111/j.1600-051x.1987.tb01518.x. [DOI] [PubMed] [Google Scholar]
- 3.Newman MG, Takei HH, Carranza FA. 10th ed. Ch. 6. Philadelphia: Elsevier; 2006. Clinical Periodontology; p. 42. [Google Scholar]
- 4.Shah HM, Shah MN, Gokani VN, Jethal BS. A comparative, qualitative and quantitative antimicrobial efficacies of mouthrinses containing chlorhexidine gluconate and essential oils. Indian J Dent Res. 1993;4:103–11. [PubMed] [Google Scholar]
- 5.Phan TN, Buckner T, Sheng J, Baldeck JD, Marquis RE. Physiologic actions of zinc related to inhibition of acid and alkali production by oral streptococci in suspensions and biofilms. Oral Microbiol Immunol. 2004;19:31–8. doi: 10.1046/j.0902-0055.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 6.Lansdown AB. Silver in health care: Antimicrobial effects and safety in use. Curr Probl Dermatol. 2006;33:17–34. doi: 10.1159/000093928. [DOI] [PubMed] [Google Scholar]
- 7.Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–53. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 8.Aydin Sevinç B, Hanley L. Antibacterial activity of dental composites containing zinc oxide nanoparticles. J Biomed Mater Res B Appl Biomater. 2010;94:22–31. doi: 10.1002/jbm.b.31620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heravi F, Ramezani M, Poosti M, Hosseini M, Shajiei A, Ahrari F. In vitro cytotoxicity assessment of an orthodontic composite containing titanium-dioxide nanoparticles. J Dent Res Dent Clin Dent Prospects. 2013;7:192–8. doi: 10.5681/joddd.2013.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadeghi R, Olia P, Rezvani MB, Taleghani F, Sharif F. Comparison of the nanosilver and chlorhexidin antimicrobial effect on Streptococcus sangius and actinomicosis viscosus. J Islamic Dent Assoc. 2010;23:225–31. [Google Scholar]
- 11.Yoon KY, Hoon Byeon J, Park JH, Hwang J. Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Sci Total Environ. 2007;373:572–5. doi: 10.1016/j.scitotenv.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Hernández-Sierra JF, Ruiz F, Pena DC, Martínez-Gutiérrez F, Martínez AE, Guillén Ade J, et al. The antimicrobial sensitivity of Streptococcus mutans to nanoparticles of silver, zinc oxide, and gold. Nanomedicine. 2008;4:237–40. doi: 10.1016/j.nano.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Wikler MA, Cockerill FR, Craig WA, Bush K, Dudley MN, Hardy D. Method for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved Standard J. 2009;29:552–8. [Google Scholar]
- 14.Michalek SM, Hirasawa M, Kiyono H, Ochiai K, McGhee JR. Oral ecology and virulence of Lactobacillus casei and Streptococcus mutans in gnotobiotic rats. Infect Immun. 1981;33:690–6. doi: 10.1128/iai.33.3.690-696.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Leary TJ, Drake RB, Naylor JE. The plaque control record. J Periodontol. 1972;43:38. doi: 10.1902/jop.1972.43.1.38. [DOI] [PubMed] [Google Scholar]
- 16.Shrivastava S, Jyung W. Characterization of enhanced antibacterial effects of nano silver nano particles. J Nanotechnol. 2010;25:103–25. [Google Scholar]
- 17.Hidalgo E, Domínguez C. Study of cytotoxicity mechanisms of silver nitrate in human dermal fibroblasts. Toxicol Lett. 1998;98:169–79. doi: 10.1016/s0378-4274(98)00114-3. [DOI] [PubMed] [Google Scholar]
- 18.Lee HG, Yeo SY. Antibacterial effect of nanosized silver colloidal solution on textile fabrics. J Biol Inorg Chem. 2003;16:44–7. [Google Scholar]
- 19.Adams LK, Lyon DY, Alvarez PJ. Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res. 2006;40:3527–32. doi: 10.1016/j.watres.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Ruparelia JP, Chatterjee AK, Duttagupta SP, Mukherji S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008;4:707–16. doi: 10.1016/j.actbio.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Liao J, Chun M, Kun WH, Zhang J, Li TB. Antibacterial activity of silver-hydroxyapatite/titania nanoparticles on oral bacteria. Key Eng Mater. 2007;16:299–302. [Google Scholar]
- 22.Jung WK, Koo HC, Kim KW, Shin S, Kim SH, Park YH. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl Environ Microbiol. 2008;74:2171–8. doi: 10.1128/AEM.02001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]


