Abstract
Background:
Many oral squamous cell carcinomas (OSCCs) arise within regions that previously had premalignant lesion. Early diagnosis and prompt treatment of premalignant lesions offers the best hope of improving the prognosis in patients with OSCC. Exfoliative cytology is a simple and non-invasive diagnostic technique that could be used for early detection of oral premalignant and malignant lesions. This study was undertaken to evaluate the quantitative changes in nuclear area (NA), cytoplasmic area (CA) and nuclear-to-cytoplasmic ratio (NA/CA) in cytological buccal smears of oral leukoplakia with dysplasia (OLD) and OSCC patients while comparing with normal healthy mucosa.
Materials and Methods:
A quantitative study was conducted over 90 subjects including 30 cases each of OLD, OSCC and clinically normal oral mucosa. The smears obtained were stained with Papanicolaou (PAP) stain and cytomorphological assessment of the keratinocytes was carried out. The statistical tools included arithmetic mean, standard deviation, Chi-square test, analysis of variance, Tukey multiple comparison. P < 0.001 was considered as significant.
Results:
The mean NA of keratinocytes in the normal mucosa was 65.47 ± 4.77 μm2 while for OLD it was 107.97 ± 5.44 μm2 and 139.02 ± 8.10 μm2 for that of OSCC. The differences show a statistically significant increment in NA (P < 0.001). There was significant reduction (P < 0.001) in the CA of keratinocytes from OSCC when compared with those from smears of OLD and normal mucosa with the values of 1535.80 ± 79.38 μm2, 1078.51 ± 56.65 μm2 and 769.70 ± 38.77 μm2 respectively. The NA/CA ratio in the smears from normal oral mucosa, OLD and OSCC showed a mean value of 0.043 ± 0.004, 0.100 ± 0.008, 0.181 ± 0.015 respectively with a significant difference among the groups (P < 0.001).
Conclusion:
Evaluation of nuclear and CA of keratinocytes by cytomorphometry can serve as a useful adjunct in the diagnosis and prognosis of a dysplastic lesion which may lead to OSCC.
Keywords: Cytomorphometry, dysplasia, exfoliative cytology, oral leukoplakia, oral squamous cell carcinoma
INTRODUCTION
Cancer is Latinized from the Greek word “Karkinos,” meaning crab, denoting how carcinoma extends its claws like a crab into the adjacent tissues.[1] Cancer being a genetic disorder involves multiple alterations of the genome progressively accumulated during a protracted period. Its overall effect of which surpasses the inherent reparative ability of the cell. During its progression, visible physical changes take place at the cellular level (atypia) and at the resultant tissue level (dysplasia). The sum total of these physical and morphological alterations are of diagnostic and prognostic relevance and is designated as “precancerous” changes. These changes are ultimately involved in driving cells further along the path to neoplastic transformation.[2]
Oral cancer is the most frequent neoplasm of the head and neck region. Among this the most common is oral squamous cell carcinoma (OSCC).[3] Cancerous lesions are usually benign in appearance and asymptomatic in nature in their early stages.[4] The diagnosis of pre-cancers is primarily based on morphology of cells and its grading on histology, i.e., dysplasia. Despite the fact that this estimation is subjective, it is still widely practiced to assess the risk of malignant potential of such lesions. Due to this inherent discrepancy, such lesions may well be designated as potentially malignant.[2]
Leukoplakia is a potentially malignant disorder, it is defined as white plaque of questionable risk having excluded (other) known disease or disorders that carry no increased risk for cancer.[5] It may occur either as a single, localized change of the oral mucosa or as diffuse, often multiple lesions.[6] As a clinical entity, it may have varied histological presentations, ranging from mildly hyperkeratotic lesions to the lesions that exhibit severe dysplastic features.[7]
Exfoliative cytology is advantageous, as it is a painless, bloodless non-invasive, quick and simple procedure. It is suitable in patients with systemic disease who are contraindicated for biopsy. It guards against false negative biopsy and post-biopsy complications can be eliminated. This procedure can be repeated a number of times for diagnosis, follow-up and research purposes.[8] Exfoliative cytology usually plays a supportive role in a properly planned and carefully executed biopsy for the diagnosis of oral cancer. The definitive diagnosis is made by biopsy.[9]
Exfoliative cytology is based on epithelial physiology. A normal epithelium is exposed to regular exfoliation, namely the loss of cell surface and the thickness of the epithelium is constant. Under normal conditions, epithelial cells are strongly held in place. The presence of benign disease or the occurrence of malignant epithelial formation causes the cells to lose their cohesive force and results in exfoliation. Loss of cohesion between the cells enables the collection of the exfoliated cells for microscopic examination.[10]
Previous studies stated that the increase in the nuclear size is related to an increase in the nuclear contents required for replication. They also mentioned that in cells with increased activity the ability of cytoplasm to mature diminishes. In addition, the amount of cytoplasm the cell makes in relation to the nucleoplasm, is less.[11]
Cytomorphometry is a quantitative technique, based on the evaluation of parameters such as nuclear area (NA), cytoplasmic area (CA), nuclear-to-cytoplasmic area ratio (NA/CA), which may increase the sensitivity of exfoliative cytology for early diagnosis of OSCC, since these techniques are precise, objective and reproducible.[12]
The purpose of this study was to conduct quantitative cytomorphometric comparison in buccal smears of oral leukoplakia with dysplasia (OLD) and OSCC.
MATERIALS AND METHODS
A prospective study was carried out on 90 patients in the Department of Oral Pathology and Microbiology, Sardar Patel Post Graduate Institute of Dental and Medical Sciences, Lucknow, India. Its approval was obtained from the Ethical Committee of the institute. Out of 90 patients, 30 cases were of OLD, 30 cases of OSCC and 30 samples of clinically normal oral mucosa from healthy individuals who were non-smokers and non-alcoholic and served as control group. The male-female ratio in the control group was 50:50 while in study groups it was 60:40 and 70:30 for OLD and OSCC respectively. The patients included in the study were over 40 years of age and non-anemic. Anemic patients, patients with history of any recent systemic disease and those on medication were excluded from the study to avoid cellular changes associated with these conditions.
Smears were taken from buccal mucosa with a cytobrush for each case from above mentioned groups and then fixed with 95% ethanol with 3% glacial acetic acid (Biofix spray). It is necessary to wash the mouth before scraping, as it removes debris from the surface and provides better staining. Smears were then stained with Papanicolaou (PAP) stain according to modified rapid PAP method (Bio-lab Diagnostics, India). The stained slides had been subjected to cytomorphometrical assessment.
PAP staining lies in the fact that the dehydration and better clearing solutions help in causing cellular transparency. This detects the overlapped cells and their individual morphology, which otherwise would be confused for bi or multinucleated cells. It also shows a stability of stain over long periods, stability of color and of course, the better reproducibility of results.[13,14]
Cytobrush is a convenient instrument to collect samples from less accessible oral sites. Cytobrush pulls together an adequate number of cells and allows uniform dispersion of cells on a microslide which facilitates an accurate cytopathologic diagnosis.[15]
Biopsies of the oral lesions were taken for making histopathological diagnosis and confirmation of disease. Only confirmed cases were included in the study. Tissues were fixed with 10% neutral buffer formalin, processed and embedded in paraffin wax. 5 μm thick sections were made and stained with hematoxylin and eosin.[16] Biopsy samples improperly fixed, processed or stained were excluded from the study. Diagnosis of the lesions was made after observation of sections using Olympus pentahead research microscope (BX 51, Japan). For image analysis, software Imagepro Express 6.0 Media Cybernetics, USA, with operating system windows XP was used.
For nuclear and cellular morphometric analysis the images were captured at magnification of X40 using Olympus E331 single-lens reflex digital camera by attaching to Olympus pentahead research microscope. For measurement, the images were recalled on to the monitor and all measurements were carried out using the measuring tools of the image analyzer software. Twenty five cells with well-defined borders were selected randomly, commencing with first representative field on left hand side and then moving the stage to the next field and continuing the selection to include 5 fields from each section. Stage readings were noted for reassessment.
Cells were measured which were only clearly defined avoiding clumped or folded cells and unusually distorted nuclei and cells. Outline of cells were traced with the pointer using the mouse. NA and CA were automatically calculated by the software [Figures 1–3]. The NA/CA was calculated by the following formula: NA/CA ratio = NA/CA. The results were tabulated and subjected to statistical analysis including arithmetic mean, standard deviation (SD), Chi-square test, analysis of variance (ANOVA) and Tukey multiple comparison test. P < 0.001 was considered as significant.
Figure 1.
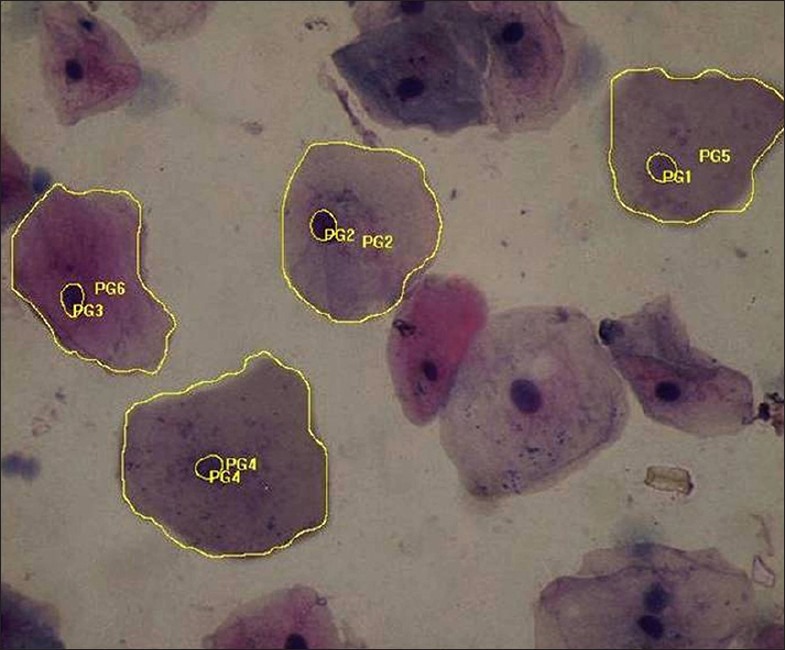
Papanicolaou stained smear of normal oral mucosa (measurement of nuclear and cytoplasmic area) (×40)
Figure 3.
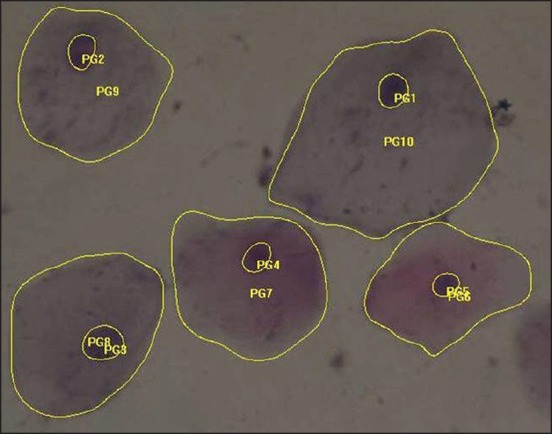
Papanicolaou stained smear of oral squamous cell carcinoma (measurement of nuclear and cytoplasmic area) (×40)
Figure 2.
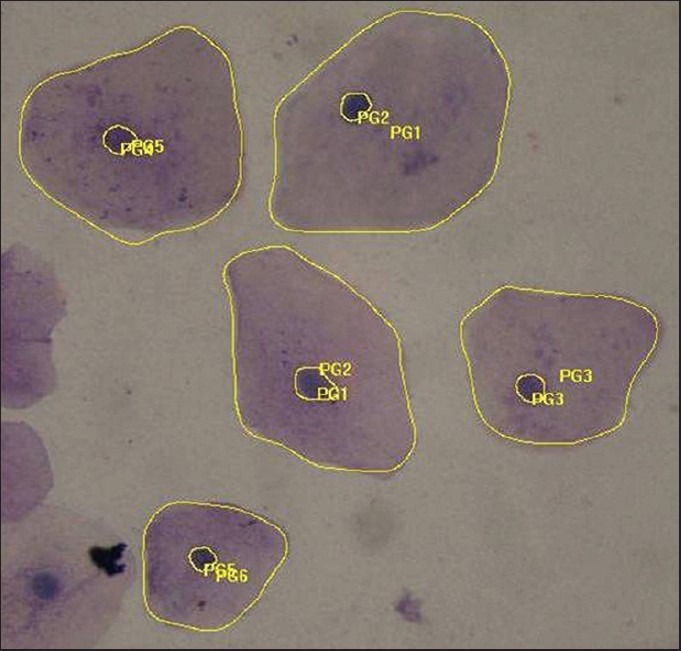
Papanicolaou stained smear of oral leukoplakia (measurement of nuclear and cytoplasmic area) (×40)
RESULTS
The NA of normal oral mucosa, OLD and OSCC groups were summarized in Table 1. The NA of three groups ranged from 52.40-72.34 μm2, 97.21-116.20 μm2 and 120.90-154.45 μm2 respectively with mean (± SD) 65.47 ± 4.77 μm2, 107.97 ± 5.44 μm2 and 139.02 ± 8.10 μm2 respectively. The differences show a statistically significant increment in NA (P < 0.001).
Table 1.
Summary (mean ± SD) of NA (μm2) of 3 groups

The CA of normal oral mucosa, OLD and OSCC groups were summarized in Table 2. The CA of three groups ranged from 1389.45-1678.96 μm2, 998.78-1200.42 μm2 and 674.45-807.10 μm2 respectively with mean (± SD) 1535.80 ± 79.38 μm2, 1078.51 ± 56.65 μm2 and 769.70 ± 38.77 μm2 respectively. There was significant reduction (P < 0.001) in the CA among the three groups.
Table 2.
Summary (mean ± SD) of CA (μm2) of 3 groups

The ratio (NA/CA) of normal oral mucosa, OLD and OSCC groups were summarized in Table 3. The NA/CA ratio of three groups ranged from 0.03-0.05, 0.08-0.11 and 0.15-0.21 respectively with mean (± SD) 0.043 ± 0.004, 0.100 ± 0.008 and 0.181 ± 0.015 μm2 respectively. There is a significant difference among the groups (P < 0.001).
Table 3.
Summary (mean ± SD) of NA/CA ratio of 3 groups

DISCUSSION
As per the normal physiology, the oral epithelium renews itself rapidly (probably every 2 weeks). The rationale of oral exfoliative cytology is based on this physiological process, examining cells that are desquamated or abraded from the surface of the oral mucosa. The superficial epithelial cells do contain nuclei and alterations in these cells can serve as reliable indicators of dysplastic or neoplastic changes.[11]
The basic defect of the alteration of any cell begins at the molecular level triggering a series of reactions and thereby affecting the entire cell system and consequently its morphology. The general biological activity is reflected best in nucleus and functional activity is reflected in cytoplasm.[13]
The ideal instrument used for making a good cytological smear should be easy to use in any location, cause minimum trauma and provide an adequate and representative number of epithelial cells. It has been shown that a cytobrush is an adequate instrument for this purpose due to its ease in sampling and it also provides cytologic samples of good quality. Brush biopsy is a simple, relatively inexpensive, highly sensitive, risk free method for screening the cancer and serves as an aid to the clinical examination.[17]
In the present study, the cytomorphometric findings demonstrated that NA from the smears of normal oral mucosa, OLD and OSCC, showed a gradual increase in the mean value with 65.47 ± 4.77, 107.97 ± 5.44 and 139.02 ± 8.10 μm2 respectively. Comparing the mean NA of three groups, ANOVA revealed significantly different NA among the groups (P < 0.001). The increase in the nuclear size may be related to an increase in the nuclear contents required for replication.
This was in accordance with the study of Weigum et al.[18] In their study of 41 cases, the mean NA of normal mucosa, dysplasia and SCC was 63.4 ± 11, 149 ± 23 and 165 ± 46 respectively. Here, the NA was significantly increased in dysplastic and SCC cytologic smears versus normal mucosa (P < 0.001). Similarly, Khandelwal and Solomon[11] in their study found that the mean NA of keratinocytes was higher in OSCC lesions when compared with those from the mucosa of tobacco users and normal mucosa. In another study conducted by Goregen et al.[10] on smokers and non-smokers, the authors observed a 16.5% increase in the NA value of smokers over non-smokers. This increase in NA can be attributed to a cellular adaptation that depends on smoking. This adaptive change in the cell nucleus tends to be dysplastic.
The values of CA from the smears of normal oral mucosa, OLD and OSCC, in the present study, showed a mean value of 1535.80 ± 79.38, 1078.51 ± 56.65 and 769.70 ± 38.77 μm2. There was a gradual decrease in CA from normal oral mucosa, OLD, OSCC respectively. ANOVA test for comparing the mean CA of three groups, revealed significantly different values among the groups (P < 0.001). This may be due to the fact that with increased activity, the maturing ability of cytoplasm diminishes. Besides, the cell makes less amount of cytoplasm in relation to the nucleoplasm.[11]
Similar results has been obtained by Khandelwal and Solomon.[11] In their study, oral smears from 60 cases with 20 cases each of normal mucosa, mucosa of tobacco users and OSCC showed a mean value of 1377.30 ± 76.68, 1120 ± 70.44 and 783.62 ± 53.33 respectively. The results revealed significantly different CA among the groups. Also, the studies done by Cowpe et al.[4] and Ogden et al.[19,20] described similar findings.
Regarding the NA/CA ratio in the present study, the smears from normal oral mucosa, OLD and OSCC showed a mean value of 0.043 ± 0.004, 0.100 ± 0.008, 0.181 ± 0.015 respectively. Comparing the mean NA/CA ratio of three groups, there is a significantly different NA/CA ratio among the groups (P < 0.001). This may be due to significant elevation in mean NA and a significant reduction in mean CA.
A significant increase in mean NA/CA ratio had also been observed in the studies of Cowpe et al.[4] and Khandelwal and Solomon.[11] On the contrary, Diniz-Freitas et al.[12] studied 10 healthy subjects and 10 patients with oral carcinoma and concluded that neither CA and NA nor NA/CA ratio differ between the oral mucosa of oral carcinoma patients and healthy patient. This may be due to the small sample size, difference in methodology employed, improper site selection and inclusion of only superficial cells.
To find OLD patients was a difficult task in the study because leukoplakia ranges from mild hyperkeratosis to the lesions that exhibit severe dysplasia and also due to the unwillingness of the patient to go for biopsy procedure. To overcome this problem, patient motivation was done explaining the patient that oral cancer is usually diagnosed when it becomes symptomatic. Small asymptomatic lesions are ignored or missed by patients. This may be due to the incomplete understanding or awareness that even small lesions can have significant malignant potential and if patient could receive treatment at an early stage, both the survival rates and quality of life could be improved.
To summarize, the study clearly indicates that an increase in the NA and decrease in the CA are characteristics of malignant keratinocytes. Cytomorphometric evaluation of keratinocytes can serve as a useful diagnostic adjunct for early detection of oral cancer. It may also aid in establishing the prognosis of a dysplastic lesion.
CONCLUSION
The major advantage of exfoliative cytology is the non-invasive character of the technique, which allows a simple and pain-free collection of intact cells from different layers in the epithelium for microscopical examination and quantitative evaluation. The technique is not intended to replace tissue biopsy, but it is a valuable supplement to biopsy. Use of cytomorphometry will improve the diagnostic reliability of exfoliative cytology. It can be indicated for the detection as well as follow-up of the patient with either a premalignant or malignant oral lesion.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.George A, Sreenivasan BS, Sunil S, Varghese SS, Thomas J, Gopakumar D, et al. Potentially malignant disorders of oral cavity. Oral Maxillofac Pathol J. 2011;2:95–100. [Google Scholar]
- 2.Rajendran R. Oral leukoplakia (leukokeratosis): Complication of facts and figures. J Oral Maxillofac Pathol. 2004;8:58–68. [Google Scholar]
- 3.Acha A, Ruesga MT, Rodríguez MJ, Martínez de Pancorbo MA, Aguirre JM. Applications of the oral scraped (exfoliative) cytology in oral cancer and precancer. Med Oral Patol Oral Cir Bucal. 2005;10:95–102. [PubMed] [Google Scholar]
- 4.Cowpe JG, Longmore RB, Green MW. Quantitative exfoliative cytology of abnormal oral mucosal smears. J R Soc Med. 1988;81:509–13. doi: 10.1177/014107688808100905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Waal I. Potentially malignant disorders of the oral and oropharyngeal mucosa; terminology, classification and present concepts of management. Oral Oncol. 2009;45:317–23. doi: 10.1016/j.oraloncology.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 6.van der Waal I, Schepman KP, van der Meij EH, Smeele LE. Oral leukoplakia: A clinicopathological review. Oral Oncol. 1997;33:291–301. doi: 10.1016/s1368-8375(97)00002-x. [DOI] [PubMed] [Google Scholar]
- 7.Usta U, Berberoglu U, Helvaci E, Altaner S, Sut N, Ozdemir C. Evaluation of cytological alterations in normal-appearing oral mucosal epithelia of smokers and non-smokers via Agnor counts and nuclear morphometry. Balkan Med J. 2008;25:110–6. [Google Scholar]
- 8.Ramaesh T, Mendis BR, Ratnatunga N, Thattil RO. Diagnosis of oral premalignant and malignant lesions using cytomorphometry. Odontostomatol Trop. 1999;22:23–8. [PubMed] [Google Scholar]
- 9.Cooke BE. Exfoliative cytology in evaluating oral lesions. J Dent Res. 1963;2:343–7. doi: 10.1177/00220345630420013901. [DOI] [PubMed] [Google Scholar]
- 10.Goregen M, Akgul HM, Gundogdu C. The cytomorphological analysis of buccal mucosa cells in smokers. Turk J Med Sci. 2011;41:205–10. [Google Scholar]
- 11.Khandelwal S, Solomon MC. Cytomorphological analysis of keratinocytes in oral smears from tobacco users and oral squamous cell carcinoma lesions — A histochemical approach. Int J Oral Sci. 2010;2:45–52. doi: 10.4248/IJOS10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diniz-Freitas M, García-García A, Crespo-Abelleira A, Martins-Carneiro JL, Gándara-Rey JM. Applications of exfoliative cytology in the diagnosis of oral cancer. Med Oral. 2004;9:355–61. [PubMed] [Google Scholar]
- 13.Sivapathasundharam B, Kalasagar M. Yet another article on exfoliative cytology. J Oral Maxillofac Pathol. 2004;8:54–7. [Google Scholar]
- 14.Zimmermann ER, Zimmermann AL. Effects of race, age, smoking habits, oral and systemic disease on oral exfoliative cytology. J Dent Res. 1965;44:627–31. doi: 10.1177/00220345650440040301. [DOI] [PubMed] [Google Scholar]
- 15.Ogden GR, Cowpe JG, Green M. Cytobrush and wooden spatula for oral exfoliative cytology. A comparison. Acta Cytol. 1992;36:706–10. [PubMed] [Google Scholar]
- 16.Bancroft JD, Gamble M. 6th ed. Philadelphia: Elsevier; 2008. Theory and Practice of Histological Techniques; pp. 126–7. [Google Scholar]
- 17.Mehrotra R, Gupta A, Singh M, Ibrahim R. Application of cytology and molecular biology in diagnosing premalignant or malignant oral lesions. Mol Cancer. 2006;5:11. doi: 10.1186/1476-4598-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Weigum SE, Floriano PN, Redding SW, Yeh CK, Westbrook SD, McGuff HS, et al. Nano-bio-chip sensor platform for examination of oral exfoliative cytology. Cancer Prev Res (Phila) 2010;3:518–28. doi: 10.1158/1940-6207.CAPR-09-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogden GR, Cowpe JG, Green MW. Quantitative exfoliative cytology of normal buccal mucosa: Effect of smoking. J Oral Pathol Med. 1990;19:53–5. doi: 10.1111/j.1600-0714.1990.tb00795.x. [DOI] [PubMed] [Google Scholar]
- 20.Ogden GR, Cowpe JG, Green MW. The effect of distant malignancy upon quantitative cytologic assessment of normal oral mucosa. Cancer. 1990;65:477–80. doi: 10.1002/1097-0142(19900201)65:3<477::aid-cncr2820650317>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]


