Abstract
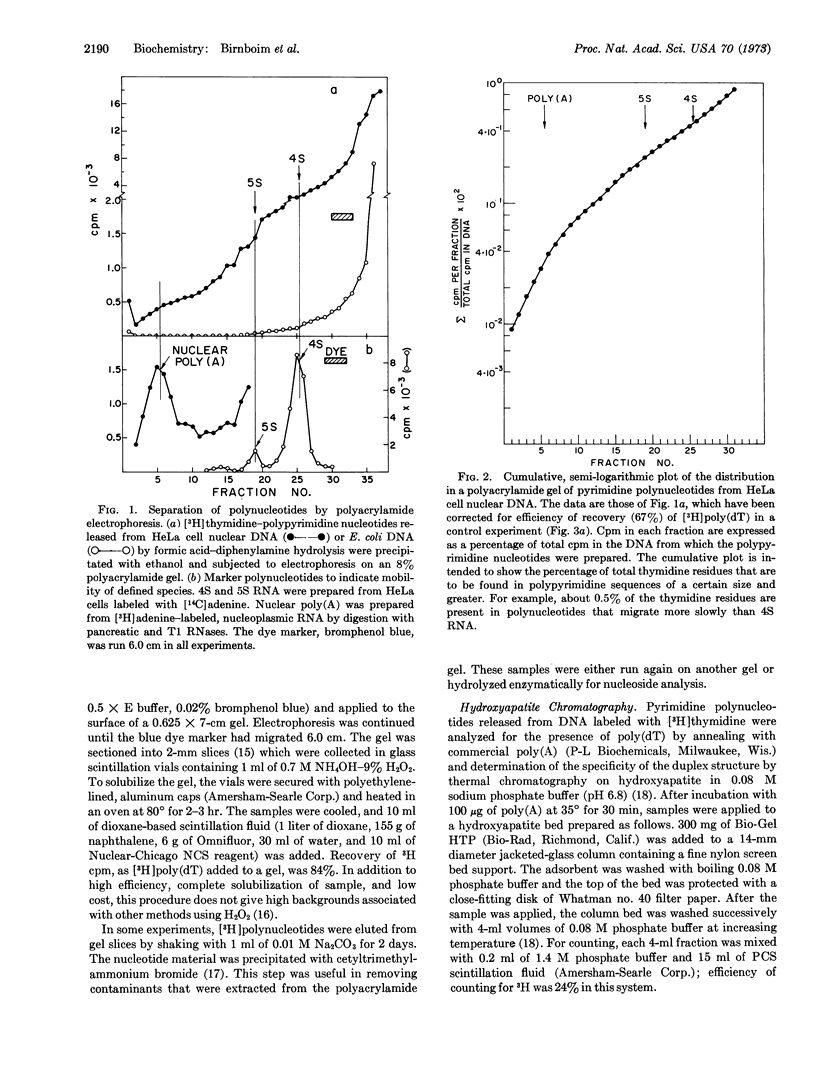
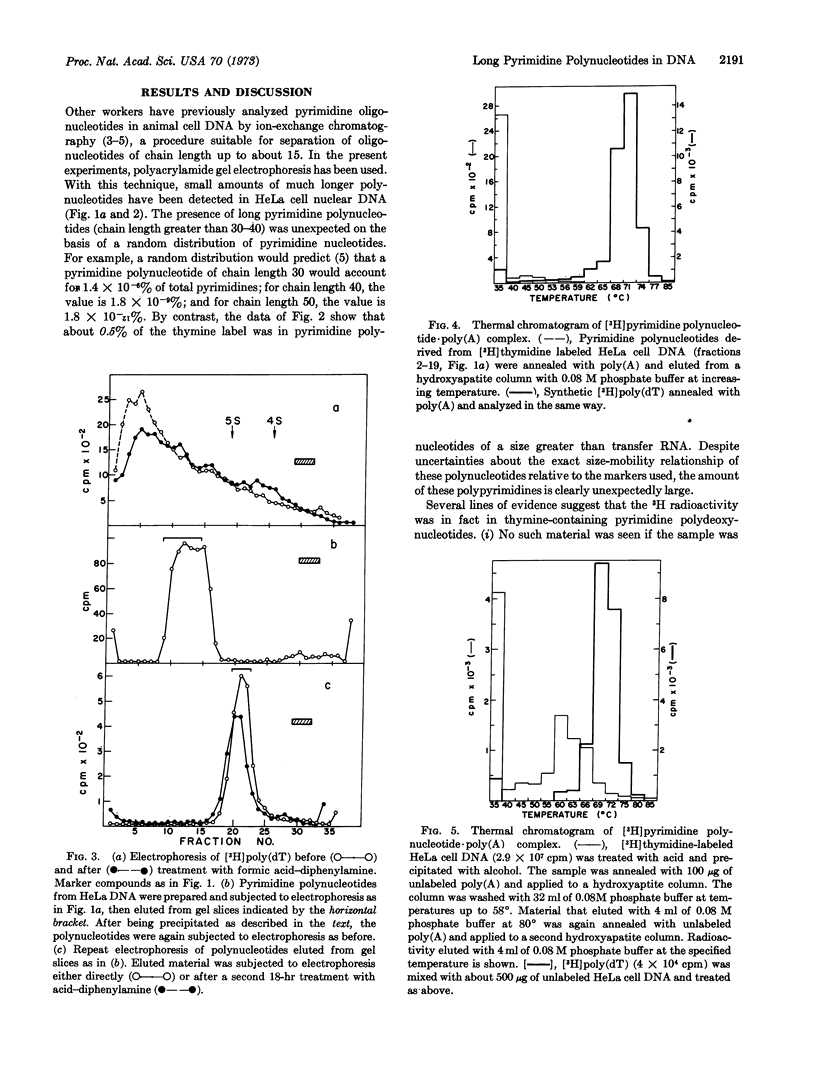
HeLa cell nuclear DNA contains an unexpectedly large amount of long pyrimidine polynucleotides. These sequences were detected in DNA labeled with [3H] thymidine by treatment with formic acid-diphenylamine and subsequent analysis by polyacrylamide gel electrophoresis. About 0.5% of the total thymine residues were found in polynucleotides that migrated more slowly than 4S RNA. No polynucleotides of comparable size were detected in Escherichia coli DNA. The pyrimidine polynucleotides contained no pure poly(dT) sequences (less than 0.0015% of the total residues), as judged by thermal chromatography of a complex formed with poly(A).
Keywords: formic acid-diphenylamine hydrolysis, hydroxyapatite chromatography, thermal elution
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Salditt M., Thomas W., Darnell J. E. Evidence that all messenger RNA molecules (except histone messenger RNA) contain Poly (A) sequences and that the Poly(A) has a nuclear function. J Mol Biol. 1972 Oct 28;71(1):21–30. doi: 10.1016/0022-2836(72)90397-x. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C. Semiautomatic fractionation of dilute polyacrylamide gels. Anal Biochem. 1969 Jun;29(3):498–504. doi: 10.1016/0003-2697(69)90334-0. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Philipson L., Wall R., Adesnik M. Polyadenylic acid sequences: role in conversion of nuclear RNA into messenger RNA. Science. 1971 Oct 29;174(4008):507–510. doi: 10.1126/science.174.4008.507. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Wall R., Tushinski R. J. An adenylic acid-rich sequence in messenger RNA of HeLa cells and its possible relationship to reiterated sites in DNA. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1321–1325. doi: 10.1073/pnas.68.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds M., Vaughan M. H., Jr, Nakazato H. Polyadenylic acid sequences in the heterogeneous nuclear RNA and rapidly-labeled polyribosomal RNA of HeLa cells: possible evidence for a precursor relationship. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1336–1340. doi: 10.1073/pnas.68.6.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. Y., Mendecki J., Brawerman G. A polynucleotide segment rich in adenylic acid in the rapidly-labeled polyribosomal RNA component of mouse sarcoma 180 ascites cells. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1331–1335. doi: 10.1073/pnas.68.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L., Canellakis E. S. Adenine-rich polymer associated with rabbit reticulocyte messenger RNA. Nature. 1970 Aug 15;227(5259):710–712. doi: 10.1038/227710a0. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The determination of the molecular weight of ribonucleic acid by polyacrylamide-gel electrophresis. The effects of changes in conformation. Biochem J. 1969 Jun;113(1):131–138. doi: 10.1042/bj1130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIYAZAWA Y., THOMAS C. A., Jr NUCLEOTIDE COMPOSITION OF SHORT SEGMENTS OF DNA MOLECULES. J Mol Biol. 1965 Feb;11:223–237. doi: 10.1016/s0022-2836(65)80053-5. [DOI] [PubMed] [Google Scholar]
- Mendecki J., Lee S. Y., Brawerman G. Characteristics of the polyadenylic acid segment associated with messenger ribonucleic acid in mouse sarcoma 180 ascites cells. Biochemistry. 1972 Feb 29;11(5):792–798. doi: 10.1021/bi00755a018. [DOI] [PubMed] [Google Scholar]
- Molloy G. R., Sporn M. B., Kelley D. E., Perry R. P. Localization of polyadenylic acid sequences in messenger ribonucleic acid of mammalian cells. Biochemistry. 1972 Aug 15;11(17):3256–3260. doi: 10.1021/bi00767a020. [DOI] [PubMed] [Google Scholar]
- Molloy G. R., Thomas W. L., Darnell J. E. Occurrence of uridylate-rich oligonucleotide regions in heterogeneous nuclear RNA of HeLa cells. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3684–3688. doi: 10.1073/pnas.69.12.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien S. J. On estimating functional gene number in eukaryotes. Nat New Biol. 1973 Mar 14;242(115):52–54. doi: 10.1038/newbio242052a0. [DOI] [PubMed] [Google Scholar]
- Ohta T., Kimura M. Functional organization of genetic material as a product of molecular evolution. Nature. 1971 Sep 10;233(5315):118–119. doi: 10.1038/233118a0. [DOI] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- Shenkin A., Burdon R. H. Deoxyadenylate-rich sequences in mammalian DNA. FEBS Lett. 1972 May 1;22(2):157–160. doi: 10.1016/0014-5793(72)80033-4. [DOI] [PubMed] [Google Scholar]
- Sneider T. W. Methylation of mammalian deoxyribonucleic acid. II. The distribution of 5-methylcytosine in pyrimidine deoxyribonucleotide clusters in Novikoff hepatoma cell deoxyribonucleic acid. J Biol Chem. 1971 Aug 10;246(15):4774–4783. [PubMed] [Google Scholar]
- Spencer J. H., Cape R. E., Marks A., Mushynski W. E. Nucleotide clusters in deoxyribonucleic acids. II. Investigation of methods for isolation of pyrimidine oligonucleotides. Can J Biochem. 1969 Mar;47(3):329–337. doi: 10.1139/o69-050. [DOI] [PubMed] [Google Scholar]



