Abstract
Background:
Neonatal respiratory distress syndrome (RDS) in premature infants who survived and its complications are a common problem. Due to high morbidity and mechanical ventilation (MV) nowadays researchers in interested minimizing MV. To determine, in very low birth weight (BW) preterm neonates with RDS, if initial treatment with nasal intermittent mandatory ventilation (early NIMV) compared with early nasal continuous positive airway pressure (early NCPAP) obtains more favorable outcomes in terms of the duration of treatment, and the need for endotracheal tube ventilation.
Methods:
In this single-center randomized control trial study, infants (BW ≤ 1500 g and/or gestational age ≤ 34 weeks) with respiratory distress were considered eligible. Forty-four infants were randomly assigned to receive early-NIMV and 54 comparable infants to early-NCPAP. Surfactants were given, when FIO2 requirement was of >30%. Primary outcomes were failure of noninvasive respiratory support, that is, the need for MV in the first 48 h of life and for the duration of noninvasive respiratory support in each group.
Results:
98 infants were enrolled (44 in the NIMV and 54 in the NCPAP group). The Preventive power of MV of NIMV usage (95.5%) was not lower than the NCPAP (98.1%) strength (hazard ratio: 0.21 (95% confidence interval: 0.02-2.66); P: 0.23). The duration of noninvasive respiratory support in the NIMV group was significantly shorter than NCPAP (the median (range) was 24 (18.00-48.00) h versus 48.00 (22.00-120.00) h in NIMV versus NCPAP groups; P < 0.001). Similarly, the duration of dependency on oxygen was less, for NIMV (the median (range) was 96.00 (41.00-504.00) h versus144.00 (70.00-1130.00) h in NIMV versus NCPAP groups; P: 0.009). Interestingly, time to full enteral feeds and length of hospital stay were more favorable in the NIMV versus the NCPAP group.
Conclusions:
Initial treatment of RDS with NIMV was safe, and well tolerated. Furthermore, NIMV had excellent benefits such as reduction of the duration of treatment, oxygen dependency period and length of hospital stay. Therefore, the primary mode with NIMV could be a feasible method of noninvasive ventilation in very premature infants.
Keywords: Nasal CPAP, noninvasive ventilation, premature infants, respiratory distress syndrome, surfactant
INTRODUCTION
In the course of time, the number of preterm labors has increased, and the more premature infants survive.[1] Therefore, neonatal respiratory distress syndrome (RDS) in premature infants who survive and its complications have become a common problem.[2]
Premature infants suffering RDS usually, need a certain degree of respiratory support.[3,4,5] Various methods of noninvasive ventilation are developed to reduce the use of mechanical ventilation (MV), possible with the use of the endotracheal tube. Therefore, the therapeutic power and side-effects of each of these methods have been evaluated and compared in different studies.[6,7,8]
Some researchers have shown that continuous positive pressure into the airways through the nose nasal continuous positive airway pressure (NCPAP) in preterm infants is effective in the treatment of RDS. Also, avoidance of MV is more possible in a relatively large number of infants with the use of NCPAP.[3,4,5,9,10] Currently, NCPAP is widely used as initial treatment for RDS. Similarly, favorable therapeutic power and reduction of the incidence of chronic lung disease (CLD), particularly in combination with a surfactant therapy, have been observed.[6,11,12,13] NCPAP was used in the treatment for apnea of prematurity (AOP) and after extubation, producing acceptable and also reduced the need for reintubation.[3,14,15]
Due to beneficial effects of merging, early surfactant administration with transient intubation (intubation, surfactant administration and extubation [INSURE approach]) by NCPAP, in several studies;[17,18,19,20] lately in the treatment of RDS, the INSURE approach has been used in numerous neonatal intensive care units (NICUs).[13,17,21] However, in some cases despite early use of NCPAP, due to treatment failure, reintubation and MV were needed.[22,23,24] In a number of studies, approximately 25-50% of RDS treatment for neonates has failed. Therefore, alternative methods of noninvasive ventilation were studied in other surveys.[8,16,25,26,27] Among noninvasive ventilation methods in RDS treatment, intermittent positive pressure ventilation via nasal (nasal intermittent positive pressure ventilation [NIPPV] or nasal intermittent mandatory ventilation [NIMV]) was also investigated.[3,6,16,28] NIMV combines NCPAP with intermittent ventilator inhalations. In this method, while ventilatory inflations are continuing; instead of the endotracheal tube, the nasal prong is used (inside the nose).[29]
In some studies such as De Paoli et al., NIMV was shown to be more effective than NCPAP after extubation in the treatment of RDS.[30,31,32,33,34] Also, NIMV was found to be more effectively in the treatment of AOP.[35,36]
Nasal intermittent mandatory ventilation has been performed by two methods; coordinated with neonatal breathing (synchronized NIMV [SNIMV]) and uncoordinated with neonatal breathing (nonsynchronized NIMV [NSNIMV]).[3,32] A relatively large number of researchers have evaluated the SNIMV. Aghai et al. reported that, in SNIMV group compared to NCPAP group, work of breathing was decreased, and minute ventilation was increased.[37]
Furthermore, usage of MV (as a result of treatment failure) during the 1st days of life is one of the important risk factors of BPD and some other morbidities.[38,39] Avery et al. and Van Marter et al. observed that the need for endotracheal tube ventilation was decreased in the NIMV group than the NCPAP group, in the first 72 h of life.[39] Finally, we hypothesized that initial treatment with NIMV in preterm neonates with RDS may obtain more favorable outcomes in terms of the duration of treatment and the endotracheal tube ventilation in comparison to ‘early NCPAP’.
METHODS
Study design and participants
In this single-center randomized control trial (RCT) study, infants born between March 2013 and January 2014 with a birth weight of (BW) ≤ 1500 g and/or gestational age ≤ 34 weeks and admitted to the tertiary referral NICUs of the Isfahan University of Medical Sciences at Alzahra and Shahid Beheshti Hospitals, were eligible for participation in the study. Gestational age was determined by the last menstrual period and ultrasound. If there was any clinical evidence of respiratory distress, very low birth weight (VLBW) infants were enrolled in a case-controlled study within our trial. RDS was defined in the presence of clinical evidence of respiratory distress and a positive chest X-ray film along with Makinen's radiologic classification.[40] Infants were excluded if there was any of the following cases: Major congenital anomalies, asphyxia, congenital cyanotic heart disease, cardiovascular instability, orofacial anomalies and consent refused or not provided.
The therapeutic effects of NSNIMV in the treatment of RDS were investigated in two groups of NIMV and NCPAP. The researchers used unequal randomization for this trial. The neonates were randomly allocated to initial treatment with either early-NIMV (NIMV group) or early-NCPAP (NCPAP group). The infants were divided into two groups according to their file number by an uninvolved employee. In order to select the neonates, randomly, those with an even digit at the end of their file numbers were placed in NIMV group and those with their file numbers ending in an odd digit were assigned to the NCPAP group. Crossover was not acceptable between groups. Group assignment and enrolment of participants were supervised by the primary author of the study. Infants were resuscitated according to standard Neonatal Resuscitation Program guidelines.
Respiratory intervention
Very low birth weight infants with clinical evidence of respiratory distress and/or reduction at arterial oxygen saturation by pulse-oximetry (SpO2), were randomly assigned to either early-NIMV or early-NCPAP treatment groups. For infants in the early-NIMV group (nonsynchronized mode), NIMV was set at peak inspiratory pressure (PIP) of 16-20cmH2O (according to infant's birth weight and chest wall expansion), positive end expiratory pressure (PEEP) of 5-6 cmH2O, rate of 40-50 breaths/min (according to PaCO2), inspiratory time (Ti) of 0.4 s and flow rate of 8-10 L/min.[16]
Nasal continuous positive airway pressure was initiated at a continuous pressure of 5-6 cmH2O with a flow of 8-10 L/min by an underwater Bubble CPAP system. Short bi-nasal prongs delivered both modes of noninvasive treatment to the infants. Settings in both groups were adjusted according to arterial blood gases (ABG), clinical parameters and to maintain SpO2 between 88% and 92%. Surfactant 100 mg/kg per dose, curosurf or survanta was administered, if studied neonates needed a fraction of inspired oxygen (FIO2) of > 30% to keep the SpO2 of >88-92%. INSURE approach, only as rescue therapy, was used in both groups. We gave a second or third dose of surfactant, if the neonates required a FIO2 of >40% to maintain the aimed saturation. Hence, surfactant was administered, if subjects required MV. The orogastric tube was used in both groups, which was kept open to decompress the stomach. ABG was achieved after 60 min of beginning respiratory support and/or surfactant administration; then, for minimal handling, as indicated. Prophylactic aminophylline wase used in both groups. Pulse oximeter saturation and heart rate were continuously monitored, and blood pressure was measured at least every 6 h. Infants were monitored as per standard NICU nursing protocols for other parameters. The medical management of the neonates followed the instructions of the attending neonatologist.
Infants on NIMV were weaned from a PIP of 14-15 cmH2O, PEEP 4-5 cmH2O, and FIO2 of < 30%, with acceptable clinical evidence and ABG. Infants on NCPAP were also weaned from a CPAP of 4 cmH2O and FIO2 of < 30%, with acceptable clinical evidence and ABG. After weaning, infants in both groups could be weaned to humidified high-flow nasal cannula (HHFNC) at 2.5-3 L/min. Efforts to wean the flow by as much as tolerated were made gradually. HHFNC was stopped completely, once the infants were able to maintain SpO2 between 88% and 92% in room air for more than at least 4 h.[41]
If the studied infants, after weaning within the first 48 h of the study, required re-initiating of respiratory support, the neonates were started on the initially allocated mode.
In both groups, the duration of noninvasive respiratory support, need to INSURE approach, the duration of dependency on oxygen, incidence of CLD (oxygen dependency at 28 days of life),[42] time to full enteral feeds, length of hospital stay, pneumothorax and other morbidities during the hospitalization such as intraventricular hemorrhage (IVH), patent ductus arteriosus (PDA) were recorded.
Primary and secondary outcomes
The primary outcomes of the study were the effect of NIMV on need for intubation/endotracheal tube ventilation (e.g. failure of noninvasive respiratory support) within the first 48 h of the study, and on the duration of noninvasive respiratory support in each group. Presence of any or more of the following was regarded as criteria for failure, in both groups: pH < 7.2 and PCO2 > 60, SpO2 of < 88% with a FIO2 of ≥ 70%, recurrent apnea > 3 times/h requiring tactile stimulation and any sever apnea, which needed bag and mask ventilation.
Secondary outcomes were need to INSURE approach, the duration of dependency to oxygen, incidence of CLD, time to full enteral feeds, length of hospital stay, pneumothorax and other morbidity during the hospitalization such as IVH, PDA. Full enteral feeds were regarded as feeds that reached 150 mL/kg/day. IVH and PDA were confirmed by brain ultrasonography (according to Papile's classification[43]) and echocardiography, respectively.
Ethics statement
This paper is derived from a residency thesis no. 392345 in the Isfahan University of Medical Sciences. The study was approved by the regional ethics review board at university. Written informed consents were obtained from parents. This trial was registered at irct.ir as IRCT2014021410026N4.
Data analyses
The sample size in our study was calculated based on the formula suggested for parallel groups randomized trials, considering the statistical power 80% (Z1-β =0.84) and two tailes significant level 5% () and for detecting a 50% difference between the studied groups[3] in terms of the rate of need to MV within first 48 h in in VLBW infants as the pivotal variable (i.e. the effect of NSNIMV on reducing the need for endotracheal ventilation in preterm infants)[1,3] led to 40 participants in each group and for compensating the possible attrition during the study period 48 neonates were recruited.
Normally, distributed and nonnormal quantitative data were presented as means (±standard deviation [SD]) and median (range), respectively. Qualitative variables were expressed as number (percent). The numeric variables were compared using the Independent-t test (for parametric) and Mann–Whitney U-test (for nonparametric). To examine the effect of the intervention on the incidence of studied outcomes, the Kaplan Meier method with log–Rank test was used and Cox regression was used to calculate hazard ratio (HR) and 95% confidence interval (CI) for HR (95% CI for HR), when adjustment was done for confounding factors, that is, gestational ages and BWs. P < 0.05 was considered as statistically significant. The data were analyzed using the SPSS statistical software ver. 20.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
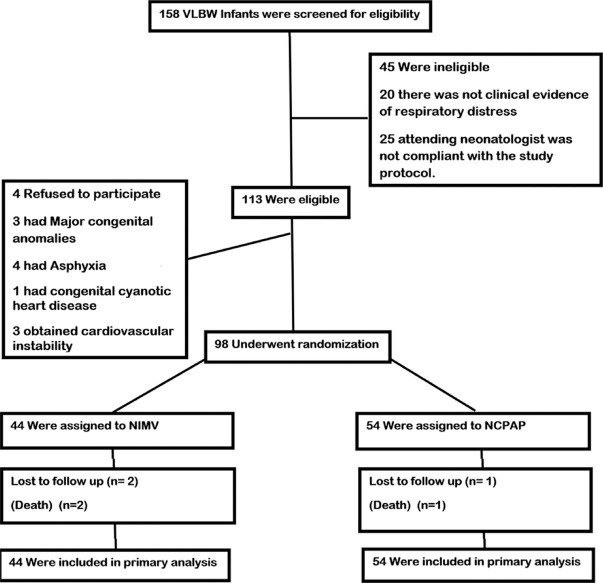
A total of 158 infants with a BW < 1500 g was assessed for eligibility, during the study period. Of these, 45 were not eligible because of poorly clinical evidence of respiratory distressor, because the attending neonatologist was not compliant with the study protocol. There was a total of 113 infants who met the eligibility criteria, and 15 infants who were excluded because of their parents’ refusal to participate, major congenital anomalies, asphyxia and cardiovascular instability. 98 infants underwent randomization (44 in the NIMV and 54 in the NCPAP groups), during the study period and completed the study [Figure 1]. The demographic characteristics of the infants in the two groups were not similar, but were adjusted for evaluation of outcomes [Tables 1 and 2]. Average gestational ages in NIMV and NCPAP groups were 30.38 ± 1.61 and 29.45 ± 1.99, respectively [P: 0.012, Table 1]. Also, average BWs in NIMV group were 1261.36 ± 75.87 g and in NCPAP group were 1156.48 ± 212.46 [P: 0.01, Table 1]. NIMV was well tolerated.
Figure 1.
Flowchart of the participants
Table 1.
Basic characteristics of study infants
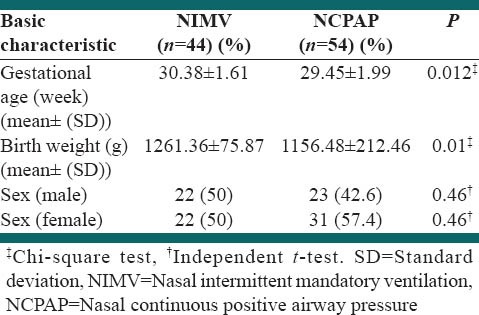
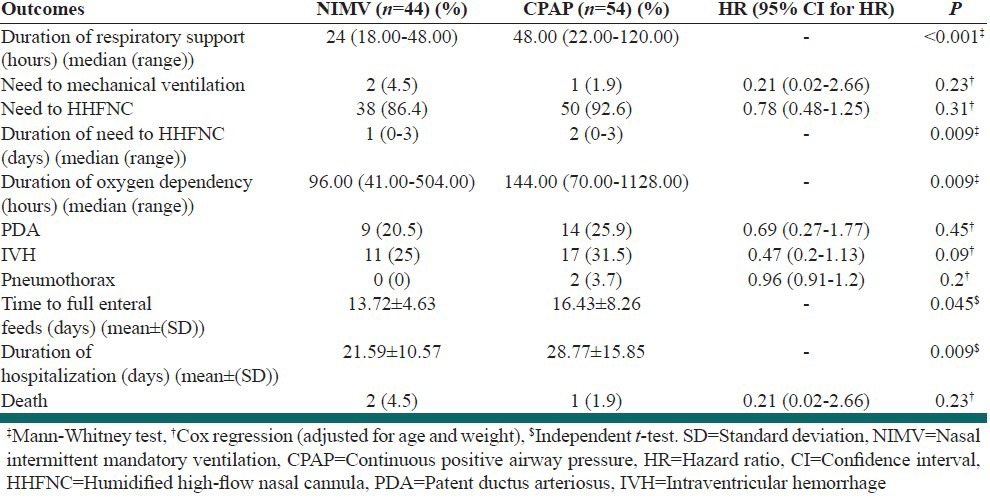
Table 2.
Outcomes and clinical characteristics of study infants
Primary outcomes
The Preventive power of MV of NIMV usage was not lower than the NCPAP strength. Need for intubation and MV (e.g. treatment failure), in the first 48 h of life, occurred in 2 of 44 infants (4.5%) in the NIMV group and 1 of 54 infants (1.9%) in the NCPAP group [HR: 0.21 (95% CI: 0.02-2.66); P: 0.23; Table 2]. The duration of noninvasive respiratory support in the NIMV group was significantly shorter than in the NCPAP. Median time of need to NIMV was 24 (18.00-48.00) h and to NCPAP was 48.00 (22.00-120.00) h [P < 0.001; Table 2]. The reason for intubation and MV in the two study groups was apnea.
Secondary outcomes
Median time of need to HHFNC in the NIMV group (1 [0-3] days) was shorter than in the NCPAP group (2 [0-3] days) (P: 0.009), while the number of infants who were required HHFNC was similar in both groups [38 (86.4%) and 50 (92.6%) infants in the NIMV and NCPAP groups respectively [HR: 0.78 (95% CI: 0.48-1.25); P: 0.31; Table 2)].
Other secondary outcomes, such as duration of dependency on oxygen and length of hospitalization, were also investigated. The duration of dependency on oxygen was less with NIMV, while the incidence of CLD was similar between the two groups. The median of duration of dependency on oxygen was 144.00 (70.00-1128.00) h and 96.00 (41.00-504.00) in the NCPAP and NIMV groups respectively [P: 0.009; Table 2].
Interestingly, we observed that time to full enteral feeds, and length of hospital stay were more favorable in NIMV group vs NCPAP. The mean ± SD of time to full enteral feeds was 13.72 ± 4.63 days and 16.43 ± 8.26 in the NIMV and NCPAP groups, respectively [P: 0.045; Table 2]. Hospitalization time in the NIMV group was shorter than the NCPAP group. Average hospital stay time was 21.59 ± 10.57 days and 28.77 ± 15.85 in the NIMV and NCPAP groups respectively [P: 0.009; Table 2].
The incidence of pneumothorax, PDA and IVH in NIMV group was 0 (0%), 9 (20.5%) and 11 (25%) respectively, and in the NCPAP group, it was 2 (3.7%), 14 (25.9%) and 17 (31.5%) [P: 0.2, 0.45 and P: 0.09, respectively; Table 2]. Infantile death was similar between the two groups; in both groups, only three neonates died during the study [HR: 0.21 (95% CI: 0.02–2.66); P: 0.23; Table 2].
DISCUSSION
According to the data obtained from other studies, the researchers hypothesized that the addition of repeated ventilatory inflations, provided by NIMV, would improve the power of treatment of RDS and oxygenation. In this study, in the 1st days after birth, the duration of noninvasive respiratory support, during treatment of RDS, was made shorter in infants who received “early NIMV” than those who were subjected to “early NCPAP.” In spite of that, in the present study, no difference was observed in treatment failure (i.e. need to MV) between the two groups.
To our knowledge, there are a few previously published studies that have compared the effect of NIMV versus NCPAP on the duration of treatment of RDS. Kishore et al. assessed 76 neonates (28-34 weeks gestation) and found that the duration of respiratory support was not statically significant between NIPPV and NCPAP groups (44 [13.5-129] and 60 [23-154] h), respectively; P: 0.33].[16] Also, Meneses et al., who assessed the duration of treatment from another point of view, was reported that total time of need for NCPAP and total time of need for nasal cannula was similar between his two study groups (P: 0.65 and P: 0.46, respectively).[6]
Several studies have investigated the preventive effect of NIMV on treatment failure (i.e. need for MV). Meneses et al. reported that the rate of need for MV was similar between NIPPV (25%) and NCPAP (34%) groups (RR: 0.71 (95% CI: 0.48-1.14)].[6] In contrast to our study, Kugelman et al.[3] and Kishore et al.[16] observed that, among infants born < 35 weeks, less endotracheal ventilation was needed in NIMV vs NCPAP groups ([25% vs. 49%, P < 0.05] and [13.5% vs. 35.9%, P: 0.02], respectively). Although, in VLBW infants, the rate of “failed nasal support” did not differ significantly between the two groups (31% vs. 62%, P: 0.06) in Kugelman et al.[3] In the review of other studies, that caught the attention of the researchers was the significantly less requirement for MV in both groups of the present study, compared to others (4.5% in the NIMV group 1.9% in the NCPAP group). Perhaps the cause of greater levels of need for MV (i.e. treatment failure) in other RCTs was the lower GA and BW in those studies (e.g. GA and BW, respectively, in NIMV vs NCPAP groups: (29.0 ± 1.4 vs. 28.2 ± 1.9 weeks) and (1155 ± 193 vs. 1039 ± 238 g) in Kugelman et al.[3] and (29 ± 1.6 vs. 30.1 ± 2.3 weeks) and (1112 ± 252 vs. 1151 ± 289 g) in Meneses et al.[6]). Nevertheless, in some instances the means of GA and BW were similar between ours and other studies (e.g. mean of GA and BW in the control groups between us and Meneses et al.).
Informed of the result of NIMV effect on the duration of oxygen requirement is attractive.
Bhandari et al. found that the total duration of supplemental oxygen was similar between SNIPPV vs. MV groups at RDS treatment (45.5 ± 6.1 vs. 46.8 ± 6.3 days; P: 0.88).[29]
In Kishore et al.,[16] to treat RDS, premature infants less than 35 weeks were assigned to two groups of SNIPPV or NCPAP and the results were compared. In NIMV, it was shown that the length of oxygen dependency during hospitalization was not shorter than NCPAP and so did not have statistical significance (72 [15, 156] 72 [36, 240] h; P: 0.39).[16] Also, no significant difference of total time of need to oxygen was found between NIPPV and NCPAP groups, in Meneses et al. (20.4 ± 16 and 23.6 ± 22.6 days; P: 0.97).[6] However, in the present study, the median of duration of need to oxygen in the NIMV group was significantly shorter although no significant difference was observed in the incidence of CLD between two groups (similar to Kishore et al. study, i.e. 2.7% and 7.7% in NIPPV and NCPAP group, respectively; P: 0.61[16]). Therefore, further studies with larger sample sizes are recommended, to further investigate the issue.
The effects of NIMV on hospitalization time and time to full enteral feeds have also been investigated. In most of the previous studies, duration of hospital stay was not significantly different between the two study groups; e.g. duration of hospitalization in NIPPV vs NCPAP was 21 (12.5-35) vs. 23.5 (11-40) days; P: 0.77, 55.9 ± 20.5 vs. 53.9 ± 19.15 days; P: 0.45 and 63 ± 23 vs. 81 ± 36 days; P: 0.16 in Kishore et al.,[16] Meneses et al.[6] and Kugelman et al.,[3] respectively, while in the present study, perhaps due to significant differences between the two groups in the course of noninvasive respiratory support, hospitalization time was more favorable in the NIMV group (21.59 ± 10.57 vs. 28.77 ± 15.85 days; P: 0.009). Furthermore, Meneses et al.[6] and Kugelman et al.,[3] have shown that using NIPPV had no positive effect on “time to full enteral feeds” (P: 0.49 and P: 0.54, respectively). In in the present experiment and some other studies,[6,16] in terms of side effects, such as the incidence of pneumothorax, no difference was found between the two study groups.
Finally, it appears that, further studies with larger sample sizes are needed to investigate these issues in more detail.
The major limitation of the study could be the rather small number of the infants included (98 premature neonates), even though the results clearly indicated a significant difference between the NIMV and control groups. The strengths of the study include the RCT design in high-risk neonates (i.e. VLBW infants) and initial treatment of RDS with NIMV.
CONCLUSIONS
Initial treatment of RDS with NIMV was safe and well tolerated. Furthermore, NIMV had excellent benefits, such as reducing the duration of treatment, oxygen dependency period and length of hospital stay. Therefore, the primary mode with NIMV could be a feasible method of noninvasive ventilation in very premature infants. Although further studies with larger sample sizes are recommended to investigate these issues.
Footnotes
Source of Support: This paper is derived from a residency thesis No. 392345 in Isfahan University of Medical Sciences. This trial was registered at IRCT.ir with a reference number as IRCT2014021410026N4.
Conflict of Interest: None declared.
REFERENCES
- 1.Seki K, Iwasaki S, An H, Horiguchi H, Mori M, Nishimaki S, et al. Early discharge from a neonatal intensive care unit and rates of readmission. Pediatr Int. 2011;53:7–12. doi: 10.1111/j.1442-200X.2010.03179.x. [DOI] [PubMed] [Google Scholar]
- 2.Mathews TJ, MacDorman MF. Infant mortality statistics from the 2008 period linked birth/infant death data set. Natl Vital Stat Rep. 2012;60:1–27. [PubMed] [Google Scholar]
- 3.Kugelman A, Feferkorn I, Riskin A, Chistyakov I, Kaufman B, Bader D. Nasal intermittent mandatory ventilation versus nasal continuous positive airway pressure for respiratory distress syndrome: A randomized, controlled, prospective study. J Pediatr. 2007;150:521–6. doi: 10.1016/j.jpeds.2007.01.032. 5261. [DOI] [PubMed] [Google Scholar]
- 4.Thomson MA IFDAS Study Group. Early nasal continuous positive pressure with prophylactic surfactant for neonates at risk of RDS. The IFDAS Multi-Center randomized trial. Pediatr Res. 2002;51:379A. [Google Scholar]
- 5.Polin RA, Sahni R. Newer experience with CPAP. Semin Neonatol. 2002;7:379–89. doi: 10.1053/siny.2002.0132. [DOI] [PubMed] [Google Scholar]
- 6.Meneses J, Bhandari V, Alves JG, Herrmann D. Noninvasive ventilation for respiratory distress syndrome: A randomized controlled trial. Pediatrics. 2011;127:300–7. doi: 10.1542/peds.2010-0922. [DOI] [PubMed] [Google Scholar]
- 7.Verder H, Bohlin K, Kamper J, Lindwall R, Jonsson B. Nasal CPAP and surfactant for treatment of respiratory distress syndrome and prevention of bronchopulmonary dysplasia. Acta Paediatr. 2009;98:1400–8. doi: 10.1111/j.1651-2227.2009.01413.x. [DOI] [PubMed] [Google Scholar]
- 8.Ammari A, Suri M, Milisavljevic V, Sahni R, Bateman D, Sanocka U, et al. Variables associated with the early failure of nasal CPAP in very low birth weight infants. J Pediatr. 2005;147:341–7. doi: 10.1016/j.jpeds.2005.04.062. [DOI] [PubMed] [Google Scholar]
- 9.Ho JJ, Subramaniam P, Henderson-Smart DJ, Davis PG. Continuous distending pressure for respiratory distress syndrome in preterm infants. Cochrane Database Syst Rev. 2000 doi: 10.1002/14651858.CD002271. CD002271. [DOI] [PubMed] [Google Scholar]
- 10.De Klerk AM, De Klerk RK. Nasal continuous positive airway pressure and outcomes of preterm infants. J Paediatr Child Health. 2001;37:161–7. doi: 10.1046/j.1440-1754.2001.00624.x. [DOI] [PubMed] [Google Scholar]
- 11.Owen LS, Morley CJ, Davis PG. Neonatal nasal intermittent positive pressure ventilation: A survey of practice in England. Arch Dis Child Fetal Neonatal Ed. 2008;93:F148–50. doi: 10.1136/adc.2007.118109. [DOI] [PubMed] [Google Scholar]
- 12.Verder H. Nasal CPAP has become an indispensable part of the primary treatment of newborns with respiratory distress syndrome. Acta Paediatr. 2007;96:482–4. doi: 10.1111/j.1651-2227.2007.00263.x. [DOI] [PubMed] [Google Scholar]
- 13.Dunn MS, Reilly MC. Approaches to the initial respiratory management of preterm neonates. Pediatric Respir Rev. 2003;4:2–8. [PubMed] [Google Scholar]
- 14.Goldsmith JP, Karotkin E. Elsevier Health Sciences. 5th ed 2010. Assisted Ventilation of the Neonate: Expert Consult. [Google Scholar]
- 15.Davis PG, Henderson-Smart DJ. Nasal continuous positive airways pressure immediately after extubation for preventing morbidity in preterm infants. Cochrane Database Syst Rev. 2003 doi: 10.1002/14651858.CD000143. CD000143. [DOI] [PubMed] [Google Scholar]
- 16.Kishore MS, Dutta S, Kumar P. Early nasal intermittent positive pressure ventilation versus continuous positive airway pressure for respiratory distress syndrome. Acta Paediatrica. 2009;98:1412–5. doi: 10.1111/j.1651-2227.2009.01348.x. [DOI] [PubMed] [Google Scholar]
- 17.Verder H, Albertsen P, Ebbesen F, Greisen G, Robertson B, Bertelsen A, et al. Nasal continuous positive airway pressure and early surfactant therapy for respiratory distress syndrome in newborns of less than 30 weeks’ gestation. Pediatrics. 1999;103:E24. doi: 10.1542/peds.103.2.e24. [DOI] [PubMed] [Google Scholar]
- 18.Bohlin K, Gudmundsdottir T, Katz-Salamon M, Jonsson B, Blennow M. Implementation of surfactant treatment during continuous positive airway pressure. J Perinatol. 2007;27:422–7. doi: 10.1038/sj.jp.7211754. [DOI] [PubMed] [Google Scholar]
- 19.Rojas MA, Lozano JM, Rojas MX, Laughon M, Bose CL, Rondon MA, et al. Very early surfactant without mandatory ventilation in premature infants treated with early continuous positive airway pressure: A randomized, controlled trial. Pediatrics. 2009;123:137–42. doi: 10.1542/peds.2007-3501. [DOI] [PubMed] [Google Scholar]
- 20.Reininger A, Khalak R, Kendig JW, Ryan RM, Stevens TP, Reubens L, et al. Surfactant administration by transient intubation in infants 29 to 35 weeks’ gestation with respiratory distress syndrome decreases the likelihood of later mechanical ventilation: A randomized controlled trial. J Perinatol. 2005;25:703–8. doi: 10.1038/sj.jp.7211381. [DOI] [PubMed] [Google Scholar]
- 21.Bohlin K, Jonsson B, Gustafsson AS, Blennow M. Continuous positive airway pressure and surfactant. Neonatology. 2008;93:309–15. doi: 10.1159/000121457. [DOI] [PubMed] [Google Scholar]
- 22.Stevens TP, Blennow M, Soll RF. Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome (Cochrane review) Cochrane Database Syst Rev. 2007;4:CD003063. doi: 10.1002/14651858.CD003063.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeena P, Pillay P, Adhikari M. Nasal CPAP in newborns with acute respiratory failure. Ann Trop Paediatr. 2002;22:201–7. doi: 10.1179/027249302125001480. [DOI] [PubMed] [Google Scholar]
- 24.Kamper J, Feilberg Jørgensen N, Jonsbo F, Pedersen-Bjergaard L, Pryds O Danish ETFOL Study Group. The Danish national study in infants with extremely low gestational age and birthweight (the ETFOL study): Respiratory morbidity and outcome. Acta Paediatr. 2004;93:225–32. doi: 10.1080/08035250310022298. [DOI] [PubMed] [Google Scholar]
- 25.Reininger A, Khalak R, Kendig JW, Ryan RM, Stevens TP, Reubens L, et al. Surfactant administration by transient intubation in infants 29 to 35 weeks’ gestation with respiratory distress syndrome decreases the likelihood of later mechanical ventilation: A randomized controlled trial. J Perinatol. 2005;25:703–8. doi: 10.1038/sj.jp.7211381. [DOI] [PubMed] [Google Scholar]
- 26.Aly H, Massaro AN, Patel K, El-Mohandes AA. Is it safer to intubate premature infants in the delivery room? Pediatrics. 2005;115:1660–5. doi: 10.1542/peds.2004-2493. [DOI] [PubMed] [Google Scholar]
- 27.Andersen T, Holm HS, Kamper J. Surfactant treatment of newborn infants receiving continuous positive airway pressure treatment. Ugeskr Laeger. 2006;168:3723–7. [PubMed] [Google Scholar]
- 28.Friedlich P, Lecart C, Posen R, Ramicone E, Chan L, Ramanathan R. A randomized trial of nasopharyngeal-synchronized intermittent mandatory ventilation versus nasopharyngeal continuous positive airway pressure in very low birth weight infants after extubation. J Perinatol. 1999;19:413–8. doi: 10.1038/sj.jp.7200205. [DOI] [PubMed] [Google Scholar]
- 29.Bhandari V, Gavino RG, Nedrelow JH, Pallela P, Salvador A, Ehrenkranz RA, et al. A randomized controlled trial of synchronized nasal intermittent positive pressure ventilation in RDS. J Perinatol. 2007;27:697–703. doi: 10.1038/sj.jp.7211805. [DOI] [PubMed] [Google Scholar]
- 30.Bhandari V. Nasal intermittent positive pressure ventilation in the newborn: Review of literature and evidence-based guidelines. J Perinatol. 2010;30:505–12. doi: 10.1038/jp.2009.165. [DOI] [PubMed] [Google Scholar]
- 31.Davis PG, Morley CJ, Owen LS. Non-invasive respiratory support of preterm neonates with respiratory distress: Continuous positive airway pressure and nasal intermittent positive pressure ventilation. Semin Fetal Neonatal Med. 2009;14:14–20. doi: 10.1016/j.siny.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Barrington KJ, Bull D, Finer NN. Randomized trial of nasal synchronized intermittent mandatory ventilation compared with continuous positive airway pressure after extubation of very low birth weight infants. Pediatrics. 2001;107:638–41. doi: 10.1542/peds.107.4.638. [DOI] [PubMed] [Google Scholar]
- 33.Khalaf MN, Brodsky N, Hurley J, Bhandari V. A prospective randomized, controlled trial comparing synchronized nasal intermittent positive pressure ventilation versus nasal continuous positive airway pressure as modes of extubation. Pediatrics. 2001;108:13–7. doi: 10.1542/peds.108.1.13. [DOI] [PubMed] [Google Scholar]
- 34.De Paoli AG, Davis PG, Lemyre B. Nasal continuous positive airway pressure versus nasal intermittent positive pressure ventilation for preterm neonates: A systematic review and meta-analysis. Acta Paediatr. 2003;92:70–5. doi: 10.1111/j.1651-2227.2003.tb00472.x. [DOI] [PubMed] [Google Scholar]
- 35.Lin CH, Wang ST, Lin YJ, Yeh TF. Efficacy of nasal intermittent positive pressure ventilation in treating apnea of prematurity. Pediatr Pulmonol. 1998;26:349–53. doi: 10.1002/(sici)1099-0496(199811)26:5<349::aid-ppul8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 36.Lemyre B, Davis PG, de Paoli AG. Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for apnea of prematurity. Cochrane Database Syst Rev. 2002 doi: 10.1002/14651858.CD002272. CD002272. [DOI] [PubMed] [Google Scholar]
- 37.Aghai ZH, Saslow JG, Nakhla T, Milcarek B, Hart J, Lawrysh-Plunkett R, et al. Synchronized nasal intermittent positive pressure ventilation (SNIPPV) decreases work of breathing (WOB) in premature infants with respiratory distress syndrome (RDS) compared to nasal continuous positive airway pressure (NCPAP) Pediatr Pulmonol. 2006;41:875–81. doi: 10.1002/ppul.20461. [DOI] [PubMed] [Google Scholar]
- 38.Avery ME, Tooley WH, Keller JB, Hurd SS, Bryan MH, Cotton RB, et al. Is chronic lung disease in low birth weight infants preventable? A survey of eight centers. Pediatrics. 1987;79:26–30. [PubMed] [Google Scholar]
- 39.Van Marter LJ, Allred EN, Ulana Sanocka MP, Marianne Moore RP, Nigel Paneth MS, Leviton A. “Do clinical markers of barotrauma and oxygen toxicity explain interhospital variation in rates of chronic lung disease.?”. Pediatrics. 2000;105:1194–201. doi: 10.1542/peds.105.6.1194. [DOI] [PubMed] [Google Scholar]
- 40.Kero PO, Mäkinen EO. Comparison between clinical and radiological classification of infants with the respiratory distress syndrome (RDS) Eur J Pediatr. 1979;130:271–8. doi: 10.1007/BF00441363. [DOI] [PubMed] [Google Scholar]
- 41.Wilkinson D, Andersen C, O’Donnell CP, De Paoli AG. High flow nasal cannula for respiratory support in preterm infants. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD006405.pub2. CD006405. [DOI] [PubMed] [Google Scholar]
- 42.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–9. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 43.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–34. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]