Abstract
Background:
Little information about the effects of conjugated linoleic acids (CLAs) on inflammation and immune function in humans is available. This study investigated the effects of CLAs, with and without Vitamin E on immunity and inflammatory parameters in adults with active rheumatoid arthritis (RA).
Methods:
In a double-blind clinical trial, 78 patients were randomly divided into four groups, each group receiving one of the following daily supplement for 3 months; group C: 2.5 g CLAs, group E: 400 mg Vitamin E, group CE: CLAs plus Vitamin E, group P: Placebo. Cytokines, matrix metalloproteinase 3 (MMP-3) and citrullinated antibody (CCP-A) were measured by ELISA method and Vitamin E by high-performance liquid chromatography.
Results:
Consider statistical methods there were no significant differences between groups in cytokines interleukin-2 (IL-2), IL-4, tumor necrosis factor-α (TNF-α), IL-1β, IL-2/IL-4, CCP-A white blood cells and neutrophils, lymphocyte, monocytes, and eosinophils numbers. TNF-α decreased in all groups, but its reduction was significant in group CE. IL-1β increased in groups P (P = 0.004) and E (P = 0.041) but the difference between group P and CE was significant. IL-4 decreased in groups C, CE and E (P = 0.03, P = 0.03, P = 0.07 respectively). IL2 did not change significantly within groups. CCP-A increased in groups P (P = 0.035) and E (P = 0.05), while it decreased in groups CE (P = 0.034). CCP-A and MMP-3 decrease were significant between groups P and CE. MMP-3 reduction was significant in group CE.
Conclusions:
Co-supplementation CLAs and Vitamin E may be effective in the level of inflammatory markers in RA patients.
Keywords: Conjugated linoleic acids, immunity, inflammatory markers, rheumatoid arthritis, Vitamin E
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic relapsing inflammatory multisystem disease with synovial proliferation and destruction of articular cartilage. It is the most common inflammatory arthritis, affecting approximately 0.5-1% of the general population worldwide.[1,2] RA has served as a useful model for the study of many inflammatory and immune-mediated diseases.[1] The exact cause of RA is not yet known. Recently, several studies have shown the possible role of reactive oxygen species (ROS) in the pathogenesis of RA.[3,4,5,6,7,8,9] The destructive reactions by ROS can be improved by antioxidants.[8] Antioxidants have beneficial effect for inhibition of inflammation related to neutrophil functions,[9] Vitamin E as a potent antioxidant has an ability to modulate host immune functions,[10] so it may be have positive effect on patients who suffer from RA.[10,11,12,13]
Conjugated linoleic acids (CLAs) are naturally occurring isomers of linoleic acid found in meat and milk of grazing animals.[14] Their anti-inflammatory effects that have been shown can protect bones from damage.[14,15] The biological activities of CLAs, much attention has been due to their anticancer,[16] antiatherogenic[17,18] and antidiabetic effects,[19] as well as their effect on increasing the bone mass.[20] The role of CLAs in oxidative stress as an antioxidant has been investigated to explain its beneficial physiological effects.[21,22,23,24,25,26,27,28] In previous articles we reported the effects of CLAs on RA by a randomized, double-blind placebo-controlled trial. Pain and morning stiffness were significantly lower in the group taking CLA or CLA plus Vitamin E, compared to placebo the group after 12 weeks of supplementation. We have concluded that CLAs may improve clinical outcomes;[28] lower lipid peroxidation[29] without negative effects on lipid profile and fasting blood sugar in patients with RA.[30]
Other studies suggest that the CLA can also inhibit the cyclooxygenase (COX-1) and COX-2 pathway enzymes that enhance inflammation in diseases such as RA.[31,32,33] It is well established that lipids can influence both the inflammatory responses and T-cell functions.[34] CLAs have been reported to have anti-inflammatory and immune-regulatory effects on healthy animals.[35,36,37] In contrast to the animal studies, clinical trials in healthy persons have reported few effects of CLA on immune function.[34,38,39,40,41,42,43,44] There are controversial results about changing Plasma C-reactive protein values.[45,46,47,48] To our knowledge there has not been any study on CLAs and/or its combination with Vitamin E on immunity and inflammation in patients with RA. The objective of the study was to determine the effects of oral administration of c9t11 and t10c12 CLA, Vitamin E supplement alone and in combination with Vitamin E supplement, on immunological and inflammatory parameters in active RA.
METHODS
Subjects
This study was a randomized, double-blind placebo-controlled trial that conducted in a 3 months period in patients with active RA. The inclusion criteria were ages between 18 and 69 years having RA for at least 2 years and fulfilling 1987 American College of Rheumatology criteria for RA.[49]
The exclusion criteria were: Taking vitamin and fatty acids/or mineral supplements, thyroid hormones, estrogens, progesterone, diuretics or β-blockers, abnormal renal and hepatic function, history of myocardial infarction, diabetes, hyperlipidemia and for the females, not being pregnant.
The subjects were fully informed of the purpose, procedures, and hazards of the trial and had the right to leave the trial at any time. Written consent was obtained from all patients. The study has been approved by the Ethics Committee on Human Experimentation of the Tehran University of Medical Sciences.
Design
The patients were randomly allocated using Random Permuted Blocks procedure to one of following four treatment groups. All patients in either treatment groups received two capsules and one pearl daily for a period of 3 months as follow. For group C: Two CLA capsules (2 capsules 1.25 g/day that contained 80% CLA, equal 2 g 50:50 mix of cis - 9, trans - 11 and trans - 10, cis - 12 glycerinated CLA and one Vitamin E placebo pearl; group CE: Two CLA capsules and One Vitamin E pearl; group E: One Vitamin E (α tocopherol) pearl (400 mg/day) and two CLA placebo capsules; for group P: Two CLA placebo capsules and one placebo pearl. Placebo capsules contained corn oil (CO) in replacing of Vitamin E and high oleic sunflower oil in replace of CLA. Vitamin E and its placebo were specially prepared for this study by Zahravey Company, and CLA and its placebo by Lipid Nutrition Company.
Biochemical analysis
Blood sample collection: A sample of 15 ml blood was obtained from each patient before the trial and at the end of it. The patients were fast for 12–14 h. Ethylene diamine tetra acetic acid as anticoagulant was used for plasma isolation.
Plasma α-tocopherol was measured by high-performance liquid chromatography (Cuesta–Sanz method) with a C15 column and ultraviolet–visible detector.[50]
Measurement of plasma inflammatory and immunity reactants: Plasma cytokines were done by ELISA method[51,52,53,54] and by human high sensitivity ELISA kits from eBioscience Company [USA] (sensitivities for interleukin-2 (IL-2), IL-4, IL-1, tumor necrosis factor-α (TNF-α) were 0.4, 0.1, 0.05, 0.13 pg/ml, respectively). Matrix metalloproteinase 3 (MMP-3) was measured by human ELISA Platinum kits from Ebioscience Company; sensitivity was 0.008 ng/ml. Citrullinated antibody (CPP-A) was measured by ELISA method for IgG Antibody citrullinated protein and kits produced by Genesis (England) company, and clinical sensitivity was 80%.[55]
Hematological values were determined by an automated blood counter (Beckman Coulter, Miami, USA).
Nutrients intakes were estimated using 24 h dietary recall questionnaire before and at the end of the trial for 3 days analyzed by Nutrition IV(San Bruno, CA, USA, Firsty Data Bank) software. The subjects were asked not to change their usual diets and physical activities throughout the study, and any changes in their medications were avoided if possible.
Compliance with the supplement intake was assessed by counting number of the capsules used and determining changes in the plasma α-tocopherol.
Statistics methods
Differences between the four groups were determined by ANOVA (one-way analysis of variance) for continuous data and the Chi-square test for group data. Log transformation was used to normalize the abnormal distributions. Differences between before and after data in each group were determined with paired-sample t-test. If the distribution of a variable was not normal, Mann–Whitney U-test was used to compare the differences between two groups and Wilcoxon signed-rank test was performed for each group to compare mean values before and after intervention. ANOCVA were used to adjust the effect of confounding factors. Correlation was determined by Pearson test. P < 0.05 was considered as statistically significant. (Version 18; SPSS Inc., Chicago, Il, USA) was used for data analysis. Quantitative values are reported as mean ± standard deviation.
RESULTS
Totally 102 subjects entered to the study, and 87 of them completed the study. Fifteen patients were excluded from the study due to either incomplete consumption of prescribed drugs (6 patients: 1 in each groups C [CLA] and CE [CLA + Vitamin E], 2 in each groups E [Vitamin E] and P [Placebo]), changing the dose of their antiinflammatory drugs (8 patients: 2 in each groups), or side-effects (1 patient in group C).
Table 1 shows demographic, anthropometric data for the 4 study groups at the baseline. There were no significant differences among the four groups at the beginning of the study regarding age, sex, BMI, daily intake of vitamin E, disease duration and diseases activity score (P > 0.05). Also, the differences between drugs intake (NSAIDS, glucocorticoid and other disease-modifying antirheumatic drugs) weren’t significant between groups (P > 0.05).[28] The Plasma level of α-tocopherol increased significantly in groups E and CE in contrast to the placebo group (P ≤ 0.017, P < 0.023 respectively) [Table 2].
Table 1.
Demographic, anthropometric, and clinical data for the four study groups at the baseline (mean±SD)

Table 2.
The plasma levels of CCP-A, MMP-3, CRP and Vitamin E in patients with active RA before and after 3 months supplementation (mean±SD)
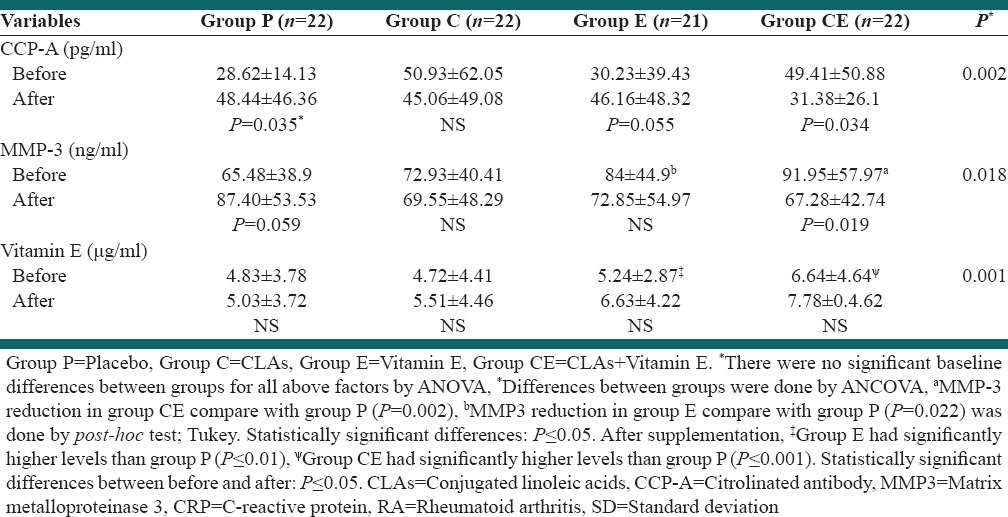
There were no significant differences between groups at the baseline in cytokines IL-2, IL-4, TNF-α, IL-1β, IL-2/IL-4 and citrullinated antibody variables [Tables 3] as well as white blood cell (WBC) [Tables 2 and 4]. In this study, significant changes were not seen in neutrophils, lymphocyte, monocytes, eosinophils numbers and BMI after treatment between groups. Decrease in WBC count was significant in group CLA plus Vitamin E, and the lymphocytes increased in group P (P ≤ 0.05) [Table 3].
Table 3.
Levels of some plasma cytokines in patients with active RA before and after 3 months supplementation (mean±SD)

Table 4.
Levels of blood immunity cells in patients with active RA before and after 3 months supplementation (mean±SD)

Although TNF-α was reduced during the study in all groups, this reduction was significant only in group CE [Table 3] (P < 0.05). IL1β increased significantly in group P and E but decreased in group CE (P > 0.05). The increase in IL-1β in group E was lower than Placebo. The IL-4 decreased in groups C, E, CE (P = 0.03, P = 0.07 and P = 0.003 respectively), but didn’t changed significantly in group P. No significant changes were seen in the plasma IL-2 levels in all groups, although increased in group P and decreased in other groups (P > 0.05). IL-2/IL-4 increased in all groups with P = 0.005, P = 0.016, P = 0.005, P = 0.006 for group P, CLAs, E and CE respectively, but differences between groups wasn’t significant. CCP-A increased in group P and decreased in group CE significantly (P ≤ 0.05). The increase in CCP-A in group E was lower than Placebo. MMP-3 increased in group P and decreased in group CE (P ≤ 0.05), and the differences between group P and group CE was significant (P = 0.018) [Tables 3]. The difference between groups for all data except for CCP-A and MMP-3 wasn’t significant.
No significant side effects were observed. There were three reports of flatulence (2 in group C and 1 in group CE). It was relieved in two patients by prescribing tablets during meals instead of before meals, but one patient in group C was needed to exclude.
DISCUSSION
Conjugated linoleic acids are groups of fatty acids with the geometrical and positional isomers of linoleic acid. CLAs were previously shown to reduce clinical signs of inflammatory diseases such as type I airway hypersensitivity,[56] lupus,[57] cancer,[58] endotoxin-induced cachexia,[59] inflammatory bowel disease,[60] and renal failure.[61] CLA reduces immune-induced TNF-α[62,63] and inducible COX2 expression, key mediators of inflammation in RA. It can also influence cell functions.[31,63,64,65] Based on previous work, it was hypothesized that dietary CLA would have anti-inflammatory effects on animal models of RA.[63,64,65,66] Some evidence suggests that CLA decreases antigen-induced cytokine production in immune competent cells, modulates the production of cytokines and leukotriene B4, has antioxidant effect,[63] and is helpful for reducing symptoms and/or adverse effects of RA.[28] CCP-A is a better index for prediction of erosive RA than ESR, CRP and MMP-3,[67] and higher CCP-A concentrations are associated with increased disease activity in RA.[68] In our study CCP-A significantly increased in group P (P = 0.035) and also E (P = 0.055) and decreased in group CE (P = 0.034). TNF-α was reduced during our study in treatment groups, but this reduction was significant only in group CE. IL-1β increased in group P (P = 0.004) and E (P = 0.041) and difference decrease was near significant between group CE, compared with group P (P = 0.061). Hence in group P increased CCP-A, MMP-3, and IL-1β, but in group E increased IL-1β and CCP-A (borderline significant, lower than placebo group) and in other groups we hadn’t significant increase in those inflammatory parameters. There is evidence for the involvement of T-cells, especially CD4+ T-cells, in the pathogenesis of RA and is thought to be a Th1 cell–associated disorders rather than Th2[69] therefore we measured IL-4 and IL-2 that respectively secreted by T helper-2 and helper-1 and their ratio. The IL4 decreased in groups C, CE and E (P = 0.03, P = 0.03 and P = 0.07 respectively), but did not change in group P significantly (P = 0.068). No significant changes were seen in the plasma levels of IL-2 in all groups [Table 5].
Song et al. studied the effect of dietary CLA supplementation (3 g/day) on the immune system in healthy humans. CLA supplementation also decreased the levels of the pro-inflammatory cytokines, TNF-α and IL-1β (P < 0.05) were similar to our findings, whereas the levels of the anti-inflammatory cytokine IL-10 increased (P < 0.05).[43] In the Butz et al. study on the effect of CLA on collagen antibody-induced arthritis (CIA), CLA-fed mice had arthritic scores 70% that of the CO fed mice.[66] Some previous studies on systemic lupus erythematos supported the effects of dietary CLAs. In one study CLA had a beneficial effect in the autoimmune NZB/W F1 mouse, because the cachectic symptom of systemic lupus erythematos was decreased by dietary CLA and survival days were increased over the control group.[59] Several studies reported that CLA can reduce TNF-α levels and TNF-α related cachexia.[70,71]
Similar to our study, the mixed CLAS isomers reduce TNF-α level in animals and human primary muscle cells.[71,72,73] CLAs have been shown to reduce pro-inflammatory cytokines, helping to decrease symptoms associated with inflammation.[33,61,62,74] However, there are reports that CLAs decreased IL-4 and increased IL-2 and interferon-γ (IFN-γ) in splenocytes and T-cells, with no effect on Plasma TNF-α levels or increased it in humans and rats,[41,75] which may reflect pro-inflammatory responses by CLAs.[47,71] The difference for the effects of CLAs on inflammatory responses may be due to the various isomer used, duration of treatment, tissue specificity and purity.[63,76,77] However, the exact mechanism by which CLAs control cytokines are still not clear.[43] CLAs may be exerting anti-inflammatory effect through peroxisome proliferator-activated receptors (PPARs). It has been reported that CLA's effects may be mediated through PPAR-α and PPAR-g.[62,78] Activation of PPAR-γ inhibits production of inflammatory cytokines, such as TNF-α, IFN-γ, IL-1 β, IL-6 and IL-8.[62,77] Some studies suggest that CLA may affect PPAR-γ through nuclear factor-kB.[63,77,78,79]
CLAs are known to inhibit dendritic cell (DC) maturation and have antiinflammatory effects on DC following activation with lipopolysaccharide.[63]
CLAs may have direct or indirect anti-inflammatory effect on the activity and expression of COX-2, thus decrease PGE2 production,[31,80] we saw a significant reduction in MMP-3 plasma levels in group CE. MMP-3 is an enzyme with abroad substrate specifies and causes joint proteoglycan and other matrix molecules degradation. The imbalance between MMP-3 and its inhibitors leads to joints destruction. MMP-3 increases in the synovial fluid of RA patients and especially in active RA disease and hence it may be a useful biomarker of response to treatment.[81]
It has been suggested that nonenzymatic and enzymatic antioxidant systems are impaired in RA; so patients with RA are exposed to oxidant stress.[10,11,12] Several studies show a beneficial effect of antioxidants in RA.[82,83] In a case–control study by our group on 59 RA patients and 60 healthy sex and age-matched controls it was shown that in patients with RA Plasma MDA level was significantly higher and plasma concentration of beta-carotene, Vitamin E and Gluthation Reductase activity were significantly lower than healthy controls (P < 0.001).[12] CLAs may confer antioxidant effect. Arab et al. studied the effects of some fatty acids on the redox status and lipid peroxidation of human fibroblasts, but only arachidonic acid and CLA enhanced Glutathion content through γ-Glutamylcysteine ligase induction. CLA was more potent than arachidonic acid in Glutathion synthesis induction.[84]
Several studies suggest positive effects of antioxidants such as Vitamin E in RA.[83,85]
The cellular responses to Vitamin E are associated with transcriptional and posttranscriptional events. Activation of diacylglycerol kinase and protein phosphatase 2α, and the inhibition of PKC, COX, lipoxygenase, tyrosine kinase phosphorylation, and cytokine release by Vitamin E are all examples of posttranscriptional regulation.[86]
Vitamin E supplementation also resulted in the suppression of pro-inflammatory cytokines such as IL-6 and chemokines such as IL-8 and monocytes chemotactic protein-1 (MCP-1). Vitamin E also modulates cyclooxygenase-2 activity and inhibits thromboxane formation.[87] In this study, we used Vitamin E supplementation as an antioxidant, and in combination with CLAs.
The lymphocytes increased in group P (P ≤ 0.05) and decreased in group CE nonsignificantly.
To the best of our knowledge, the effect of Vitamin E on cytokines in RA patients has not been investigated up to now. Pallest study (1999) in elderly person shows that Vitamin E increases IL4 and IL2 nonsignificantly,[88] but alber study) 2003 (on rats shows Vitamin E reduces IL4 and INFγ in spleen and lymph nodes.[40] In van Tits et al. study α-tocopherol supplementation decreased production of cytokines and superoxide by leukocytes ex vivo in both hypertriglyceridemic and normolipidemic persons.[89] In Mol et al. study was shown that the supplementation of 600 IU/d for four weeks decreased plasma IL-1α, TNF-α, and IL-1 β in smokers but not in diabetic patients.[10]
In our study, Vitamin E supplementation for three months reduced IL4 and IL1 β significantly. The suggested mechanism may be either directly change in T-cell surface receptors or indirectly effect on macrophages, reduction in PGE2 and H2O2 (product of activated macrophages).[90,91] Some studies show the effectiveness of Vitamin E on reducing the pain[29,85,86] inflammation and other clinical outcomes that may verify our data.
CONCLUSIONS
In our study, 3-month co-supplementation with CLA and Vitamin E resulted in a significant reduction of WBC count, MMP-3, and TNF-α in patients with active RA. It seems that CLAs may decrease inflammation in patients with RA. Co-supplementation with Vitamin E could be helpful in increasing the antiinflammatory effects of CLAs on active RA, but further investigations are needed to determine the exact effects of CLAs on inflammation and Immunity in humans.
ACKNOWLEDGMENTS
This study was funded through a research grant from the Research Deputy of the Tehran University of Medical Sciences (with Proposal No., 2804). We are thankful for the cooperation of both Lipid Nutrition Company (Netherlands) for providing CLA capsules as Clarinol G-80 and Zahravi Company (Iran) for providing Vitamin E pearls for this research. We are thankful from Nafeese Aryaeian for editing the article.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Firestein GS. Kelley's Textbook of Rheumatology. 8th ed. Vol. 2. Philadelphia: Saunders; 2008. Etiology and pathogenesis of rheumatoid arthritis; p. 1035. [Google Scholar]
- 2.Clair E, Pisetsky W, Haynes F. 2nd ed. USA: Lippincott and Wilkins Inc; 2004. Rheumatoid Arthritis. [Google Scholar]
- 3.Heliövaara M, Knekt P, Aho K, Aaran RK, Alfthan G, Aromaa A. Serum antioxidants and risk of rheumatoid arthritis. Ann Rheum Dis. 1994;53:51–3. doi: 10.1136/ard.53.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hadjigogos K. The role of free radicals in the pathogenesis of rheumatoid arthritis. Panminerva Med. 2003;45:7–13. [PubMed] [Google Scholar]
- 5.Kalpakcioglu B, Senel K. The interrelation of glutathione reductase, catalase, glutathione peroxidase, superoxide dismutase, and glucose-6-phosphate in the pathogenesis of rheumatoid arthritis. Clin Rheumatol. 2008;27:141–5. doi: 10.1007/s10067-007-0746-3. [DOI] [PubMed] [Google Scholar]
- 6.Afonso V, Champy R, Mitrovic D, Collin P, Lomri A. Reactive oxygen species and superoxide dismutases: Role in joint diseases. Joint Bone Spine. 2007;74:324–9. doi: 10.1016/j.jbspin.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Mirshafiey A, Mohsenzadegan M. The role of reactive oxygen species in immunopathogenesis of rheumatoid arthritis. Iran J Allergy Asthma Immunol. 2008;7:195–202. [PubMed] [Google Scholar]
- 8.Hitchon CA, El-Gabalawy HS. Oxidation in rheumatoid arthritis. Arthritis Res Ther. 2004;6:265–78. doi: 10.1186/ar1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauerová K, Bezek A. Role of reactive oxygen and nitrogen species in etiopathogenesis of rheumatoid arthritis. Gen Physiol Biophys. 1999;18(Spec No):15–20. [PubMed] [Google Scholar]
- 10.Mol MJ, de Rijke YB, Demacker PN, Stalenhoef AF. Plasma levels of lipid and cholesterol oxidation products and cytokines in diabetes mellitus and cigarette smoking: Effects of vitamin E treatment. Atherosclerosis. 1997;129:169–76. doi: 10.1016/s0021-9150(96)06022-4. [DOI] [PubMed] [Google Scholar]
- 11.Kamanli A, Naziroglu M, Aydilek N, Hacievliyagil C. Plasma lipid peroxidation and antioxidant levels in patients with rheumatoid arthritis. Cell Biochem Funct. 2004;22:53–7. doi: 10.1002/cbf.1055. [DOI] [PubMed] [Google Scholar]
- 12.Aryaeian N, Djalali M, Shahram F, Jazayeri Sh, Chamari M, Nazari S. Beta-Carotene, Vitamin E, MDA, Glutathione Reductase and Arylesterase Activity Levels in Patients with Active Rheumatoid Arthritis. Iran J Public Health. 2011;40:102–9. [PMC free article] [PubMed] [Google Scholar]
- 13.Knekt P, Heliövaara M, Aho K, Alfthan G, Marniemi J, Aromaa A. Serum selenium, serum alpha-tocopherol, and the risk of rheumatoid arthritis. Epidemiology. 2000;11:402–5. doi: 10.1097/00001648-200007000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Kelly O, Cashman KD. The effect of conjugated linoleic acid on calcium absorption and bone metabolism and composition in adult ovariectomised rats. Prostaglandins Leukot Essent Fatty Acids. 2004;71:295–301. doi: 10.1016/j.plefa.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Kelly O, Cusack S, Jewell C, Cashman KD. The effect of polyunsaturated fatty acids, including conjugated linoleic acid, on calcium absorption and bone metabolism and composition in young growing rats. Br J Nutr. 2003;90:743–50. doi: 10.1079/bjn2003951. [DOI] [PubMed] [Google Scholar]
- 16.Ip C, Dong Y, Ip MM, Banni S, Carta G, Angioni E, et al. Conjugated linoleic acid isomers and mammary cancer prevention. Nutr Cancer. 2002;43:52–8. doi: 10.1207/S15327914NC431_6. [DOI] [PubMed] [Google Scholar]
- 17.Valeille K, Gripois D, Blouquit MF, Souidi M, Riottot M, Bouthegourd JC, et al. Lipid atherogenic risk markers can be more favourably influenced by the cis-9, trans-11-octadecadienoate isomer than a conjugated linoleic acid mixture or fish oil in hamsters. Br J Nutr. 2004;91:191–9. doi: 10.1079/BJN20031057. [DOI] [PubMed] [Google Scholar]
- 18.Valeille K, Férézou J, Amsler G, Quignard-Boulangé A, Parquet M, Gripois D, et al. A cis-9, trans-11-conjugated linoleic acid-rich oil reduces the outcome of atherogenic process in hyperlipidemic hamster. Am J Physiol Heart Circ Physiol. 2005;289:H652–9. doi: 10.1152/ajpheart.00130.2005. [DOI] [PubMed] [Google Scholar]
- 19.Houseknecht KL, Vanden Heuvel JP, Moya-Camarena SY, Portocarrero CP, Peck LW, Nickel KP, et al. Dietary conjugated linoleic acid normalizes impaired glucose tolerance in the Zucker diabetic fatty fa/fa rat. Biochem Biophys Res Commun. 1998;244:678–82. doi: 10.1006/bbrc.1998.8303. [DOI] [PubMed] [Google Scholar]
- 20.Banu J, Bhattacharya A, Rahman M, O’Shea M, Fernandes G. Effects of conjugated linoleic acid and exercise on bone mass in young male Balb/C mice. Lipids Health Dis. 2006;5:7. doi: 10.1186/1476-511X-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kung FC, Yang MC. The effect of covalently bonded conjugated linoleic acid on the reduction of oxidative stress and blood coagulation for polysulfone hemodialyzer membrane. Int J Biol Macromol. 2006;38:157–64. doi: 10.1016/j.ijbiomac.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 22.Kung FC, Chang JJ, Yang MC. The reduction of oxidative stress, anticoagulation of platelets, and inhibition of lipopolysaccharide by conjugated linoleic acid bonded on a polysulfone membrane. Polym Adv Technol. 2007;18:286–91. [Google Scholar]
- 23.Rossary A, Arab K, Steghens JP. Polyunsaturated fatty acids modulate NOX4 anion superoxide production in human fibroblasts. Biochem J. 2007;406:77–83. doi: 10.1042/BJ20061009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergamo P, Luongo D, Maurano F, Mazzarella G, Stefanile R, Rossi M. Conjugated linoleic acid enhances glutathione synthesis and attenuates pathological signs in MRL/MpJ-Fas (lpr) mice. J Lipid Res. 2006;47:2382–91. doi: 10.1194/jlr.M600187-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Su ND, Liu XW, Kim MR, Jeong TS, Sok DE. Protective action of CLA against oxidative inactivation of paraoxonase 1, an antioxidant enzyme. Lipids. 2003;38:615–22. doi: 10.1007/s11745-003-1106-9. [DOI] [PubMed] [Google Scholar]
- 26.Yu L. Free radical scavenging properties of conjugated linoleic acids. J Agric Food Chem. 2001;49:3452–6. doi: 10.1021/jf010172v. [DOI] [PubMed] [Google Scholar]
- 27.Kim HK, Kim SR, Ahn JY, Cho IJ, Yoon CS, Ha TY. Dietary conjugated linoleic acid reduces lipid peroxidation by increasing oxidative stability in rats. J Nutr Sci Vitaminol (Tokyo) 2005;51:8–15. doi: 10.3177/jnsv.51.8. [DOI] [PubMed] [Google Scholar]
- 28.Aryaeian N, Shahram F, Djalali M, Eshragian MR, Djazayeri A, Sarrafnejad A, et al. Effect of conjugated linoleic acids, vitamin E and their combination on the clinical outcome of Iranian adults with active rheumatoid arthritis. Int J Rheum Dis. 2009;12:20–8. doi: 10.1111/j.1756-185X.2009.01374.x. [DOI] [PubMed] [Google Scholar]
- 29.Aryaeian N, Shahram F, Djalali M, Djazayeri A, Eshragian MR, Naderi N, et al. The effect of conjugated linoleic acids, vitamin e and their combination on lipid peroxidation in active rheumatoid arthritis. Iran J Public Health. 2009;38:79–89. [Google Scholar]
- 30.Aryaeian N, Shahram F, Djalali M, Djazayeri A, Eshragian MR, Sarrafnejad A, et al. Effect of conjugated linoleic acid, vitamin E and their combination on lipid profiles and blood pressure of Iranian adultswith active rheumatoid arthritis. Vasc Health Risk Manag. 2008;4:1423–32. doi: 10.2147/vhrm.s3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li G, Barnes D, Butz D, Bjorling D, Cook ME. 10th, 12c-conjugated linoleic acid inhibits lipopolysaccharide-induced cyclooxygenase expression in vitro and in vivo. J Lipid Res. 2005;46:2134–42. doi: 10.1194/jlr.M500064-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Clare MR, Draper E, Keogh B, Rahman A, Moloney AP, Kingston HG, et al. A Conjugated linoleic acid-enriched beef diet attenuates lipopolysaccharide-induced inflammationin mice through pparγ mediated suppregion of toll-likereceptor 4. J Nutr. 2009;139:2351–7. doi: 10.3945/jn.109.113035. [DOI] [PubMed] [Google Scholar]
- 33.Changhua L, Jindong Y, Defa L, Lidan Z, Shiyan Q, Jianjun X. Conjugated linoleic acid attenuates the production and gene expression of proinflammatory cytokines in weaned pigs challenged with lipopolysaccharide. J Nutr. 2005;135:239–44. doi: 10.1093/jn/135.2.239. [DOI] [PubMed] [Google Scholar]
- 34.O’Shea M, Bassaganya-Riera J, Mohede IC. Immunomodulatory properties of conjugated linoleic acid. Am J Clin Nutr. 2004;79:1199S–206. doi: 10.1093/ajcn/79.6.1199S. [DOI] [PubMed] [Google Scholar]
- 35.Sugano M, Tsujita A, Yamasaki M, Noguchi M, Yamada K. Conjugated linoleic acid modulates tissue levels of chemical mediators and immunoglobulins in rats. Lipids. 1998;33:521–7. doi: 10.1007/s11745-998-0236-4. [DOI] [PubMed] [Google Scholar]
- 36.Yu Y, Correll PH, Vanden Heuvel JP. Conjugated linoleic acid decreases production of pro-inflammatory products in macrophages: Evidence for a PPAR gamma-dependent mechanism. Biochim Biophys Acta. 2002;1581:89–99. doi: 10.1016/s1388-1981(02)00126-9. [DOI] [PubMed] [Google Scholar]
- 37.Cook ME, Butz DE, Li G, Pariza MW, Whigham LD, Yang M. Advances in Conjugated Linoleic Acid. USA: AOCS Press; 2003. Conjugated linoleic acid enhances immune responses but protects against the collateral dammage of immune events; pp. 283–91. [Google Scholar]
- 38.Fritsche K. Fatty acids as modulators of the immune response. Annu Rev Nutr. 2006;26:45–73. doi: 10.1146/annurev.nutr.25.050304.092610. [DOI] [PubMed] [Google Scholar]
- 39.Cook ME. 19, NIH, Bethesda, Maryland: Lister Hill Auditorium; 2002. Conjugated linoleic acid's role in immunity and immune related disorders. Perspectives on conjugated linoleic acid research: Current status and future directions. [Google Scholar]
- 40.Albers R, van der Wielen RP, Brink EJ, Hendriks HF, Dorovska-Taran VN, Mohede IC. Effects of cis-9, trans-11 and trans-10, cis-12 conjugated linoleic acid (CLA) isomers on immune function in healthy men. Eur J Clin Nutr. 2003;57:595–603. doi: 10.1038/sj.ejcn.1601585. [DOI] [PubMed] [Google Scholar]
- 41.Kelley DS, Simon VA, Taylor PC, Rudolph IL, Benito P, Nelson GJ, et al. Dietary supplementation with conjugated linoleic acid increased its concentration in human peripheral blood mononuclear cells, but did not alter their function. Lipids. 2001;36:669–74. doi: 10.1007/s11745-001-0771-z. [DOI] [PubMed] [Google Scholar]
- 42.Calder PC, Grimble RF. Polyunsaturated fatty acids, inflammation and immunity. Eur J Clin Nutr. 2002;56(Suppl 3):S14–9. doi: 10.1038/sj.ejcn.1601478. [DOI] [PubMed] [Google Scholar]
- 43.Song HJ, Grant I, Rotondo D, Mohede I, Sattar N, Heys SD, et al. Effect of CLA supplementation on immune function in young healthy volunteers. Eur J Clin Nutr. 2005;59:508–17. doi: 10.1038/sj.ejcn.1602102. [DOI] [PubMed] [Google Scholar]
- 44.Nugent AP, Roche HM, Noone EJ, Long A, Kelleher DK, Gibney MJ. The effects of conjugated linoleic acid supplementation on immune function in healthy volunteers. Eur J Clin Nutr. 2005;59:742–50. doi: 10.1038/sj.ejcn.1602132. [DOI] [PubMed] [Google Scholar]
- 45.Smedman A, Basu S, Jovinge S, Fredrikson GN, Vessby B. Conjugated linoleic acid increased C-reactive protein in human subjects. Br J Nutr. 2005;94:791–5. doi: 10.1079/bjn20041419. [DOI] [PubMed] [Google Scholar]
- 46.Risérus U, Basu S, Jovinge S, Fredrikson GN, Arnlöv J, Vessby B. Supplementation with conjugated linoleic acid causes isomer-dependent oxidative stress and elevated C-reactive protein: A potential link to fatty acid-induced insulin resistance. Circulation. 2002;106:1925–9. doi: 10.1161/01.cir.0000033589.15413.48. [DOI] [PubMed] [Google Scholar]
- 47.Moloney F, Yeow TP, Mullen A, Nolan JJ, Roche HM. Conjugated linoleic acid supplementation, insulin sensitivity, and lipoprotein metabolism in patients with type 2 diabetes mellitus. Am J Clin Nutr. 2004;80:887–95. doi: 10.1093/ajcn/80.4.887. [DOI] [PubMed] [Google Scholar]
- 48.Raff M, Tholstrup T, Basu S, Nonboe P, Sørensen MT, Straarup EM. A diet rich in conjugated linoleic acid and butter increases lipid peroxidation but does not affect atherosclerotic, inflammatory, or diabetic risk markers in healthy young men. J Nutr. 2008;138:509–14. doi: 10.1093/jn/138.3.509. [DOI] [PubMed] [Google Scholar]
- 49.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 50.Cuesta Sanz D, Castro Santa-Cruz M. Simultaneous measurement of retinol and alpha-tocopherol in human serum by high-performance liquid chromatography with ultraviolet detection. J Chromatogr. 1986;380:140–4. doi: 10.1016/s0378-4347(00)83634-8. [DOI] [PubMed] [Google Scholar]
- 51.O’Brien SM, Scully P, Dinan TG. Increased tumor necrosis factor-alpha concentrations with interleukin-4 concentrations in exacerbations of schizophrenia. Psychiatry Res. 2008;160:256–62. doi: 10.1016/j.psychres.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 52.Furuzawa-Carballeda J, Macip-Rodríguez PM, Cabral AR. Osteoarthritis and rheumatoid arthritis pannus have similar qualitative metabolic characteristics and pro-inflammatory cytokine response. Clin Exp Rheumatol. 2008;26:554–60. [PubMed] [Google Scholar]
- 53.Mullen A, Moloney F, Nugent AP, Doyle L, Cashman KD, Roche HM. Conjugated linoleic acid supplementation reduces peripheral blood mononuclear cell interleukin-2 production in healthy middle-aged males. J Nutr Biochem. 2007;18:658–66. doi: 10.1016/j.jnutbio.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 54.Fukuda K, Fujitsu Y, Kumagai N, Nishida T. Inhibition of matrix metalloproteinase-3 synthesis in human conjunctival fibroblasts by interleukin-4 or interleukin-13. Invest Ophthalmol Vis Sci. 2006;47:2857–64. doi: 10.1167/iovs.05-1261. [DOI] [PubMed] [Google Scholar]
- 55.Vincent C, Nogueira L, Sebbag M, Chapuy-Regaud S, Arnaud M, Letourneur O, et al. Detection of antibodies to deiminated recombinant rat filaggrin by enzyme-linked immunosorbent assay: A highly effective test for the diagnosis of rheumatoid arthritis. Arthritis Rheum. 2002;46:2051–8. doi: 10.1002/art.10436. [DOI] [PubMed] [Google Scholar]
- 56.MacRedmond R, Singhera G, Attridge S, Bahzad M, Fava C, Lai Y, et al. Conjugated linoleic acid improves airway hyper-reactivity in overweight mild asthmatics. Clin Exp Allergy. 2010;40:1071–8. doi: 10.1111/j.1365-2222.2010.03531.x. [DOI] [PubMed] [Google Scholar]
- 57.Yang M, Pariza MW, Cook ME. Dietary conjugated linoleic acid protects against end stage disease of systemic lupus erythematosus in the NZB/W F1 mouse. Immunopharmacol Immunotoxicol. 2000;22:433–49. doi: 10.3109/08923970009026004. [DOI] [PubMed] [Google Scholar]
- 58.Park HS, Ryu JH, Ha YL, Park JH. Dietary conjugated linoleic acid (CLA) induces apoptosis of colonic mucosa in 1,2-dimethylhydrazine-treated rats: A possible mechanism of the anticarcinogenic effect by CLA. Br J Nutr. 2001;86:549–55. doi: 10.1079/bjn2001445. [DOI] [PubMed] [Google Scholar]
- 59.Yang M, Cook ME. Dietary conjugated linoleic acid decreased cachexia, macrophage tumor necrosis factor-alpha production, and modifies splenocyte cytokines production. Exp Biol Med (Maywood) 2003;228:51–8. doi: 10.1177/153537020322800107. [DOI] [PubMed] [Google Scholar]
- 60.Hontecillas R, Wannemeulher MJ, Zimmerman DR, Hutto DL, Wilson JH, Ahn DU, et al. Nutritional regulation of porcine bacterial-induced colitis by conjugated linoleic acid. J Nutr. 2002;132:2019–27. doi: 10.1093/jn/132.7.2019. [DOI] [PubMed] [Google Scholar]
- 61.Yang M, Cook ME. Dietary CLA decreased weight loss and extended survival following the onset of kidney failure in NZB/W F1 mice. Lipids. 2003;38:21–4. doi: 10.1007/s11745-003-1026-8. [DOI] [PubMed] [Google Scholar]
- 62.Turek JJ, Li Y, Schoenlein IA, Allen KG, Watkins BA. Modulation of macrophage cytokine production by conjugated linoleic acids. J Nutr Biochem. 1998;9:258–66. [Google Scholar]
- 63.Hur SJ, Park Y. Effect of conjugated linoleic acid on bone formation and rheumatoid arthritis. Eur J Pharmacol. 2007;568:16–24. doi: 10.1016/j.ejphar.2007.04.056. [DOI] [PubMed] [Google Scholar]
- 64.Degner SC, Kemp MQ, Bowden GT, Romagnolo DF. Conjugated linoleic acid attenuates cyclooxygenase-2 transcriptional activity via an anti-AP-1 mechanism in MCF-7 breast cancer cells. J Nutr. 2006;136:421–7. doi: 10.1093/jn/136.2.421. [DOI] [PubMed] [Google Scholar]
- 65.Huebner SM, Campbell JP, Butz DE, Fulmer TG, Gendron-Fitzpatrick A, Cook ME. Individual isomers of conjugated linoleic acid reduce inflammation associated with established collagen-induced arthritis in DBA/1 mice. J Nutr. 2010;140:1454–61. doi: 10.3945/jn.109.120527. [DOI] [PubMed] [Google Scholar]
- 66.Butz DE, Li G, Huebner SM, Cook ME. A mechanistic approach to understanding conjugated linoleic acid's role in inflammation using murine models of rheumatoid arthritis. Am J Physiol Regul Integr Comp Physiol. 2007;293:R669–76. doi: 10.1152/ajpregu.00005.2007. [DOI] [PubMed] [Google Scholar]
- 67.Shovman O, Gilburd B, Zandman-Goddard G, Sherer Y, Orbach H, Gerli R, et al. The diagnostic utility of anti-cyclic citrullinated peptide antibodies, matrix metalloproteinase-3, rheumatoid factor, erythrocyte sedimentation rate, and C-reactive protein in patients with erosive and non-erosive rheumatoid arthritis. Clin Dev Immunol. 2005;12:197–202. doi: 10.1080/17402520500233510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miriovsky BJ, Michaud K, Thiele GM, O’Dell JR, Cannon GW, Kerr G, et al. Anti-CCP antibody and rheumatoid factor concentrations predict greater disease activity in men with rheumatoid arthritis. Ann Rheum Dis. 2010;69:1292–7. doi: 10.1136/ard.2009.122739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–61. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 70.Akahoshi A, Goto Y, Murao K, Miyazaki T, Yamasaki M, Nonaka M, et al. Conjugated linoleic acid reduces body fats and cytokine levels of mice. Biosci Biotechnol Biochem. 2002;66:916–20. doi: 10.1271/bbb.66.916. [DOI] [PubMed] [Google Scholar]
- 71.Park Y, Yang M, Storkson JM, Albrigh KJ, Liu W, Cook ME, et al. Effects of conjugated linoleic acid (CLA) isomers on serum tumor necrosis factor-α concentration in mice. J. Food Biochem. 2007;31:252–65. [Google Scholar]
- 72.Kim KH, Kim DI, Kim SH, Jung EM, Kang JH, Jeung EB, et al. Trans-10, cis-12-conjugated linoleic acid attenuates tumor necrosis factor-a production by lipopolysaccharide-stimulated porcine peripheral blood mononuclear cells through induction of interleukin-10. Cytokine. 2011;56:224–30. doi: 10.1016/j.cyto.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 73.Jaudszus A, Foerster M, Kroegel C, Wolf I, Jahreis G. Cis-9, trans-11-CLA exerts anti-inflammatory effects in human bronchial epithelial cells and eosinophils: Comparison to trans-10, cis-12-CLA and to linoleic acid. Biochim Biophys Acta. 2005;1737:111–8. doi: 10.1016/j.bbalip.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 74.Zhao L, Yin J, Li D, Lai C, Chen X, Ma D. Conjugated linoleic acid can prevent tumor necrosis factor gene expression by inhibiting nuclear factor binding activity in peripheral blood mononuclear cells from weaned pigs challenged with lipopolysaccharide. Arch Anim Nutr. 2005;59:429–38. doi: 10.1080/17450390500353333. [DOI] [PubMed] [Google Scholar]
- 75.Hayek MG, Han SN, Wu D, Watkins BA, Meydani M, Dorsey JL, et al. Dietary conjugated linoleic acid influences the immune response of young and old C57BL/6NCrlBR mice. J Nutr. 1999;129:32–8. doi: 10.1093/jn/129.1.32. [DOI] [PubMed] [Google Scholar]
- 76.Roche HM, Noone E, Sewter C, Mc Bennett S, Savage D, Gibney MJ, et al. Isomer-dependent metabolic effects of conjugated linoleic acid: Insights from molecular markers sterol regulatory element-binding protein-1c and LXRalpha. Diabetes. 2002;51:2037–44. doi: 10.2337/diabetes.51.7.2037. [DOI] [PubMed] [Google Scholar]
- 77.Parra P, Serra F, Palou A. Moderate doses of conjugated linoleic acid isomers mix contribute to lowering body fat content maintaining insulin sensitivity and a noninflammatory pattern in adipose tissue in mice. J Nutr Biochem. 2010;21:107–15. doi: 10.1016/j.jnutbio.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 78.Moya-Camarena SY, Vanden Heuvel JP, Blanchard SG, Leesnitzer LA, Belury MA. Conjugated linoleic acid is a potent naturally occurring ligand and activator of PPARalpha. J Lipid Res. 1999;40:1426–33. [PubMed] [Google Scholar]
- 79.Evans NP, Misyak SA, Schmelz EM, Guri AJ, Hontecillas R, Bassaganya-Riera J. Conjugated linoleic acid ameliorates inflammation-induced colorectal cancer in mice through activation of PPARgamma. J Nutr. 2010;140:515–21. doi: 10.3945/jn.109.115642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cheng WL, Lii CK, Chen HW, Lin TH, Liu KL. Contribution of conjugated linoleic acid to the suppression of inflammatory responses through the regulation of the NF-kappaB pathway. J Agric Food Chem. 2004;52:71–8. doi: 10.1021/jf0348626. [DOI] [PubMed] [Google Scholar]
- 81.Stachowska E, Dolegowska B, Dziedziejko V, Rybicka M, Kaczmarczyk M, Bober J, et al. Prostaglandin E2 (PGE2) and thromboxane A2 (TXA2) synthesis is regulated by conjugated linoleic acids (CLA) in human macrophages. J Physiol Pharmacol. 2009;60:77–85. [PubMed] [Google Scholar]
- 82.Wener MH. Auto antibodies and other laboratoty tests. In: William ST, Pisetsky DS, Hayens BF, editors. Rheumatoid Arthritis. Philadelphia: Lipincott Williams; 2004. pp. 64–79. [Google Scholar]
- 83.Comstock GW, Burke AE, Hoffman SC, Helzlsouer KJ, Bendich A, Masi AT, et al. Serum concentrations of alpha tocopherol, beta carotene, and retinol preceding the diagnosis of rheumatoid arthritis and systemic lupus erythematosus. Ann Rheum Dis. 1997;56:323–5. doi: 10.1136/ard.56.5.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arab K, Rossary A, Soulère L, Steghens JP. Conjugated linoleic acid, unlike other unsaturated fatty acids, strongly induces glutathione synthesis without any lipoperoxidation. Br J Nutr. 2006;96:811–9. doi: 10.1017/bjn20061910. [DOI] [PubMed] [Google Scholar]
- 85.Edmonds SE, Winyard PG, Guo R, Kidd B, Merry P, Langrish-Smith A, et al. Putative analgesic activity of repeated oral doses of vitamin E in the treatment of rheumatoid arthritis. Results of a prospective placebo controlled double blind trial. Ann Rheum Dis. 1997;56:649–55. doi: 10.1136/ard.56.11.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singh U, Devaraj S, Jialal I. Vitamin E, oxidative stress, and inflammation. Annu Rev Nutr. 2005;25:151–74. doi: 10.1146/annurev.nutr.24.012003.132446. [DOI] [PubMed] [Google Scholar]
- 87.Meydani SN. Vitamin E modulation of cardiovascular disease. Ann N Y Acad Sci. 2004;1031:271–9. doi: 10.1196/annals.1331.027. [DOI] [PubMed] [Google Scholar]
- 88.Pallast EG, Schouten EG, de Waart FG, Fonk HC, Doekes G, von Blomberg BM, et al. Effect of 50- and 100-mg vitamin E supplements on cellular immune function in noninstitutionalized elderly persons. Am J Clin Nutr. 1999;69:1273–81. doi: 10.1093/ajcn/69.6.1273. [DOI] [PubMed] [Google Scholar]
- 89.van Tits LJ, Demacker PN, de Graaf J, Hak-Lemmers HL, Stalenhoef AF. alpha-tocopherol supplementation decreases production of superoxide and cytokines by leukocytes ex vivo in both normolipidemic and hypertriglyceridemic individuals. Am J Clin Nutr. 2000;71:458–64. doi: 10.1093/ajcn/71.2.458. [DOI] [PubMed] [Google Scholar]
- 90.Beharka AA, Wu D, Han SN, Meydani SN. Macrophage prostaglandin production contributes to the age-associated decrease in T cell function which is reversed by the dietary antioxidant vitamin E. Mech Ageing Dev. 1997;93:59–77. doi: 10.1016/s0047-6374(96)01819-2. [DOI] [PubMed] [Google Scholar]
- 91.Beharka AA, Wu D, Serafini M, Meydani SN. Mechanism of vitamin E inhibition of cyclooxygenase activity in macrophages from old mice: Role of peroxynitrite. Free Radic Biol Med. 2002;32:503–11. doi: 10.1016/s0891-5849(01)00817-6. [DOI] [PubMed] [Google Scholar]


