Abstract
Background:
Multiple Sclerosis (MS) is a chronic demyelinating disease of the central nervous system which has been identifies more prevalent in economically developed countries than in the developing countries. Low prevalence of parasitic infections (which can activate immune response and prevent or modulate damage to host antigens) in these areas is among the possible responsible factors for such a difference. In this study we aimed to compare frequency of blood-tissue parasitic infections in patients with MS, as compared to their healthy family members.
Methods:
This study was conducted on 50 relapsing remitting MS patients and 50 family members attending MS clinic at Alzahra Hospital. IgM and IgG anti-Toxoplasma gondii were measured. Given the high prevalence of cutaneous leishmaniasis in Isfahan, all the participants were also examined for protozoan leishmania microscopically. Furthermore malaria parasite was investigated.
Results:
Eighteen patients and 24 healthy family members had positive test in IgG Toxoplasma gondii(P = 0.09). In both groups, there was no positive IgM Toxoplasma gondii. In investigating leishmania, only 3 participants in the case group and 2 in the control tested positive (P = 0.25). No case of malaria was found among the participants.
Conclusion:
Our results showed a mismatch with hygiene hypotheses examined. However, considering that the prevalence of parasites varies with time, and depends on numerous epidemiological factors; these results do not discredit the theory investigated.
Keywords: Blood-tissue parasitic, cutaneous leishmaniasis, malaria, multiple sclerosis, toxoplasmosis
INTRODUCTION
Multiple sclerosis (MS) is a demyelinating inflammatory disease of the central nervous system that is identified with the triad of inflammation, demyelination, and gliosis. It has been a long time since identifying much higher prevalence of MS in economically developed countries than in the developing countries. One of the theories proposed to explain this increase in developed regions is a low prevalence of parasitic infections in these areas. This theory, named “old friends hypothesis” as an amendment to the well-known “hygiene hypothesis” was first proposed by Strachan in 1998 and widely received by the world's medical community. This theory states that microorganisms such as lactobacilli and parasitic worms play an important role in regulating the immune system in the human body as their host. Hence, their elimination from modern life leads to diseases induced by deregulation of the immune system such as autoimmune, and allergy.[1,2] In other words, these biological factors can activate the immune response against them, and prevent or modulate damage to own antigens due to the high antigenic power including the immune system.[3,4] To confirm the above theory, the last decade was full of studies investigating the regulation of host immunity at cellular and molecular scales that suggested numerous mechanisms and paths for this regulation.[5,6,7] Most drugs used, including mitoxantrone, Avonex, and beta-interferon are immune modulators and prevent the incidence of symptoms and progress of the disease.[8,9] Given issues discussed, and mechanisms of drugs mentioned, methods that can prevent attacks on self-antigens and the incidence of autoimmune diseases without suppressing the immune system could be effective in control of the disease. In animal models, there is a correlation between parasitic infections and autoimmune diseases such as MS, and these infections can cause modulation of the immune system, and consequently remitting the symptoms of autoimmune diseases.[3,4] In this study we aimed to compare the frequency of blood tissue parasitic infections in patients with MS as compared to their family members.
METHODS
This descriptive-analytical study was conducted on 50 relapsing-remitting MS patients resident in Isfahan Province and 50 family members cohabiting with the patients, attending MS clinic at Alzahra Hospital. The study commenced after explaining the objectives of the project, completing the questionnaires and obtaining their written consents. To prepare serums for measuring IgM and IgG anti-Toxoplasma gondii, blood samples were centrifuged without the anticoagulation agent, and serums were tested for anti-T. gondii IgG and IgM antibodies titers by commercially available enzyme immunoassays (Euroimmun®, Germany) with an automatic microplate spectrophotometer (Bio-Tek Instruments Inc., USA). Analyses were performed as instructed by the manufacturers, at the School of Medicine, Isfahan University of Medical Sciences. Positive and negative controls were used with each series of ant-T. gondii IgG/IgM test. Results were obtained by comparison with a cut-off value measured at 450 nm absorbance. According to the manufacturer instruction of the kit, an ISR ≥ 13.05 was considered as positive. Meanwhile, given the prevalence of cutaneous leishmaniasis in Isfahan region, the participants were examined for protozoan leishmania microscopically. The samples were taken of those participants with fresh wounds, and after preparing smear slides, they received final approval using giemsa staining, but the other group with only a scar was analyzed in the group with the history of this disease.
To investigate malaria parasite, peripheral blood smears were prepared from blood samples taken from the participants. The prepared smears were examined for malaria infection by trained personnel. We used SPSS 20.0 software (SPSS Inc., Chicago, Illinois, USA) for analyzing data. The Chi-square and Student's t-test was used to test statistically significant differences. Statically significance is accepted at (P > 0.05).
RESULTS
The distribution of IgG antibody titers of serum samples measured by ELISA is shown in Table 1. A total of 18 patients and 24 of their family members had a positive test in IgG T. gondii. Chi-square test revealed not significant differences between the two groups. In both groups, there was no positive IgM T. gondii, indicating the absence of active disease in the two groups. Of the total population, 42.4% of tests are positive IgG T. gondii (P = 0.09).
Table 1.
Comparison of the results of the IgM and IgG ELISA in the case and control groups
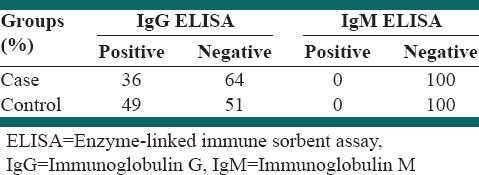
The mean duration of MS disease in the participants that tested positive for IgG T. gondii was 44.6 ± 10.1 months, and for the participants with negative test results, the mean duration was 44.3 ± 8.9 months. Independent t-test showed that the mean duration of disease had no significant difference with positive IgG T. gondii test (P = 0.98). In investigating leishmania, only 3 participants A total of 18 patients and 24 of their family members positive, and Chi-square test showed no significant difference between the two groups (P = 0.25). No case of malaria was found among the participants.
Nine persons in patient's group and 22 in the control group had a history of parasitic diseases. The Chi-square test showed that this difference was not significant (P = 0.05). A history of parasitic disease in the case group 18.4% and the control group was 44.9%. A significant relationship between a parasitic disease history and risk of MS between the two groups was observed (P = 0.001).
DISCUSSION
In this study, attempts were made to examine the relationship between MS and a few parasitic diseases. This current information reflects the endemicity of protozoan blood and tissue infections in 50 MS patients and 50 family members of these patients.
In both groups, there was no positive IgM T. gondii, indicating the absence of active disease in the two groups. It was reported that T. gondii seroprevalence ranges from 12% to 91% in Iranian population.[10] In the present study, we have shown that the prevalence of toxoplasmosis in patients and their family members was 36% and 49% respectively. This rate was similar to previous data of Mostafavi et al. who reported a 41.4% positive rate in Isfahan (center of Iran) in the total population.[11] In man, evidence of infection has been found in all population groups investigated prevalence rates vary from place to place. The prevalence of toxoplasmosis is related to several factors, including culture, nutritional habits and type of diet, age, and a rural or urban setting. Drinking contaminated water, the role of domestic and cats has been not fully assessed.[10]
The results shed light the relationship between these diseases and a mismatch with hypotheses examined. However, considering that the prevalence of toxoplasmosis, leishmaniasis, and malaria varies with time, and depends on numerous epidemiological factors, hence, at the time of this study, this rate was low in the control group, as well. These results do not discredit the theory investigated. It is recommended that these theories should be verified with a larger sample size, or by infecting animal models with these parasites to examine the clinical changes in MS disease in them.
CONCLUSIONS
Our results showed a mismatch with hygiene hypotheses examined. However, considering that the prevalence of parasites varies with time, and depends on numerous epidemiological factors; these results do not discredit the theory investigated.
ACKNOWLEDGMENTS
The results of this study are dedicated to MS patients who bravely cope with illness.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Ahlgren C, Lycke J, Odén A, Andersen O. High risk of MS in Iranian immigrants in Gothenburg, Sweden. Mult Scler. 2010;16:1079–82. doi: 10.1177/1352458510376777. [DOI] [PubMed] [Google Scholar]
- 2.La Flamme AC, Ruddenklau K, Bäckström BT. Schistosomiasis decreases central nervous system inflammation and alters the progression of experimental autoimmune encephalomyelitis. Infect Immun. 2003;71:4996–5004. doi: 10.1128/IAI.71.9.4996-5004.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ascherio A, Munger KL. Epstein-barr virus infection and multiple sclerosis: A review. J Neuroimmune Pharmacol. 2010;5:271–7. doi: 10.1007/s11481-010-9201-3. [DOI] [PubMed] [Google Scholar]
- 4.Koch-Henriksen N, Sørensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 2010;9:520–32. doi: 10.1016/S1474-4422(10)70064-8. [DOI] [PubMed] [Google Scholar]
- 5.Osada Y, Kanazawa T. Parasitic helminths: New weapons against immunological disorders. J Biomed Biotechnol 2010. 2010 doi: 10.1155/2010/743758. 743758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carvalho L, Sun J, Kane C, Marshall F, Krawczyk C, Pearce EJ. Review series on helminths, immune modulation and the hygiene hypothesis: Mechanisms underlying helminth modulation of dendritic cell function. Immunology. 2009;126:28–34. doi: 10.1111/j.1365-2567.2008.03008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Everts B, Smits HH, Hokke CH, Yazdanbakhsh M. Helminths and dendritic cells: Sensing and regulating via pattern recognition receptors, Th2 and Treg responses. Eur J Immunol. 2010;40:1525–37. doi: 10.1002/eji.200940109. [DOI] [PubMed] [Google Scholar]
- 8.Kingwell E, Koch M, Leung B, Isserow S, Geddes J, Rieckmann P, et al. Cardiotoxicity and other adverse events associated with mitoxantrone treatment for MS. Neurology. 2010;74:1822–6. doi: 10.1212/WNL.0b013e3181e0f7e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nonchev BI. Cases of interferon-alpha and interferon-beta-induced thyroiditis. Folia Med (Plovdiv) 2010;52:5–12. doi: 10.2478/v10153-010-0001-6. [DOI] [PubMed] [Google Scholar]
- 10.Mostafavi S.N, Jalali Monfared L. “Toxoplasmosis epidemiology in Iran: A systematic review”. J Isfahan Med School. 2012;30:1–15. [Google Scholar]
- 11.Mostafavi SN, Ataei B, Nokhodian Z, Yaran M, Babak A. Seroepidemiology of Toxoplasma gondii infection in Isfahan province, central Iran: A population based study. J Res Med Sci. 2011;16:496–501. [PMC free article] [PubMed] [Google Scholar]


