Abstract
Background:
Nephrotoxicity is the major side-effect of cisplatin (CDDP), and it is reported to be gender-related. We evaluated the effects of pomegranate flower extract (PFE) as an antioxidant on CDDP-induced nephrotoxicity in female rats.
Methods:
Twenty-three adult female rats in four groups treated as following. Groups 1 and 2 received PFE at doses of 25 and 50 (mg/kg/day), respectively, for 9 days, and from day 3 on, they also received cisplatin (CDDP) (2.5 mg/kg) daily. Group 3 was treated as group 1 expects saline instead of PFE, and group 4 received PFE (25 mg/kg/day) alone.
Results:
Cisplatin alone increased the serum levels of blood urea nitrogen, creatinine, and nitrite; and kidney tissue damage score and kidney weight. However, PFE not only did not ameliorate the induced nephrotoxicity, but also aggravated renal tissue damage.
Conclusions:
Pomegranate extract as an antioxidant did not ameliorate CDDP-induced nephrotoxicity in female rats.
Keywords: Cisplatin, female rats, nephrotoxicity, pomegranate
INTRODUCTION
Cisplatin (CDDP), as an antitumor drug, is widely used for chemotherapy.[1] CDDP-induced nephrotoxicity is related to its accumulation in the proximal tubule cells,[2] and synthetic or herbal agents have been investigated as supplementations against CDDP-induced nephrotoxicity.[3,4] Previous studies showed that some antioxidant agents have protective effect against CDDP-induced nephrotoxicity in male but not in female[5,6,7] while nephrotoxicity induced by CDDP may be gender-related,[8,9] and presence of estrogen aggravates nephrotoxicity induced by CDDP in ovariectomized female rats.[10,11,12]
Herbs are often administrated in combination with chemical drugs, which may enhance the potential of herb-drug antioxidant activity.[13] Several flavonoids, including quercetin and some other polyphenols are found in Morus alba, Zingiber, and garlic, which have been used against kidney toxicity induced by nephrotoxins.[14,15,16]
Pomegranate has antioxidant, antiinflammatory, and antimicrobial effects.[17,18,19,20] Thus, the current study was designed to investigate whether pomegranate flower extract (PFE) could have nephroprotective role against CDDP in female rats.
METHODS
To prepare PFE, 500 g of pomegranate flower was provided and powdered. The hydroalcoholic extract was prepared by ethanol:water (70:30) mixture using percolation method. The extract was concentrated and dried to obtain 123 g pure powder. Total phenolic content of the extract was estimated by the Folin–Ciocalteu method. Briefly, 20 μl extract 85% plus 1.58 ml deionized water and 100 μl Folin–Ciocalteu reagents were mixed. After 30 s, 30 μl Na2CO3 was added to the mixture and incubated at 20°C for 2 h. Finally, the absorbance was read at 765 nm. Total phenolic content of the extract was reported to be 9.98% of gallic acid.
Twenty-three adult female Wistar rats weighting 166.1 ± 2.3 g in four groups were housed at the temperature of 23–25°C, and they had free access to water and rat chow. They were treated as following. Groups 1 (n = 6) and 2 (n = 6) respectively received PFE at the doses of 25 and 50 mg/kg/day for 9 days, and from day 3 on they also received CDDP (2.5 mg/kg/day, intraperitoneal injection). CDDP was purchased from EBEWE Pharma Ges.m.b.H, Austria. Group 3 (n = 6) was treated as group 1 expect saline instead of PFE, and group 4 (n = 5) received PFE (25 mg/kg/day) alone. At the end of the study, all animals were sacrificed after blood sampling. The kidneys and uterus were excised and weighed immediately. The left kidney was used for histopathological investigations via hematoxylin and eosin staining. The intensity of tubular injuries was graded by a pathologist from 1 to 4, while zero score was assigned to normal tissue without damage.
Serum levels of creatinine (Cr) and blood urea nitrogen (BUN) were determined using quantitative diagnostic kits (Pars Azmoon, Iran). The serum level of nitrite (stable NO metabolite) was measured using a colorimetric assay method that involves the Griess reaction. Serum and kidney levels of malondialdehyde (MDA) were quantified according to the manual method.
Statistical analysis
The data obtained are presented as mean ± standard error of the mean. The quantitative data was compared by analysis of variance using latent difference score as the posttest. Since the scoring was qualitative, the Mann–Whitney test was applied to compare the pathology damage scores among the groups. P < 0.05 was considered as statistically significant.
RESULTS
CDDP induced body weight loss and increased kidney weight significantly (P < 0.05). This agent itself also significantly elevated the serum levels of BUN, Cr, and nitrite; and also kidney tissue damage score (P < 0.05). Furthermore, it increased the serum level of MDA insignificantly but it did not alter the uterus weight. Administration of PFE did not ameliorate the levels of the aforementioned parameters. However, combination of PFE and CDDP increased and decreased the serum levels of BUN and nitrite, respectively (P < 0.05) [Figure 1].
Figure 1.
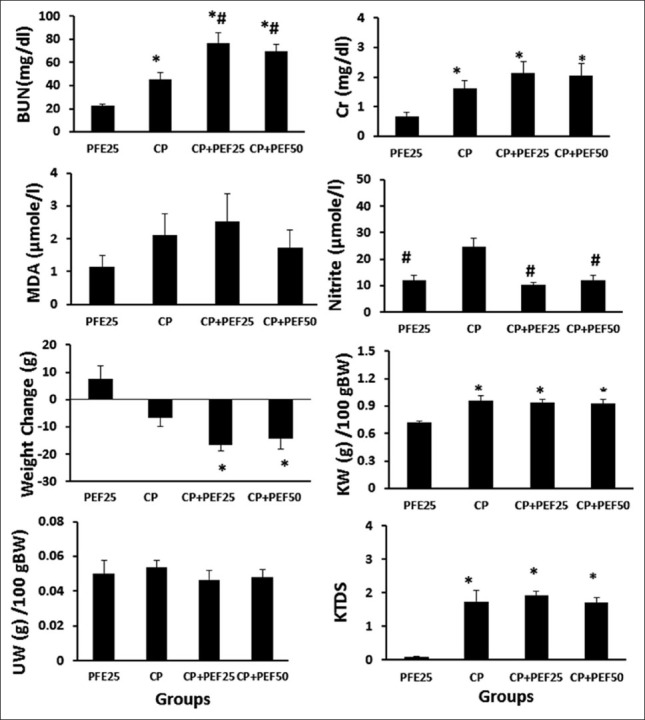
Serum levels of blood urea nitrogen, creatinine, malondialdehyde, and nitrite; weight change, kidney weight/100 g body weight, uterus weight/100 g body weight and kidney tissue damage score in the four experimental groups. *and #indicate significant difference from pomegranate flower extract 25 and concentrated pomegranate groups, respectively (P < 0.05)
DISCUSSION
Today, employment of herbal compounds has become an accepted approach to ameliorate side effects induced by chemotherapy drugs.[21,22] Pomegranate contains polyphenols and antioxidant capacity.[23,24,25,26] The main purpose of this study was to investigate the protective role of PFE against CDDP-induced nephrotoxicity in female rats. Our results showed that PFE had no protective effect on nephrotoxicity induced by CDDP in female rats. Recently, we had performed a series of studies to investigate the role of gender difference in nephrotoxicity induced by CDDP.[6,7,9] We showed that CDDP-induced nephrotoxicity is gender-related, and males are vulnerable more than females.[10,11] CDDP induced nephrotoxicity due to free radicals and reactive oxygen species that are involved in CDDP-induced oxidative stress.[27]
Previously, we used both single (7 mg/kg) and continuous dose of CDDP in female rats[5,6,7,8,9,28] which was accompanied with different responses. Moreover, administration of PFE did not ameliorate tissue damage and renal dysfunction. Antioxidants such as losartan, Vitamin E, Vitamin C, and L-argentine did not ameliorate nephrotoxicity induced by CDDP in female rats while they were nephroprotective in male rats.[6,10,29] Evidences also showed that the presence of estrogen may alter the effects of these supplementations.[10,11] It is reported that the low dose of PFE 25 mg/kg/day exhibits a protective role against CDDP in male rats,[30] however such results was not seen in the current study in female rats.
Nitric oxide, as a vasodilator agent, involves in kidney circulation.[31] CDDP-induces endothelial dysfunction and increases inducible nitric oxide synthase (iNOS) level and iNOS involves in CDDP-induced toxicity.[32,33] We observed that CDDP increases the serum level of nitrite probably by increasing the iNOS level, but PFE could ameliorate this increase. This finding is in agreement with other studies, which indicated the effect of pomegranate juice on NO level.[34]
CONCLUSIONS
It is concluded that administration of PFE not only did not ameliorate nephrotoxicity induced by CDDP in female rats, but also aggravated renal damage, while the extract was protective against CDDP in male rats.[30]
ACKNOWLEDGMENTS
This research was supported by Isfahan University of Medical Sciences.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Florea AM, Büsselberg D. Cisplatin as an anti-tumor drug: Cellular mechanisms of activity, drug resistance and induced side effects. Cancers (Basel) 2011;3:1351–71. doi: 10.3390/cancers3011351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Townsend DM, Deng M, Zhang L, Lapus MG, Hanigan MH. Metabolism of Cisplatin to a nephrotoxin in proximal tubule cells. J Am Soc Nephrol. 2003;14:1–10. doi: 10.1097/01.asn.0000042803.28024.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atessahin A, Yilmaz S, Karahan I, Ceribasi AO, Karaoglu A. Effects of lycopene against cisplatin-induced nephrotoxicity and oxidative stress in rats. Toxicology. 2005;212:116–23. doi: 10.1016/j.tox.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 4.Atessahín A, Ceríbasi AO, Yuce A, Bulmus O, Cikim G. Role of ellagic acid against cisplatin-induced nephrotoxicity and oxidative stress in rats. Basic Clin Pharmacol Toxicol. 2007;100:121–6. doi: 10.1111/j.1742-7843.2006.00015.x. [DOI] [PubMed] [Google Scholar]
- 5.Haghighi M, Nematbakhsh M, Talebi A, Nasri H, Ashrafi F, Roshanaei K, et al. The role of angiotensin II receptor 1 (AT1) blockade in cisplatin-induced nephrotoxicity in rats: Gender-related differences. Ren Fail. 2012;34:1046–51. doi: 10.3109/0886022X.2012.700886. [DOI] [PubMed] [Google Scholar]
- 6.Eshraghi-Jazi F, Nematbakhsh M, Nasri H, Talebi A, Haghighi M, Pezeshki Z, et al. The protective role of endogenous nitric oxide donor (L-arginine) in cisplatin-induced nephrotoxicity: Gender related differences in rat model. J Res Med Sci. 2011;16:1389–96. [PMC free article] [PubMed] [Google Scholar]
- 7.Eshraghi-Jazi F, Nematbakhsh M, Pezeshki Z, Nasri H, Talebi A, Safari T, et al. Sex differences in protective effect of recombinant human erythropoietin against cisplatin-induced nephrotoxicity in rats. Iran J Kidney Dis. 2013;7:383–9. [PubMed] [Google Scholar]
- 8.Nematbakhsh M, Talebi A, Nasri H, Safari T, Dolatkhah S, Ashrafi F, et al. Some evidence for sex-based differences in cisplatin-induced nephrotoxicity in rats. Clin Exp Med Lett. 2012;53:29–32. [Google Scholar]
- 9.Nematbakhsh M, Ebrahimian S, Tooyserkani M, Eshraghi-Jazi F, Talebi A, Ashrafi F. Gender difference in Cisplatin-induced nephrotoxicity in a rat model: Greater intensity of damage in male than female. Nephrourol Mon. 2013;5:818–21. doi: 10.5812/numonthly.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nematbakhsh M, Pezeshki Z, Eshraghi-Jazi F, Ashrafi F, Nasri H, Talebi A, et al. Vitamin E, vitamin C, or losartan is not nephroprotectant against cisplatin-induced nephrotoxicity in presence of estrogen in ovariectomized rat model. Int J Nephrol. 2012;2012:1–7. doi: 10.1155/2012/284896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pezeshki Z, Nematbakhsh M, Mazaheri S, Eshraghi-Jazi F, Talebi A, Nasri H, et al. Estrogen Abolishes Protective Effect of Erythropoietin against Cisplatin-Induced Nephrotoxicity in Ovariectomized Rats. ISRN Oncol 2012. 2012 doi: 10.5402/2012/890310. 890310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pezeshki Z, Nematbakhsh M, Nasri H, Talebi A, Pilehvarian AA, Safari T, et al. Evidence against protective role of sex hormone estrogen in Cisplatin-induced nephrotoxicity in ovarectomized rat model. Toxicol Int. 2013;20:43–7. doi: 10.4103/0971-6580.111568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Surh YJ. Anti-tumor promoting potential of selected spice ingredients with antioxidative and anti-inflammatory activities: A short review. Food Chem Toxicol. 2002;40:1091–7. doi: 10.1016/s0278-6915(02)00037-6. [DOI] [PubMed] [Google Scholar]
- 14.Nematbakhsh M, Hajhashemi V, Ghannadi A, Talebi A, Nikahd M. Protective effects of the Morus alba L. leaf extracts on cisplatin-induced nephrotoxicity in rat. Res Pharm Sci. 2013;8:71–7. [PMC free article] [PubMed] [Google Scholar]
- 15.Nasri H, Nematbakhsh M, Rafieian-Kopaei M. Ethanolic extract of garlic for attenuation of gentamicin-induced nephrotoxicity in Wistar rats. Iran J Kidney Dis. 2013;7:376–82. [PubMed] [Google Scholar]
- 16.Nasri H, Nematbakhsh M, Ghobadi S, Ansari R, Shahinfard N, Rafieian-Kopaei M. Preventive and curative effects of ginger extract against histopathologic changes of gentamicin-induced tubular toxicity in rats. Int J Prev Med. 2013;4:316–21. [PMC free article] [PubMed] [Google Scholar]
- 17.Boroushaki MT, Asadpour E, Sadeghnia HR, Dolati K. Effect of pomegranate seed oil against gentamicin-induced nephrotoxicity in rat. J Food Sci Technol. 2014;51:3510–14. doi: 10.1007/s13197-012-0881-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibrahium M. Efficiency of pomegranate peel extract as antimicrobial, antioxidant and protective agents. World J Agric Sci. 2010;6:338–44. [Google Scholar]
- 19.Ali NAM, Saeed SZ. Nephro-protective effect of Punica granatum in gentamicin-induced nephrotoxicity in rats. Medical Journal of Babylon. 2012;9:220–8. [Google Scholar]
- 20.Zhang L, Yang X, Zhang Y, Wang L, Zhang R. In vitro antioxidant properties of different parts of pomegranate flowers. Food and Bioproducts Process. 2011;89:234–40. [Google Scholar]
- 21.Mohana L, Kiran U, Rani KS. A review on medicinal plants for nephroprotective activity. Asian J Pharm Clin Res. 2012;5:8–14. [Google Scholar]
- 22.Cayir K, Karadeniz A, Simsek N, Yildirim S, Karakus E, Kara A, et al. Pomegranate seed extract attenuates chemotherapy-induced acute nephrotoxicity and hepatotoxicity in rats. J Med Food. 2011;14:1254–62. doi: 10.1089/jmf.2010.0286. [DOI] [PubMed] [Google Scholar]
- 23.Chalfoun-Mounayar A, Nemr R, Yared P, Khairallah S, Chahine R. Antioxidant and weight loss effects of pomegranate molasses. J Appl Pharm Sci. 2012;2:45–50. [Google Scholar]
- 24.Kaur G, Jabbar Z, Athar M, Alam MS. Punica granatum (pomegranate) flower extract possesses potent antioxidant activity and abrogates Fe-NTA induced hepatotoxicity in mice. Food Chem Toxicol. 2006;44:984–93. doi: 10.1016/j.fct.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Patel C, Dadhaniya P, Hingorani L, Soni MG. Safety assessment of pomegranate fruit extract: Acute and subchronic toxicity studies. Food Chem Toxicol. 2008;46:2728–35. doi: 10.1016/j.fct.2008.04.035. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed MM, Ali SE. Protective effect of pomegranate peel ethanol extract against ferric nitrilotriacetate induced renal oxidative damage in rats. J Cell Mol Biol. 2010;7,8:35–43. [Google Scholar]
- 27.Matsushima H, Yonemura K, Ohishi K, Hishida A. The role of oxygen free radicals in cisplatin-induced acute renal failure in rats. J Lab Clin Med. 1998;131:518–26. doi: 10.1016/s0022-2143(98)90060-9. [DOI] [PubMed] [Google Scholar]
- 28.Rastghalam R, Nematbakhsh M, Bahadorani M, Eshraghi-Jazi F, Talebi A, Moeini M, et al. Angiotensin Type-1 Receptor Blockade May Not Protect Kidney against Cisplatin-Induced Nephrotoxicity in Rats. ISRN Nephrol 2014. 2014 doi: 10.1155/2014/479645. 479645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jilanchi S, Nematbakhsh M, Bahadorani M, Talebi A, Eshraghi-Jazi F, Mansouri A, et al. Vitamin e is a nephroprotectant agent in male but not in female in a model of Cisplatin-induced nephrotoxicity. ISRN Nephrol 2013. 2013 doi: 10.5402/2013/280395. 280395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Motamedi F, Nematbakhsh M, Monajemi R, Pezeshki Z, Talebi A, Zolfaghari B, et al. Effect of pomegranate flower extract on cisplatin-induced nephrotoxicity in rats. J Nephropathol. 2014:3. doi: 10.12860/jnp.2014.26. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garg UC, Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989;83:1774–7. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watanabe K, Hess A, Bloch W, Michel O. Expression of inducible nitric oxide synthase (iNOS/NOS II) in the vestibule of guinea pigs after the application of cisplatin. Anticancer Drugs. 2000;11:29–32. doi: 10.1097/00001813-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Srivastava RC, Farookh A, Ahmad N, Misra M, Hasan SK, Husain MM. Evidence for the involvement of nitric oxide in cisplatin-induced toxicity in rats. Biometals. 1996;9:139–42. doi: 10.1007/BF00144618. [DOI] [PubMed] [Google Scholar]
- 34.de Nigris F, Williams-Ignarro S, Lerman LO, Crimi E, Botti C, Mansueto G, et al. Beneficial effects of pomegranate juice on oxidation-sensitive genes and endothelial nitric oxide synthase activity at sites of perturbed shear stress. Proc Natl Acad Sci U S A. 2005;102:4896–901. doi: 10.1073/pnas.0500998102. [DOI] [PMC free article] [PubMed] [Google Scholar]


