Abstract
The collimator in single photon emission computed tomography (SPECT), is an important part of the imaging chain. One of the most important collimators that used in research, preclinical study, small animal, and organ imaging is the pinhole collimator. Pinhole collimator can improve the tradeoff between sensitivity and resolution in comparison with conventional parallel-hole collimator and facilities diagnosis. However, a major problem with pinhole collimator is a small field of view (FOV). Multi-pinhole collimator has been investigated in order to increase the sensitivity and FOV with a preserved spatial resolution. The geometry of pinhole and multi-pinhole collimators is a critical factor in the image quality and plays a key role in SPECT imaging. The issue of the material and geometry for pinhole and multi-pinhole collimators have been a controversial and much disputed subject within the field of SPECT imaging. On the other hand, recent developments in collimator optimization have heightened the need for appropriate reconstruction algorithms for pinhole SPECT imaging. Therefore, iterative reconstruction algorithms were introduced to minimize the undesirable effect on image quality. Current researches have focused on geometry and configuration of pinhole and multi-pinhole collimation rather than reconstruction algorithm. The lofthole and multi-lofthole collimator are samples of novel designs. The purpose of this paper is to provide a review on recent researches in the pinhole and multi-pinhole collimators for SPECT imaging.
Keywords: Image quality, multi-pinhole collimator, pinhole collimator, reconstruction algorithm, single photon emission computed tomography
Introduction
Single photon emission computed tomography (SPECT) is a nuclear medicine imaging modality where two-dimensional projections are acquired with a gamma camera and the projections are used for reconstruction of a three-dimensional image volume. The projections are distorted by several factors, including attenuation and scattering of gamma ray, collimator structure, data acquisition, reconstruction method, and organ motions. The collimator in SPECT is a crucial component of the imaging chain that controls the noise, resolution, and sensitivity of the final functional image.[1,2,3,4,5,6,7,8] Specialized collimators are used for a specific imaging task, for example the fan beam collimator for brain imaging,[9] cone beam collimator for posterior portion of the thalamus,[10] slit slat collimator for radionuclide imaging with high-energy gamma ray emission,[11,12] and parallel-hole collimators that routinely use in SPECT and planar imaging. The pinhole and multi-pinhole collimators used for research, preclinical study, small animal, and focal uptake nuclear medicine imaging. The main objective of this research is to review the pinholes and multi-pinholes that recently have been used in clinical and preclinical nuclear medicine imaging.
Pinhole Collimator
Pinhole collimators have a single hole that drilled into the sheet with high atomic number material. Lead and tungsten generally used for fabricating the pinhole collimator. This collimator generates magnified images.[13] Smaller pinhole diameter leads to an improvement the spatial resolution, but also a loss in sensitivity.[14] Pinhole collimators are widely used for SPECT imaging of small organ or small region of interest such as the thyroid,[15] the parathyroid glands,[16,17] the breast,[18,19,20,21,22] the knee joints and shoulder,[23,24] or physiological imaging for the small animals.[25,26,27,28,29] Pinhole collimator is used to obtain a superior spatial resolution compared to conventional parallel-hole collimator [Figure 1].[30,31] Pinhole collimator provides a smaller field of view (FOV) in comparison with a parallel-hole collimator, so these collimators are suited to image the small organs of the body or focal uptake.[32] In the pinhole collimator, penetration and scattering of gamma radiation at the edge of the aperture, also the attenuation coefficient of the aperture material have a significant effect on the spatial resolution of the system.[33,34] Small pinhole opening angle makes reduce penetration and short object to collimator distance improve sensitivity and spatial resolution.[35] The sensitivity of a parallel-hole collimator is almost constant as a function of distance from the collimator; however, the sensitivity of a pinhole collimator depends on the inverse square of the distance from the pinhole aperture. It also increases as the square of the pinhole diameter with simultaneous linearly loss in spatial resolution. The magnification factor of a pinhole is determined by the ratio of the pinhole-to-detector distance relative to the pinhole-to-source distance.[36] With increased magnification factor, the detector intrinsic resolution has decreased effect on the total system spatial resolution. Therefore, a pinhole collimator is the most effective for imaging a small object placed close to the pinhole aperture.[37]
Figure 1.

The images of 99mTc-sestamibi from thyroide at 15 min in a patient with hyperparathyroidism acquired with (a) pinhole and (b) parallel-hole collimators
Multi-Pinhole Collimator
Multi-pinhole collimator is used for superior spatial resolution trade off sensitivity compared to pinhole collimator. Since the single pinhole collimator suffers from the low detection efficiency, it has been investigated to increase the sensitivity and FOV with preserved spatial resolution. In multi-pinhole collimator, multiple projections through the different pinholes provide relatively efficient coverage of the detector area while multiplexing effect can make a problem for image reconstruction. If the projections from different apertures overlap, then the multiplexing artifacts occurred. The primary limitation in multi-pinhole collimator design includes the overlap of the projections from different pinholes.[38,39] Irregular apertures configuration could suppress this artifact.[40] In some cases, perpendicular lead septa were employed to acquire nonoverlapping projections.[41,42] Multi-pinhole collimation was used in the early days of nuclear cardiology to provide multiple angle views of the myocardium.[43,44] With advances in iterative image reconstruction, multi-pinhole collimators have made a come-back in nuclear cardiology.[45] The new model assumes that the multi-pinhole collimator and the detector with spherical layer shape can optimal resolution and sensitivity.[46] Multi-pinhole SPECT provides for sub-millimeter resolution imaging of the radio molecule distribution in small laboratory animals.[47]
Pinhole Geometry
The most important reasons of error in quantitative pinhole SPECT are the photon penetrating throughout the edge of the pinhole aperture, photon scattered by the object, photon scattered by the collimator material, and attenuation of photons within the object. The magnitudes of these components highly depend on collimator geometry.[48] Two types of pinhole collimators commonly used in SPECT imaging, channel-edge, and knife-edge shaped [Figure 2]. The channel-edge pinholes were designed in order to reduce the penetration of gamma rays through the edge of the pinhole aperture caused by the large acceptance angle of knife-edge pinhole.[49,50] Compared to the channel-edge, the penetration, object scatter, and collimator scatter for knife-edge are higher than channel-edge.[48] At perpendicular incidence of the photons, the channel-edge has lower penetration and scatter fractions rather than knife-edge.[51] The advantage from channel-edge pinhole of lower penetration and scatter contributions significantly diminishes at nonperpendicular incident. Furthermore, the channel-edge pinholes have a lower sensitivity than knife-edge pinholes.[52] By increasing the pinhole diameter, the penetration and the scattered photon approximately decreased by the linear function for the knife-edge micro-pinhole. For the knife edge micro-pinhole, platinum and gold are superior rather than tungsten and lead with regard to pinhole scatter while platinum performs slightly better than gold.[51] Song et al. showed the penetration and the scatter fraction for pinhole collimators with a tungsten aperture in channel shape is preferable over knife edge and the optimal range of channel height have an average from 0.3 to 0.6 mm.[53] 131I imaging is challenging because the primary photon is emitted at 364 keV, and the penetration through the pinhole aperture is significant, which will degrade SPECT image resolution. The tungsten pinhole with channel edge shapes has an advantage over lead for high resolution 131I imaging due to reduced photon penetration near the pinhole aperture.[54] Briefly, the purpose of designing a channel height collimator is to reduce photon penetration near the pinhole aperture.
Figure 2.

Two types of the pinhole collimators that routinely used in pinhole single photon emission computed tomography imaging: (a) Knife-edge collimator and (b) channel-edge pinhole collimator
Reconstruction Algorithms for Pinhole Single Photon Emission Computed Tomography
The most reconstruction algorithms used in pinhole imaging are the filtered back projection (FBP) method proposed by Feldkamp, Davis, and Kress (FDK).[55] Ramp filter routinely used during the reconstruction.[56,57] If a FBP method was used for pinhole SPECT combined with mechanical shift correction, the doughnut type artifact could correct.[58] The pinhole SPECT data with a circular orbit acquisition do not satisfy the data sufficiency condition, so pinhole SPECT restricted by artifacts, mainly axial blurring in the noncentral slices. The iterative reconstruction algorithm[59] can reduce these artifacts in comparison with FBP. The expectation maximization (EM) algorithm could improve the quality of pinhole SPECT, but the long reconstruction times make this method less practical in clinical usage in comparison with FDK algorithm.[57] The maximum likelihood EM (ML-EM) reconstruction algorithm can use in the pinhole SPECT.[60] This method improves image quality compared with FBP, but the major drawback of ML-EM is increased noise along the iteration. Postreconstruction filters are used to remove noise, but these filters reduce the spatial resolution, contrast and have a tendency to blur image edge. The noise created by ML-EM can also reduce using Bayesian method.[61] The median root prior (MPR) is a new Bayesian reconstruction algorithm which has mainly used in positron emission tomography.[62] MPR is adapted for pinhole SPECT and can reduce the noise generated from ML-EM algorithm.[63] The ordered subsets EM (OS-EM) algorithm is a type of iterative reconstruction algorithm, and its function depends on the number of subset and iteration. The processing time with one iteration and eight subsets is only 2.5 times longer compared with FDK. This algorithm significantly improves the reconstructed image quality, also suppresses the streak artifacts in comparison with FBP.[64] The primary attraction of OS-EM versus FBP is the absence of noise amplification.[65] The insufficient projection data acquired by a single circular pinhole orbit are the mainly cause of blurring. A pinhole SPECT by two circular orbits and a three-dimensional OS-EM can provide complete projection data, furthermore this method disappears the blurring edge in the reconstructed image. This imaging method can improve axial blurring and resolution uniformity in pinhole SPECT imaging and have a potential to become the standard tool for in vivo quantification of physiological functions in small animal laboratory [Figure 3].[66]
Figure 3.

The whole body scan images of 99mTc-hydroxymethylene diphosphonate from a mouse were acquired with a pinhole collimator and reconstructed by (a) the filtered back projection method for single-orbit data and (b) three-dimensional ordered subsets expectation maximization method for two-orbit data
The main goal of SPECT in preclinical molecular imaging by multi-pinhole collimator is to measure the concentration radiolabeled biomolecules in in vivo, so the quantification is important. Accuracy of the reconstructed image is not sufficient due to the partial volume effect. Anatomy-based reconstruction using micro-computed tomography information has ability to decrease the partial volume effect and improve the quantifying accuracy in multi-pinhole SPECT.[67]
The New Configuration of Pinhole and Multi-Pinhole Collimator
A blended collimator uses the two different collimators, pinhole and multi-pinhole collimator, installed on each detector in the SPECT imaging system. The specific reconstruction software applied to create a single image that combined from the two collimators data acquisitions, so a high quality image can be achieved.[68] Most multi-pinhole collimators prevent the multiplexing effect by using an additional shielding or by spacing far apart the pinholes, but additional shielding has the drawback of increase weight, designing complexity and cost, also spacing far apart the pinholes causes the noneffective use of detector. This is due to the circular projections from pinholes on the detector. New pinhole collimator geometry, the lofthole collimator, has a circular aperture and a rectangular entrance/exit opening, so it has a rectangular projection on the detector. The lofthole collimator irradiates precisely the surface of the square detector [Figure 4]. This has the benefit that no extra shielding is necessary in case of a multi-pinhole collimator and that the amount of penetrating photons is lower than with an equivalent pinhole. The lofthole collimator has less penetration than the knife-edge pinhole collimator. Multi-lofthole collimator has high detector coverage without additional shielding. The most advantage of this collimator is the lower amount of penetration compared to multi-pinhole collimator [Figure 5].[69,70,71,72,73,74] The drawback of the pinhole and multi-pinhole collimators includes cone beam imaging geometry, so that does not provide a complete data as the detector rotates around the object. For reducing the data-insufficiency artifacts, the SPECT imaging needs the small cone angle in the axial direction. A multi skew-slit collimator has a single vertical slit and several horizontal slits. The slits prepare separate projections with small cone angle and small magnification in the axial direction, which makes less severe data-insufficiency artifacts; also partly larger cone angle and magnification in the transaxial direction provide a better detector area usage comparison with multi-pinhole collimator [Figure 6]. Thus, multi skew-slit collimator provided an appropriate transaxial resolution, detector area usage, and minimum multiplexing effect.[75,76]
Figure 4.
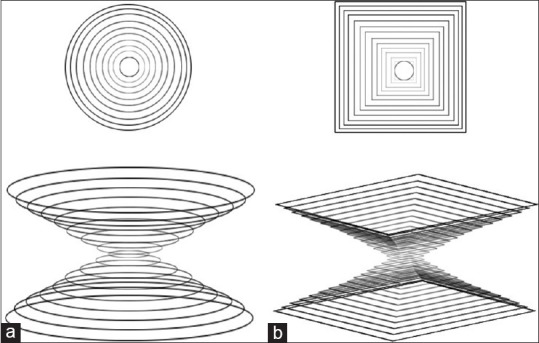
Schematic of (a) a pinhole collimator and (b) a lofthole collimator with a circular aperture and a rectangular entrance/exit opening. This type of collimator creates the rectangular projection on the detector
Figure 5.

Projections from (a) a multi-pinhole collimator that do not fully coverage the detector, (b) multiplexing effect in a multi-pinhole collimator, and (c) projections created from a multi lofthole collimator without the multiplexing effect
Figure 6.

A spheric object is imaged by (a) a pinhole collimator; a circular projection was created, (b) a skew slit collimator; an elliptical projection was created. A small cone angle in the axial direction could reduce the data insufficiency artifacts, (c) a multipinhole collimator, and (d) a multi skew slit collimator provide a desire transaxial resolution, detector area usage, and minimum multiplexing effect
Conclusion
Collimator is a very important component in SPECT as critical equipment in sensitivity and resolution of the system. Many types of collimators are used for nuclear medicine imaging. Pinhole collimators used for SPECT imaging of small animals or small organs like thyroid. Pinhole collimator often provides a superior resolution-sensitivity tradeoff for radionuclide imaging compared to SPECT with parallel-hole collimator. Multi-pinhole collimator has been invented to increase the FOV, sensitivity, and detection efficiency without degrading spatial resolution. Channel-edge and knife-edge pinhole collimators are the routinely collimators that used in pinhole SPECT imaging. The magnitude of penetration and scatter for channel-edge or Knife-edge pinhole collimators depends on the collimator material and the energy of radionuclide. The type of reconstruction algorithms in pinhole SPECT imaging is very important because the quality of the final image is relevant to this item. The most reconstruction algorithm that used in pinhole SPECT is the FDK FBP algorithm. The interested reconstruction algorithm for utilizing in pinhole SPECT imaging is OS-EM algorithm. This algorithm significantly improves reconstructed image quality, also suppresses streak artifacts in comparison with FBP. To eliminate the multiplexing effect in multi-pinhole collimators, the additional shielding or spacing the pinholes has been used but these adjustment methods themselves have some disadvantages. The new collimators such as skew slit and lofthole collimators produce noncircular projections; therefore, provide high detector coverage, spatial resolution, and sensitivity without multiplexing effect.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Islamian JP, Toossi MT, Momennezhad M, Zakavi SR, Sadeghi R, Ljungberg M. Monte carlo study of the effect of collimator thickness on T-99m source response in single photon emission computed tomography. World J Nucl Med. 2012;11:70–4. doi: 10.4103/1450-1147.103419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu Y. Collimator Optimization for Single Photon Emission Computed Tomography Using Detection and Localization Tasks. Stony Brook, NY: The Graduate School, Stony Brook University; 2011. [Google Scholar]
- 3.Capote RM, Matela N, Conceição RC, Almeida P. Optimization of convergent collimators for pixelated SPECT systems. Med Phys. 2013;40:062501. doi: 10.1118/1.4804053. [DOI] [PubMed] [Google Scholar]
- 4.McQuaid SJ, Southekal S, Kijewski MF, Moore SC. Joint optimization of collimator and reconstruction parameters in SPECT imaging for lesion quantification. Phys Med Biol. 2011;56:6983–7000. doi: 10.1088/0031-9155/56/21/014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleming JS, Alaamer AS. Influence of collimator characteristics on quantification in SPECT. J Nucl Med. 1996;37:1832–6. [PubMed] [Google Scholar]
- 6.Mok SP, Wang Y, Tsui BM. Design of a novel pinhole collimator system for SPECT imaging of small animals with different sizes. IEEE Nucl Sci Symp Conf Rec. 2005;5:2649–52. [Google Scholar]
- 7.Accorsi R, Metzler SD. Analytic determination of the resolution-equivalent effective diameter of a pinhole collimator. IEEE Trans Med Imaging. 2004;23:750–63. doi: 10.1109/tmi.2004.826951. [DOI] [PubMed] [Google Scholar]
- 8.Bahreyni Toossi MT, Islamian JP, Momennezhad M, Ljungberg M, Naseri SH. SIMIND Monte Carlo simulation of a single photon emission CT. J Med Phys. 2010;35:42–7. doi: 10.4103/0971-6203.55967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherry SR, Sorenson JA, Phelps ME. Physics in Nuclear Medicine. 4th ed. Philadelphia: Elsevier Health Sciences; 2012. p. 202. [Google Scholar]
- 10.Li J, Jaszczak RJ, Turkington TG, Metz CE, Gilland DR, Greer KL, et al. An evaluation of lesion detectability with cone-beam, fanbeam and parallel-beam collimation in SPECT by continuous ROC study. J Nucl Med. 1994;35:135–40. [PubMed] [Google Scholar]
- 11.Kamali-Asl A, Sarkar S, Shahriari M, Agha-Hosseini H. Slit slat collimator optimization with respect to MTF. Int J Rad Appl Instrum A. 2005;62:461–8. doi: 10.1016/j.apradiso.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Metzler SD, Accorsi R, Novak JR, Ayan AS, Jaszczak RJ. On-axis sensitivity and resolution of a slit-slat collimator. J Nucl Med. 2006;47:1884–90. [PubMed] [Google Scholar]
- 13.Powsner RA, Powsner ER. Essential Nuclear Medicine Physics. 2nd ed. Massachusetts: John Wiley and Sons; 2008. p. 80. [Google Scholar]
- 14.Van Audenhaege K, Van Holen R, Vandenberghe S. Analysis of the trade-off between sensitivity and resolution of a pinhole collimator for SPECT. Soc Nucl Med. 2012;53:2410. [Google Scholar]
- 15.Wanet PM, Sand A, Abramovici J. Physical and clinical evaluation of high-resolution thyroid pinhole tomography. J Nucl Med. 1996;37:2017–20. [PubMed] [Google Scholar]
- 16.Tomas MB, Pugliese PV, Tronco GG, Love C, Palestro CJ, Nichols KJ. Pinhole versus parallel-hole collimators for parathyroid imaging: An intraindividual comparison. J Nucl Med Technol. 2008;36:189–94. doi: 10.2967/jnmt.108.055640. [DOI] [PubMed] [Google Scholar]
- 17.Gabriel M, Erler H, Profanter C, Moncayo R, Riccabona G. Evaluation of parathyroid nodules with thallium/technetium pinhole SPECT. Eur J Nucl Med. 2000;27:1070. [Google Scholar]
- 18.Scarfone C, Jaszczak RJ, Li J, Soo MS, Smith MF, Greer KL, et al. Breast tumour imaging using incomplete circular orbit pinhole SPET: A phantom study. Nucl Med Commun. 1997;18:1077–86. doi: 10.1097/00006231-199711000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Tornai MP, Bowsher JE, Jaszczak RJ, Pieper BC, Greer KL, Hardenbergh PH, et al. Mammotomography with pinhole incomplete circular orbit SPECT. J Nucl Med. 2003;44:583–93. [PubMed] [Google Scholar]
- 20.Bricou A, Duval MA, Charon Y, Barranger E. Mobile gamma cameras in breast cancer care-A review. Eur J Surg Oncol. 2013;39:409–16. doi: 10.1016/j.ejso.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Liu H, Chan C, Grobshtein Y, Ma T, Liu Y, Wang S, et al. 3D molecular breast imaging using a high-resolution dedicated cardiac SPECT camera. IEEE Nucl Sci Symp Conf Rec. 2013;1:1–4. [Google Scholar]
- 22.Bae J, Bae S, Lee K, Choi Y, Kim Y, Joung J. Design and performance investigation of a multi-pinhole collimator for a small field of view gamma imaging system. J Korean Phys Soc. 2014;64:970–5. [Google Scholar]
- 23.Seret A, Defrise M, Blocklet D. 180 degree pinhole SPET with a tilted detector and OS-EM reconstruction: Phantom studies and potential clinical applications. Eur J Nucl Med. 2001;28:1836–41. doi: 10.1007/s002590100629. [DOI] [PubMed] [Google Scholar]
- 24.An B, Jang E, Min BJ, Woo S-K, Lee K. Usefulness of 24-hour delayed bone scintigraphy of knee joint using pinhole collimator. Soc Nucl Med. 2014;55:2625. [Google Scholar]
- 25.Beekman FJ, van der Have F, Vastenhouw B, van der Linden AJ, van Rijk PP, Burbach JP, et al. U-SPECT-I: A novel system for submillimeter-resolution tomography with radiolabeled molecules in mice. J Nucl Med. 2005;46:1194–200. [PubMed] [Google Scholar]
- 26.Hirai T, Nohara R, Hosokawa R, Tanaka M, Inada H, Fujibayashi Y, et al. Evaluation of myocardial infarct size in rat heart by pinhole SPECT. J Nucl Cardiol. 2000;7:107–11. doi: 10.1016/s1071-3581(00)90030-8. [DOI] [PubMed] [Google Scholar]
- 27.Wollenweber T, Zach C, Rischpler C, Fischer R, Nowak S, Nekolla SG, et al. Myocardial perfusion imaging is feasible for infarct size quantification in mice using a clinical single-photon emission computed tomography system equipped with pinhole collimators. Mol Imaging Biol. 2010;12:427–34. doi: 10.1007/s11307-009-0281-5. [DOI] [PubMed] [Google Scholar]
- 28.Mok GS, Yu J, Du Y, Wang Y, Tsui BM. Evaluation of a multi-pinhole collimator for imaging small animals with different sizes. Mol Imaging Biol. 2012;14:60–9. doi: 10.1007/s11307-011-0472-8. [DOI] [PubMed] [Google Scholar]
- 29.Deleye S, Van Holen R, Verhaeghe J, Vandenberghe S, Stroobants S, Staelens S. Performance evaluation of small-animal multipinhole μSPECT scanners for mouse imaging. Eur J Nucl Med Mol Imaging. 2013;40:744–58. doi: 10.1007/s00259-012-2326-2. [DOI] [PubMed] [Google Scholar]
- 30.Beekman F, van der Have F. The pinhole: Gateway to ultra-high-resolution three-dimensional radionuclide imaging. Eur J Nucl Med Mol Imaging. 2007;34:151–61. doi: 10.1007/s00259-006-0248-6. [DOI] [PubMed] [Google Scholar]
- 31.Klingensmith WC, 3rd, Koo PJ, Summerlin A, Fehrenbach BW, Karki R, Shulman BC, et al. Parathyroid imaging: The importance of pinhole collimation with both single- and dual-tracer acquisition. J Nucl Med Technol. 2013;41:99–104. doi: 10.2967/jnmt.112.118208. [DOI] [PubMed] [Google Scholar]
- 32.Smith MF, Majewski S, Weisenberger AG. Optimizing pinhole and parallel hole collimation for scintimammography with compact pixellated detectors. IEEE Trans Med Imaging. 2003;50:321–6. [Google Scholar]
- 33.Rajaee A, Shahriari M, Asl AK, Hosseini S. Simulation study of the influence of collimator material on image quality improvement for high energy photons in nuclear medicine using MCNP code. J Theor Appl Phys. 2011;4:13–8. [Google Scholar]
- 34.Bom V, Goorden M, Beekman F. Comparison of pinhole collimator materials based on sensitivity equivalence. Phys Med Biol. 2011;56:3199–214. doi: 10.1088/0031-9155/56/11/003. [DOI] [PubMed] [Google Scholar]
- 35.Shokouhi S, Metzler SD, Wilson DW, Peterson TE. Multi-pinhole collimator design for small-object imaging with SiliSPECT: A high-resolution SPECT. Phys Med Biol. 2009;54:207–25. doi: 10.1088/0031-9155/54/2/003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franc BL, Acton PD, Mari C, Hasegawa BH. Small-animal SPECT and SPECT/CT: Important tools for preclinical investigation. J Nucl Med. 2008;49:1651–63. doi: 10.2967/jnumed.108.055442. [DOI] [PubMed] [Google Scholar]
- 37.Peterson TE, Shokouhi S. Advances in preclinical SPECT instrumentation. J Nucl Med. 2012;53:841–4. doi: 10.2967/jnumed.111.099853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vunckx K, Suetens P, Nuyts J. Effect of overlapping projections on reconstruction image quality in multipinhole SPECT. IEEE Trans Med Imaging. 2008;27:972–83. doi: 10.1109/TMI.2008.922700. [DOI] [PubMed] [Google Scholar]
- 39.Mok GS, Tsui BM, Beekman FJ. The effects of object activity distribution on multiplexing multi-pinhole SPECT. Phys Med Biol. 2011;56:2635–50. doi: 10.1088/0031-9155/56/8/019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nuyts J, Vunckx K, Defrise M, Vanhove C. Small animal imaging with multi-pinhole SPECT. Methods. 2009;48:83–91. doi: 10.1016/j.ymeth.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 41.Min BJ, Choi Y, Lee N-Y, Song TY, Jung JH, Hong KJ, et al. An investigation to design high performance multi-pinhole collimator. IEEE Nucl Sci Symp Conf Rec. 2006;5:3004–7. [Google Scholar]
- 42.Min BJ, Choi Y, Lee N-Y, Jung JH, Hong KJ, Kang JH, et al. A miniature SPECT using multi-pinhole collimator with vertical septa. IEEE Nucl Sci Symp Conf Rec. 2009;1:3116–20. [Google Scholar]
- 43.Hasegawa B, Kirch D, Stern D, Adams M, Sklar J, Johnson T, et al. Single-photon emission tomography with a 12-pinhole collimator. J Nucl Med. 1982;23:606–12. [PubMed] [Google Scholar]
- 44.Steele PP, Kirch DL, Koss JE. Comparison of simultaneous dual-isotope multipinhole SPECT with rotational SPECT in a group of patients with coronary artery disease. J Nucl Med. 2008;49:1080–9. doi: 10.2967/jnumed.107.040915. [DOI] [PubMed] [Google Scholar]
- 45.Holly TA, Abbott BG, Al-Mallah M, Calnon DA, Cohen MC, DiFilippo FP, et al. Single photon-emission computed tomography. J Nucl Cardiol. 2010;17:941–73. doi: 10.1007/s12350-010-9246-y. [DOI] [PubMed] [Google Scholar]
- 46.Rentmeester MC, van der Have F, Beekman FJ. Optimizing multi-pinhole SPECT geometries using an analytical model. Phys Med Biol. 2007;52:2567–81. doi: 10.1088/0031-9155/52/9/016. [DOI] [PubMed] [Google Scholar]
- 47.Cao Z, Bal G, Accorsi R, Acton PD. Optimal number of pinholes in multi-pinhole SPECT for mouse brain imaging - a simulation study. Phys Med Biol. 2005;50:4609–24. doi: 10.1088/0031-9155/50/19/013. [DOI] [PubMed] [Google Scholar]
- 48.Deloar HM, Watabe H, Aoi T, Iida H. Evaluation of penetration and scattering components in conventional pinhole SPECT: Phantom studies using Monte Carlo simulation. Phys Med Biol. 2003;48:995–1008. doi: 10.1088/0031-9155/48/8/303. [DOI] [PubMed] [Google Scholar]
- 49.van der Have F, Beekman FJ. Penetration, scatter and sensitivity in channel micro-pinholes for SPECT: A Monte Carlo investigation. IEEE Trans Nucl Sci. 2006;53:2635–45. [Google Scholar]
- 50.Smith MF, Jaszczak RJ, Wang H, Li J. Lead and tungsten pinhole inserts for I-131 SPECT tumor imaging: Experimental measurements and photon transport simulations. IEEE Trans Nucl Sci. 1997;44:74–82. [Google Scholar]
- 51.van der Have F, Beekman FJ. Photon penetration and scatter in micro-pinhole imaging: A Monte Carlo investigation. Phys Med Biol. 2004;49:1369–86. doi: 10.1088/0031-9155/49/8/001. [DOI] [PubMed] [Google Scholar]
- 52.van der Have F, Beekman FJ. Penetration and scatter in channel micro-pinholes for SPECT: A Monte Carlo investigation. IEEE Nucl Sci Symp Conf Rec. 2004;1:2575–8. [Google Scholar]
- 53.Song TY, Choi Y, Chung YH, Jung JH, Choe YS, Lee K-H, et al. Optimization of pinhole collimator for small animal SPECT using Monte Carlo simulation. IEEE Trans Nucl Sci. 2003;50:327–32. [Google Scholar]
- 54.Smith M, Jaszczak R, Wang H. Pinhole aperture design for 131 I tumor imaging. IEEE Trans Nucl Sci. 1997;44:1154–60. [Google Scholar]
- 55.Feldkamp L, Davis L, Kress J. Practical cone-beam algorithm. J Opt Soc Am A. 1984;1:612–9. [Google Scholar]
- 56.Habraken JB, de Bruin K, Shehata M, Booij J, Bennink R, van Eck Smit BL, et al. Evaluation of high-resolution pinhole SPECT using a small rotating animal. J Nucl Med. 2001;42:1863–9. [PubMed] [Google Scholar]
- 57.Jaszczak RJ, Li J, Wang H, Zalutsky MR, Coleman RE. Pinhole collimation for ultra-high-resolution, small-field-of-view SPECT. Phys Med Biol. 1994;39:425–37. doi: 10.1088/0031-9155/39/3/010. [DOI] [PubMed] [Google Scholar]
- 58.Li J, Jaszczak RJ, Greer KL, Coleman RE. A filtered backprojection algorithm for pinhole SPECT with a displaced centre of rotation. Phys Med Biol. 1994;39:165–76. doi: 10.1088/0031-9155/39/1/010. [DOI] [PubMed] [Google Scholar]
- 59.Vandenberghe S, D'Asseler Y, Van de Walle R, Kauppinen T, Koole M, Bouwens L, et al. Iterative reconstruction algorithms in nuclear medicine. Comput Med Imaging Graph. 2001;25:105–11. doi: 10.1016/s0895-6111(00)00060-4. [DOI] [PubMed] [Google Scholar]
- 60.Shepp LA, Vardi Y. Maximum likelihood reconstruction for emission tomography. IEEE Trans Med Imaging. 1982;1:113–22. doi: 10.1109/TMI.1982.4307558. [DOI] [PubMed] [Google Scholar]
- 61.Green PJ. Bayesian reconstructions from emission tomography data using a modified EM algorithm. IEEE Trans Med Imaging. 1990;9:84–93. doi: 10.1109/42.52985. [DOI] [PubMed] [Google Scholar]
- 62.Bettinardi V, Pagani E, Gilardi MC, Alenius S, Thielemans K, Teras M, et al. Implementation and evaluation of a 3D one-step late reconstruction algorithm for 3D positron emission tomography brain studies using median root prior. Eur J Nucl Med Mol Imaging. 2002;29:7–18. doi: 10.1007/s002590100651. [DOI] [PubMed] [Google Scholar]
- 63.Sohlberg A, Kuikka JT, Ruotsalainen U. Pinhole single-photon emission tomography reconstruction based on median root prior. Eur J Nucl Med Mol Imaging. 2003;30:217–21. doi: 10.1007/s00259-002-1015-y. [DOI] [PubMed] [Google Scholar]
- 64.Vanhove C, Defrise M, Franken PR, Everaert H, Deconinck F, Bossuyt A. Interest of the ordered subsets expectation maximization (OS-EM) algorithm in pinhole single-photon emission tomography reconstruction: A phantom study. Eur J Nucl Med. 2000;27:140–6. doi: 10.1007/s002590050019. [DOI] [PubMed] [Google Scholar]
- 65.Leong LK, Kruger RL, O'Connor MK. A comparison of the uniformity requirements for SPECT image reconstruction using FBP and OSEM techniques. J Nucl Med Technol. 2001;29:79–83. [PubMed] [Google Scholar]
- 66.Zeniya T, Watabe H, Aoi T, Kim KM, Teramoto N, Hayashi T, et al. A new reconstruction strategy for image improvement in pinhole SPECT. Eur J Nucl Med Mol Imaging. 2004;31:1166–72. doi: 10.1007/s00259-004-1510-4. [DOI] [PubMed] [Google Scholar]
- 67.Vanhove C, Defrise M, Bossuyt A, Lahoutte T. Improved quantification in multiple-pinhole SPECT by anatomy-based reconstruction using microCT information. Eur J Nucl Med Mol Imaging. 2011;38:153–65. doi: 10.1007/s00259-010-1627-6. [DOI] [PubMed] [Google Scholar]
- 68.Cerami J. Introduction to the proceedings of the 2012 World Molecular imaging Congress, Dublin, Ireland, September 5-8, 2012. Mol Imaging Biol. 2012;1:1–8. doi: 10.1007/s11307-012-0598-3. [DOI] [PubMed] [Google Scholar]
- 69.Deprez K, Pato LR, Vandenberghe S, Van Holen R. Characterization of a SPECT pinhole collimator for optimal detector usage (the lofthole) Phys Med Biol. 2013;58:859–85. doi: 10.1088/0031-9155/58/4/859. [DOI] [PubMed] [Google Scholar]
- 70.Xia D, Park M, Moore SC, Metzler SD. Preliminary investigation of imaging properties for sub-millimeter square pinholes. IEEE Nucl Sci Symp Conf Rec. 2013;1:1–5. [Google Scholar]
- 71.Van Audenhaege K, Vandenberghe S, Deprez K, Vandeghinste B, Van Holen R. Design and simulation of a full-ring multi-lofthole collimator for brain SPECT. Phys Med Biol. 2013;58:6317–36. doi: 10.1088/0031-9155/58/18/6317. [DOI] [PubMed] [Google Scholar]
- 72.Van Holen R, Deprez K, Vandeghinste B, Vandenberghe S. High sensitivity multi-lofthole microSPECT imager using minifying projections. Soc Nucl Med. 2012;53:2393. [Google Scholar]
- 73.Deprez K, Vandenberghe S, Vandeghinste B, Van Holen R. FlexiSPECT: A SPECT system consisting of a compact high-resolution scintillation detector (SPECTatress) and a lofthole collimator. IEEE Trans Nucl Sci. 2013;60:53–64. [Google Scholar]
- 74.Deprez K, Van Holen R, Vandenberghe S. The lofthole: A novel shaped pinhole geometry for optimal detector usage without multiplexing and without additional shielding. IEEE Nucl Sci Symp Med Imaging Conf. 2011;1:3317–22. [Google Scholar]
- 75.Zeng GL. A skew-slit collimator for small-animal SPECT. J Nucl Med Technol. 2008;36:207–12. doi: 10.2967/jnmt.108.055582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zeng G, Piatt J. First phantom experiment of skew-slit SPECT. Soc Nucl Med. 2007;48:93. [Google Scholar]


