Abstract
Fertility after orchidopexied undescended testes (UDT) is impaired. Although fertility parameters are known to be more favorable in unilateral cases than in bilateral cases, the exact contribution of the unilateral orchidopexied UDT to fertility is unknown. We used testicular 18F-fluoro-2-deoxyglucose (18F-FDG)-uptake assessed by positron emission tomography/computed tomography (PET/CT) to investigate the function of the orchidopexied unilateral congenital UDT, compared to its normally descended counterpart. We hypothesize that the contribution of the orchidopexied unilateral congenital UDT to fertility in adulthood is low. Eleven men who underwent orchidopexy for congenital UDT at the age of 1.9 ± 1.4 (range, 4.5 months -4.0) years were seen in follow-up at the age of 24.1 ± 2.3 (20.6-28.0) years. All underwent physical examination, testicular ultrasonography and PET/CT. Testicular 18F-FDG-uptake was expressed as the peak Standardized Uptake Value (SUVpeak). The mean SUVpeak of the orchidopexied UDT was 2.74 ± 0.48 (2.13-3.47), which was significantly lower than its counterpart (P = 0.021). Besides, there was no correlation between the testicular volume and the SUVpeak. The orchidopexied congenital UDT has been shown to be less metabolically active than its contralateral counterpart. Nevertheless, we suggest that the operated testes function to some degree.
Keywords: 18F-fluoro-2-deoxyglucose-uptake, congenital undescended testis, long-term follow-up
Introduction
Congenital undescended testis (UDT) is one of the most common urological anomalies and is associated with an enhanced risk of testicular cancer and impaired spermatogenesis.[1,2] Long-term follow-up studies show a decreased fertility in adulthood. Fertility potential after unilateral orchidopexied congenital UDT is better compared to orchidopexied bilateral congenital UDTs.[3,4,5,6,7] The main parameters used to assess this fertility have been paternity rate, semen quality and hormone levels. Those parameters measure the function of both testes and are not able to discriminate the role of each testis independently. Therefore, the contribution of the unilateral orchidopexied UDT to fertility remains uncertain.
Positron emission tomography (PET) imaging with 18F-fluoro-2-deoxyglucose (FDG) in combination with computed tomography (CT) scan is a promising new method to evaluate testicular function. FDG-uptake reflects tissue metabolism and in normal testicular tissue it accumulates.[8,9,10] Besides, testicular FDG-uptake can be measured accurately, shows a high symmetry in healthy testes[9,10,11] and is correlated with sperm quality.[11] As the FDG-uptake can be assessed for both testes separately it is possible to evaluate the function of both testes independently.
Although case reports of detection of undescended testes on PET/CT scans have been published,[12,13,14](FDG-uptake of) orchidopexied testes on PET/CT scan have never been described before.
We speculate that the contribution of the orchidopexied unilateral congenital UDT to fertility in adulthood is low. The aim of this project is to evaluate this long-term independent function of the orchidopexied congenital UDT. Therefore, we used a testicular PET/CT scan.
To calculate the number of individuals needed to compare the FDG-uptake of the orchidopexied versus its counterpart, we performed a pilot study to be able to achieve a power-analysis (sample size calculation).
Materials and Methods
Population
From a cohort of 386 boys, who underwent orchidopexy on congenital UDT between 1986 and 2006 at our hospital, we requested 50 men by mail to participate in this long-term follow-up study. Eleven men with a mean ± standard deviation (SD) age of 24.1 ± 2.3 (range, 20.6-28.0) years took part. Written informed consent was obtained before participation.
Orchidopexy
Orchidopexy was performed by the same surgeon. After inguinal exploration, if present, the open processus vaginalis was separated from the cord structures and ligated. Separation of the cremaster muscle and retroperitoneal funiculolysis were performed to mobilize the cord. Finally, the testis was fixated scrotally in a created Dartos pouch.
Follow-up data
Follow-up included one visit at our hospital, whereby medical history, physical examination, testicular ultrasound and an 18F-FDG PET/CT scan were performed.
Medical history
A questionnaire was used, including the medical history, inguinal or scrotal complaints and use of medication. Furthermore, we inquired after fatherhood or the desire to father a child as well as how long it took to conceive a child.
Physical examination
Physical examination included testis position, which was classified as low scrotal, high scrotal, inguinal, or nonpalpable.
Testicular ultrasound
All testicular ultrasound examinations were performed with the same equipment (Falco Auto Image; Falco Software Co., Tomsk, Russia) using a 12-MHz linear array transducer by a single investigator. To measure testicular volume, the transducer was placed on the scrotum while exerting light pressure to avoid distortion of the testicular shape. Images of the testes were obtained in the transverse and longitudinal planes. The epididymis was not included in the images. Length, width and height were measured 3 times, and the volume was calculated with the formula for an ellipsoid = π/6 × length × width × height. For each testis, the highest value of the three testicular volumes was taken as volume measurement. Additional findings, such as hydrocele, varicocele, and microlithiasis, were recorded. If necessary, the patient was referred for further follow-up.
18F-fluoro-2-deoxyglucose positron emission tomography/computed tomography scan
Testicular 18F-FDG PET/CT scanning was performed using a Biograph 16 TruePoint PET/CT scanner (Siemens Healthcare, Knoxville, USA). All participants received an intravenous injection of 18F-FDG. The average injected dose was 1.01 MBq/kg body weight (range, 0.96-1.05 MBq/kg), and the average time between FDG administration and the start of the PET acquisition was 44 min (range, 31-65 min). The testes were positioned longitudinally using two plastic tubes, which also prevented direct contact between both testes or improper position between the legs.
A low-dose CT scan was performed for localization and attenuation correction. Scanning parameters included 20 ref. mAs and 130 kV with 4D Care Dose (Siemens Healthcare, Erlangen, Germany). No intravenous radio contrast was administered.
For PET scanning, a three-dimensional emission scan was acquired, using 10 min at one bed position (the axial field of view of 21.6 cm). Images with CT-based attenuation correction were reconstructed, using ordered-subset expectation maximization three-dimensional reconstruction with four iterations, eight subsets and a Gaussian postsmoothing filter of 5 mm.
Images were interpreted on syngo. via VA20A equipped workstations, using the MM Oncology software package (version 1.0; Siemens Healthcare, Erlangen, Germany), which can display CT, PET and fused PET/CT images simultaneously.
In order to measure testicular volume by CT, the testes were selected semi-automatically using the generic segmentation tool in this software package. The resulting selected area was checked visually in all orthogonal planes and reshaped manually in case of obvious errors. Subsequently, the CT volume of each testis was recorded. SUVs were calculated from the PET images [Figure 1] as the ratio of the activity (kBq) in tissue per ml to the activity in the injected dose, corrected by residual activity in the syringe, per patient body weight in kg. Volume of Interest (VOI)s were selected on the PET images using the VOI isocontour tool in the oncology software package mentioned above, with a threshold of 50%. Spheres were placed manually around each testis on the three-dimensional PET images and rotated to the correct orientation of the testes. Within the resulting isocontour, SUVmax (SUV of single pixel with highest uptake in the VOI), SUVpeak (mean SUV of 1 cm3 with highest uptake in VOI), SUVmean (mean SUV in whole VOI) were measured and recorded.
Figure 1.
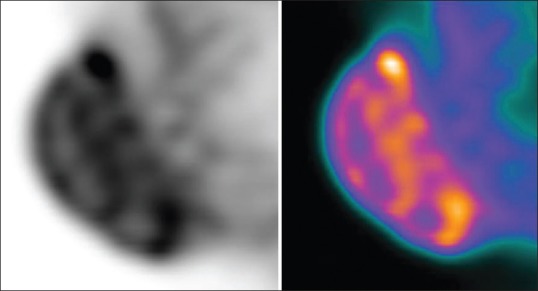
Testicular positron emission tomography/computed tomography image
Statistical analysis
All data were managed and analyzed using SPSS, version 14.0 (SPSS Inc, Chicago, III). The differences in volume and SUVs between the orchidopexied testis and its counterpart were calculated using the Wilcoxon signed rank test. The correlation between the testicular volume measured with ultrasound and with CT, as well as between testicular volume and SUVpeak, was assessed with Pearson's correlation coefficient test. A correlation coefficient (r) >0.7 was regarded as a good correlation. P < 0.05 was considered to be statistically significant.
Power-analysis was based on a paired t-test with the parameter SUVpeak and a power of >0.8 was regarded to be good.
Ethical approval
This study was approved by the Ethical Committee.
Results
The eleven participating men with a mean ± SD age of 24.1 ± 2.3 (range, 20.6-28.0) years underwent an orchidopexy for congenital UDT at a mean age ± SD of 1.9 ± 1.4 (range, 4.5 months –4.0) years. All congenital UDTs were right-sided.
Medical history
General medical history involved one man with ulcerative colitis, one with hemophilia. Furthermore, one had received a contralateral inguinal hernia repair, one an appendectomy and another a circumcision. No relevant medication was used. None of the men did mention any relevant complaints of the inguinoscrotal region. Two patients had fathered a child; both pregnancies occurred within a month after stopping anticonception. Of the men without children, one wished to have fathered, for the time of a year.
Testicular position and abnormalities
All, but one, testes were located low scrotal. One of the orchidopexied testes was in a high scrotal position. Intra- and extratesticular varicocele were seen in one of the operated testis. No other abnormalities were observed.
Testicular volume
Mean testicular volume measured with ultrasound of the orchidopexied testes was not significantly different from the contralateral testes; 13.3 ± 5.8 (4.9-24.4) versus 20.0 ± 11.3 (11.2-52.4) ml, P = 0.13 (Wilcoxon signed ranks test).
Mean testicular volume assessed on CT was not significantly different for the orchidopexied testes, 24.0 ± 9.8 (10.4-48.6) ml, in comparison to the contralateral testes, 28.3 ± 7.5 (18.0-44.5) ml, P = 0.33 (Wilcoxon signed ranks test).
Testicular volume measurement assessed by ultrasound correlated well with the assessment on CT, r = 0.729 [Figure 2].
Figure 2.
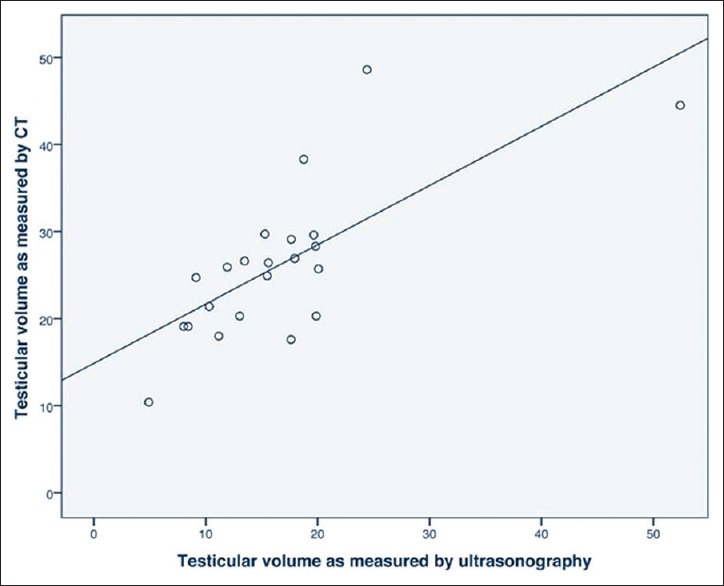
Correlation between testicular volume measurements as assessed by ultrasound and computed tomography; r = 0.729; P < 0.00
Testicular 18F-fluoro-2-deoxyglucose-uptake
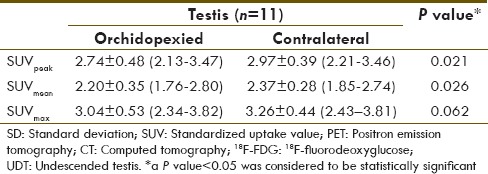
The 11 orchidopexied testes had a mean SUVpeak of 2.74 ± 0.48 (2.13-3.47), which was significantly smaller than the SUVpeak of its counterpart: 2.97 ± 0.39 (2.21-3.46), P = 0.021 (Wilcoxon signed ranks test). Table 1 lists the results of the SUVpeak, SUVmean and SUVmax.
Table 1.
Long-term mean±SD (range) of SUVpeak, SUVmean and SUVmax of orchidopexied congenital UDT and its contralateral counterpart as assessed on 18F-FDG-PET/CT scan
There was no correlation between testicular volume measured by ultrasound and CT and FDG-uptake (VolUS~ SUVpeak r = 0.209; VolCT~ SUVpeak r = 0.269).
Power-analysis
Based on a paired t-test calculator with the following parameters; SDx = 0.48, SDy = 0.39, R (x.y) =0.85, α =0.05, β =0.2, δ =0.23, the power of the current study, involving 11 men, was 0.85.
Discussion
This study shows that, with a mean SUVpeak of 2.74 ± 0.48, the congenital UDT after orchidopexy at early age, functions in adulthood, but at a significantly lower level than its counterpart, P = 0.021. Furthermore, we saw no correlation between the testicular volume and SUVpeak.
The fertility of men treated for UDT has been the subject of study for many years. Although impaired, the fertility in men is better after unilateral compared with bilateral cryptorchidism.[3,4,5,6,7,15,16] Since this fertility has been expressed as paternity rate, semen quality and hormone levels, it reflects the function of the whole genital tract and both testes. Hence until date, the contribution of the unilateral orchidopexied congenital UDT has not been elucidated.
This is the first study that evaluates the function of orchidopexied UDTs expressed as 18F-FDG-uptake assessed by PET/CT-scan. This relative new parameter for testicular function was introduced by Dierickx et al.[11] The testicular uptake of FDG is probably linked to glucose transporter 3 (GLUT 3) receptors which are situated in Sertoli cells and early spermatocytes,[17] as illustrated in Figure 1. In rats, surgically induced unilateral abdominal cryptorchidism give degenerative changes associated with decreased GLUT 3 receptors.[18] A study by Dierickx et al. they found correlations between testicular FDG-uptake and its function. Several studies support the symmetry in healthy testes and usability of the FDG-uptake, expressed as SUV.[10,11]
In a previous study, the normative testicular SUV in 20 young men and found a mean SUVpeak of 3.06 ± 0.54 (1.81-4.14) with a high symmetry for both testes was evaluated.[19]
The SUVpeak of the orchidopexied congenital UDT is lower than these normative values, P = 0.043, whereas the SUVpeak of the contralateral counterpart is comparable, P = 0.588 (Mann-Whitney U-test). Nevertheless, we should be careful comparing these data; absolute uptake values such as SUVs are known to be affected by many technical and physiological factors. Therefore, measurements from different studies cannot reliably be compared.[20,21,22]
Though, all orchidopexied testes in this study show a SUVpeak above 2.13. Therefore, we seem to be justified to conclude that the orchidopexied congenital UDT is functioning to some degree.
Regarding the testicular volumes, Sijstermans et al.[23] described smaller volumes of orchidopexied congenital UDTs compared to its counterparts, as assessed by Prader orchidometry (P < 0.01) and ultrasound (P < 0.01). We did not find this difference in volume (P = 0.13, for ultrasound). The number of testes studied (152 vs. 163 in Sijstermans et al.[23] and 11 vs. 11 in our study) can be a good explanation for this contrary finding.
The limitations of this study need to be addressed. First, the number of 11 men is small. Because this study was meant as a pilot study we performed a sample size calculation which showed that this current study has a power of 0.85. This means that the number of 11 participants was sufficient to prove the difference in SUVpeak of the orchidopexied congenital UDT compared to its counterpart. Second, the meaning of the SUV should be interpreted with care. The correlation between the testicular FDG-uptake and the main sperm parameters described by Dierickx et al.[8] is, so far, the only justification for using the SUV as a parameter for testicular function. As studies have shown a decline in FDG-uptake with age and other studies show a decline in fertility with age, they assumed that decreasing FDG uptake may reflect a decline in male spermatogenesis. Dierickx et al. selected 20 men (14-35 years) treated for nontesticular cancer, who had undergone a PET/CT and had provided semen samples. Then, they correlated functional volume (on PET/CT) and FDG-uptake with sperm parameters and found that testicular FDG-uptake reflects testicular function. Namely, FDG-uptake was correlated to total sperm count, which is an independent parameter for testicular function.
The orchidopexied congenital UDT has been shown to be less metabolically active than its contralateral counterpart. Nevertheless, with a mean SUVpeak of 2.74 ± 0.48 we suggest that the operated testes function to some degree.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Lip SZ, Murchison LE, Cullis PS, Govan L, Carachi R. A meta-analysis of the risk of boys with isolated cryptorchidism developing testicular cancer in later life. Arch Dis Child. 2013;98:20–6. doi: 10.1136/archdischild-2012-302051. [DOI] [PubMed] [Google Scholar]
- 2.Hutson JM, Balic A, Nation T, Southwell B. Cryptorchidism. Semin Pediatr Surg. 2010;19:215–24. doi: 10.1053/j.sempedsurg.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Lee PA, Coughlin MT. Fertility after bilateral cryptorchidism. Evaluation by paternity, hormone, and semen data. Horm Res. 2001;55:28–32. doi: 10.1159/000049960. [DOI] [PubMed] [Google Scholar]
- 4.Lee PA, Coughlin MT. The single testis: Paternity after presentation as unilateral cryptorchidism. J Urol. 2002;168:1680–2. doi: 10.1097/01.ju.0000028222.74363.ad. [DOI] [PubMed] [Google Scholar]
- 5.Cortes D, Thorup J, Lindenberg S, Visfeldt J. Infertility despite surgery for cryptorchidism in childhood can be classified by patients with normal or elevated follicle-stimulating hormone and identified at orchidopexy. BJU Int. 2003;91:670–4. doi: 10.1046/j.1464-410x.2003.04177.x. [DOI] [PubMed] [Google Scholar]
- 6.Trsinar B, Muravec UR. Fertility potential after unilateral and bilateral orchidopexy for cryptorchidism. World J Urol. 2009;27:513–9. doi: 10.1007/s00345-009-0406-0. [DOI] [PubMed] [Google Scholar]
- 7.van Brakel J, Kranse R, de Muinck Keizer-Schrama SM, Hendriks AE, de Jong FH, Bangma CH, et al. Fertility potential in men with a history of congenital undescended testes: A long-term follow-up study. Andrology. 2013;1:100–8. doi: 10.1111/j.2047-2927.2012.00024.x. [DOI] [PubMed] [Google Scholar]
- 8.Kosuda S, Fisher S, Kison PV, Wahl RL, Grossman HB. Uptake of 2-deoxy-2-[18F] fluoro-D-glucose in the normal testis: Retrospective PET study and animal experiment. Ann Nucl Med. 1997;11:195–9. doi: 10.1007/BF03164763. [DOI] [PubMed] [Google Scholar]
- 9.Kitajima K, Nakamoto Y, Senda M, Onishi Y, Okizuka H, Sugimura K. Normal uptake of 18F-FDG in the testis: An assessment by PET/CT. Ann Nucl Med. 2007;21:405–10. doi: 10.1007/s12149-007-0041-z. [DOI] [PubMed] [Google Scholar]
- 10.Goethals I, De Vriendt C, Hoste P, Smeets P, Ham H. Normal uptake of F-18 FDG in the testis as assessed by PET/CT in a pediatric study population. Ann Nucl Med. 2009;23:817–20. doi: 10.1007/s12149-009-0308-7. [DOI] [PubMed] [Google Scholar]
- 11.Dierickx LO, Huyghe E, Nogueira D, Zerdoud S, Filleron T, Brillouet S, et al. Functional testicular evaluation using PET/CT with 18F-fluorodeoxyglucose. Eur J Nucl Med Mol Imaging. 2012;39:129–37. doi: 10.1007/s00259-011-1929-3. [DOI] [PubMed] [Google Scholar]
- 12.Purandare NC, Rangarajan V, Sharma AR, Shah S. (18) F-FDG uptake in undescended testis mimicking inguinal adenopathy in a case of melanoma. Hell J Nucl Med. 2008;11:130–1. [PubMed] [Google Scholar]
- 13.Iwamura H, Hatakeyama S, Fukushi K, Sato T, Kojima Y, Murasawa H, et al. Testicular tumor arising in intra-abdominal testis which was not detected at prior orchidopexy: A case report. Hinyokika Kiyo. 2013;59:189–93. [PubMed] [Google Scholar]
- 14.Groheux D, Teyton P, Vercellino L, Ferretti A, Rubello D, Hindié E. Cryptorchidism as a potential source of misinterpretation in 18 FDG-PET imaging in restaging lymphoma patients. Biomed Pharmacother. 2013;67:533–8. doi: 10.1016/j.biopha.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Miller KD, Coughlin MT, Lee PA. Fertility after unilateral cryptorchidism. Paternity, time to conception, pretreatment testicular location and size, hormone and sperm parameters. Horm Res. 2001;55:249–53. doi: 10.1159/000050005. [DOI] [PubMed] [Google Scholar]
- 16.Murphy F, Paran TS, Puri P. Orchidopexy and its impact on fertility. Pediatr Surg Int. 2007;23:625–32. doi: 10.1007/s00383-007-1900-3. [DOI] [PubMed] [Google Scholar]
- 17.Kokk K, Veräjänkorva E, Laato M, Wu XK, Tapfer H, Pöllänen P. Expression of insulin receptor substrates 1-3, glucose transporters GLUT-1-4, signal regulatory protein 1alpha, phosphatidylinositol 3-kinase and protein kinase B at the protein level in the human testis. Anat Sci Int. 2005;80:91–6. doi: 10.1111/j.1447-073x.2005.00091.x. [DOI] [PubMed] [Google Scholar]
- 18.Farooqui SM, Al-Bagdadi F, O'Donnell JM, Stout R. Degenerative changes in spermatogonia are associated with loss of glucose transporter (Glut 3) in abdominal testis of surgically induced unilateral cryptorchidism in rats. Biochem Biophys Res Commun. 1997;236:407–12. doi: 10.1006/bbrc.1997.6954. [DOI] [PubMed] [Google Scholar]
- 19.Meij-de Vries A, Knol RJ, Lazarenko SV, Meijer RW, Van der Plas EM, Heij HA. Uptake of 18F-FDG in the healthy testes of young men as assessed by PET/CT; including the interobserver and intraobserver variation. World J Nucl Med. 2014;13:88–93. doi: 10.4103/1450-1147.139137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boellaard R, Krak NC, Hoekstra OS, Lammertsma AA. Effects of noise, image resolution, and ROI definition on the accuracy of standard uptake values: A simulation study. J Nucl Med. 2004;45:1519–27. [PubMed] [Google Scholar]
- 21.Boellaard R. Standards for PET image acquisition and quantitative data analysis. J Nucl Med. 2009;50(Suppl 1):11S–20. doi: 10.2967/jnumed.108.057182. [DOI] [PubMed] [Google Scholar]
- 22.Park HH, Park DS, Kweon DC, Lee SB, Oh KB, Lee JD, et al. Inter-comparison of 18F-FDG PET/CT standardized uptake values in Korea. Appl Radiat Isot. 2011;69:241–6. doi: 10.1016/j.apradiso.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Sijstermans K, Hack WW, van der Voort-Doedens LM, Meijer RW. Long-term testicular growth and position after orchidopexy for congenital undescended testis. Urol Int. 2009;83:438–45. doi: 10.1159/000251185. [DOI] [PubMed] [Google Scholar]


