Abstract
Positron emission tomography (PET) is currently the most advanced technique of metabolic imaging available for tumor diagnosis and follow-up. The aim of this study was to examine the versatility and accuracy of fluorodeoxyglucose (FDG) PET/computed tomography (CT) in the metastasis detection of renal cell carcinoma (RCC). We also compared our findings to other similar studies from the literature. This is the biggest study so far to examine the sensitivity and specificity of FDG PET/CT in the management of RCC. A retrospective review was carried out on all the FDG PET/CT studies done from January 1999 to January 2014 at our institution. Biopsy results were considered the gold standard. For our patients (n = 315) with biopsy results, FDG PET/CT studies exhibited 100% sensitivity, 100% specificity. Our results were better than results achieved by other studies. The use of FDG PET/CT in restaging and metastasis detection of RCC has many advantages, in addition to high accuracy. This imaging technique has great potential in influencing treatment decisions. We recommend the incorporation of FDG PET/CT in routine standard protocols for RCC.
Keywords: Fluorodeoxyglucose, metastasis, positron emission tomography/computed tomography, renal cell carcinoma, renal cell carcinoma, sensitivity, specificity
Introduction
The incidence of renal cell carcinoma (RCC) is increasing steadily in Western countries.[1] In the United States, approximately 56,000 cases of renal cancer are diagnosed, and 13,000 deaths occur annually.[2] RCC most commonly originates in the proximal convoluted tubules. The most common subtype, clear cell RCC, is associated with autosomal dominant mutation of the Von Hippel-Lindau gene (VHL).[3] Smoking and obesity are other known risk factors.[3] Papillary RCC is the second most common subtype. Other uncommon types of RCC include collecting duct (Bellini) tumors, medullary RCC, multilocular cystic RCC, and unclassified types.[4] The peak incidence is in the sixth decade of life.[5] It is two times more common in males than in females.[5] The incidence and mortality of RCC are higher in African Americans than in whites.[5] Hematuria is the most common symptom of this type of cancer. Laboratory abnormalities can include anemia and hypercalcemia, and they are identified as poor prognostic factors.[5] The most common sites of distant metastases are lung, bone, skin, liver, and brain.[6] RCC spreads by both hematogenous and lymphatic routes.
For RCC with widespread metastasis, no effective chemotherapy is available. As many as 85% of patients with VHL will experience tumor recurrence at 10 years.[7] RCC has a tendency of late recurrence and about 20–40% of patients develop metastases after radical nephrectomy.[8] Hence, it is very important to continuously monitor with imaging studies for tumor recurrence in RCC. The only established curative treatment for RCC remains surgery, with radical or partial nephrectomy.[9] RCC is the most lethal of urological cancers. 25% of patients with RCC will die from their cancer, compared with the 20% or lower mortality rates associated with prostate and bladder cancers.[2] If metastasis is present at the time of initial diagnosis, the median survival is only 10 months.[2]
Although fluorodeoxyglucose (FDG) positron emission tomography (PET) has proved to be an invaluable tool in staging a variety of cancer types such as lung, breast, lymphoma, colorectal, and head and neck, it currently has a limited role in evaluating RCC. FDG accumulation inside RCC cells depends on the expression of glucose transporter-1.[10] Previous studies have reported various sensitivity and specificity rates of PET/computed tomography (CT) in utilization for RCC. In this paper, we evaluated the role of (PET) in metastasis detection of RCC at our institution by retrospective review and compared it to the published literature. This is the biggest study so far to examine the sensitivity and specificity of FDG PET/CT in the management of RCC.
Methods
A retrospective review was done involving all the patients with RCC, who had an FDG-PET/CT exam at our institution from January 1999 to January 2014. The inclusion criteria were defined as follows: Patients who were diagnosed with primary RCC, but unknown status of RCC metastasis, patients who had FDG-PET/CT for staging of RCC and patients who had biopsies. Two nuclear medicine physicians independently reviewed the FDG-PET/CT exams with full agreement on the final findings. All FDG PET/CT exams in our study were backed by biopsy reports. We also collected the demographic information of the patients. We did a literature search on PubMed to compare our results with other studies. IBM SPSS version 20 (SPSS, Chicago, Illinois, USA) and WinPepi version 11.25 programs were used to analyze the data. The 2 × 2 table [Table 1] was constructed based on the biopsy data and FDG-PET/CT results of individual lesions.
Table 1.
Cross-tabulation of the FDG PET/CT results with biopsy findings

FDG-PET/CT imaging.
The patients were intravenously administered with 10-12 mCi of F18-FDG depending on the body weight. Imaging was performed in a PET/CT scanner (GE STE 64 slice CT scanner, GE healthcare, Waukesha, WI). A transmission scan (5 mm contiguous axial cuts) was obtained using an integrated multi-slice helical nonenhanced CT from vertex to toes for attenuation correction and anatomic localization. The PET emission scan was corrected using segmented attenuation data of the conventional transmission scan. A Gaussian filtering (6.4 mm) was performed for smoothing of images. The PET images were reconstructed with a standard iterative algorithm (OSEM, two iterative steps, 24 subsets) using GE software release 5.0 VUE Point FX intelligent reconstruction. CT data were reduced to an image matrix of 128 × 128. FDG and CT images were “hardware” co-registered. The voxel size of the final co-registered PET/CT image was 3.75 × 3.91 × 4.25 mm. All images were reformatted into axial, coronal, and sagittal views.
Results
The characteristics of the study population (n = 315) are shown in Table 2. The mean age of patients in our study was 47.5 years. For our patients, there was 100% sensitivity (95% confidence interval (CI), 0.87–1.00), 100% specificity (95% CI, 0.65–1.00) associated with FDG PET/CT [Table 1]. The smallest metastatic lesion detected in our study was 7 mm. Our results are compared to other studies from the literature [Table 3]. In our study, lymph nodes are the second most common place for chromophobe RCC metastasis [Figure 1]. Bone was the common location of RCC metastasis in this study [Figures 2 and 3]. In addition, chromophobe RCC represents 15% (47/315) of our sample population, which was quite unusual. Chromophobe RCC is the least aggressive type, and as expected, 30% of the suspected lesions were benign [Table 1].
Table 2.
Characteristics of the study population

Table 3.
Comparison of sensitivity and specificity rates of different studies

Figure 1.
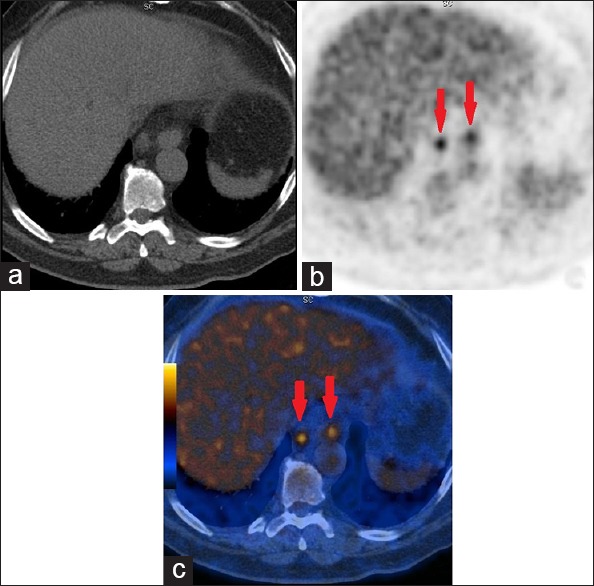
(a) CTAC (CT for attenuation correction and anatomic localization) image showing no signs of metastasis. (b) PET image showing metabolically active paraaortic lymph nodes (arrows). (c) PET/CT fusion image showing FDG uptake in the paraaortic lymph nodes (arrows). Biopsy confirmed the lesions as metastatic chromophobe RCC
Figure 2.
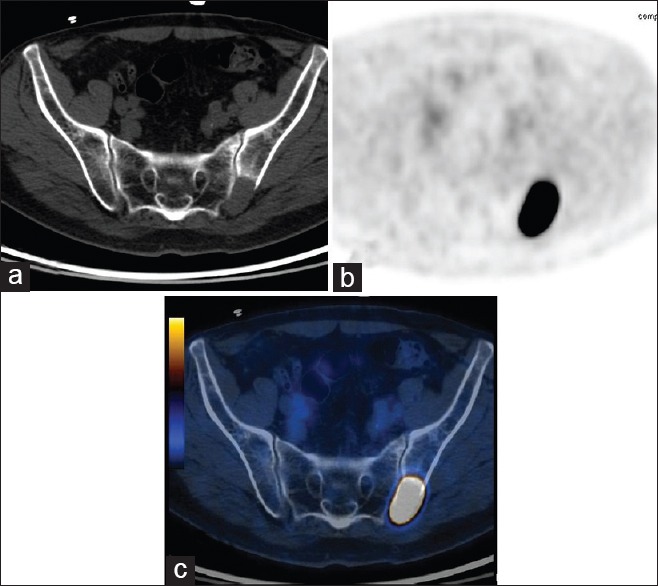
(a) The CT image showed a lesion in the left iliac bone, suggesting the possibility of metastasis. (b,c) On PET/CT, the hypermetabolic lesion measured SUVmax 11.3. Biopsy revealed clear cell RCC metastasis
Figure 3.
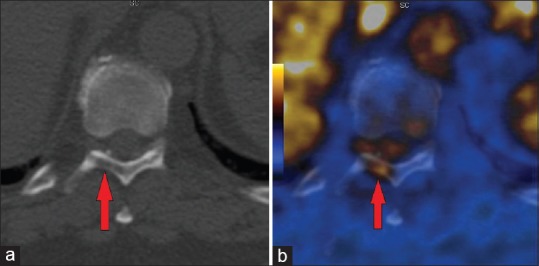
(a,b) Papillary RCC metastasis to the laminae of T10 vertebra
Discussion
To our knowledge, this is the first paper to report 100% sensitivity and 100% specificity of FDG PET/CT in metastasis detection of RCC. The results of the FDG PET/CT exams were backed by biopsy reports. Other studies gave lower sensitivity and specificity rates compared to ours [Table 3]. The sensitivity rates range from 64% to 90%, and the specificity rates range from 50% to 100%. But, no other study has reported 100% for both sensitivity and specificity. Combined PET/CT is an imaging modality that allows the acquisition of spatially registered PET and CT data in one imaging procedure. FDG is eliminated by the kidneys, and this can produce high background. It can be overcome by increasing diuresis with hydration or by administering diuretics. Primary and metastatic lesions can be detected with FDG-PET within an hour of FDG injection.[18]
Gallbladder involvement with RCC has been reported at a rate of less than 0.6%, and this is usually detected only at autopsy.[19] Clinical diagnosis of gallbladder metastasis is even rarer. Chung et al. reported that gallbladder metastasis is associated with clear cell type RCC.[20] In this study, there were a total of five gallbladder metastasis cases, and they were seen in papillary and chromophobe RCC cases. Incidence of metastatic disease in chromophobe RCC is only about 0.6%.[21] Women have a higher proportion of chromophobe RCC than in men.[22] However, from our results, men outnumber women in chromophobe RCC cases. Liver and lungs are the most common sites of metastasis for chromophobe RCC.[21]
Currently, CT is the method of choice for detection and staging of RCC.[2,14] PET/CT provides combined anatomical and functional imaging information and it has higher sensitivity and specificity than PET or CT alone.[23] Aide et al. stated that PET is more efficient than CT in detecting distant metastasis in RCC.[24] PET/CT is particularly useful for lymph node metastases which can often be falsely negative using the CT size criteria (1 cm).[25] In this study, the smallest metastatic lesion in a lymph node measured 7mm. RCC can have hypo, Iso or hyperdense appearance on unenhanced CT.[25] CT interpretation of the renal bed is difficult due to migration of the adjacent normal organs into the renal fossa, postoperative scar, and artifacts from surgical clips. The metabolic activity of tumor is not altered by these factors. Therefore, FDG-PET was found to be superior for evaluation of renal bed recurrence.[18] Contrasted CT of the chest, abdomen, and pelvis is routinely performed in separate exams to stage RCC.[6] Hence, it is more expensive and causes inconvenience for the patients. FDG PET/CT can scan the whole body in one procedure noninvasively and there is no need for contrast agents. Noncontrast CT has difficulty detecting metastasis in pancreas and muscle.[12] Lack of contrast agents in PET/CT is especially beneficial for renal cell cancer patients who frequently have impaired renal function or who are on dialysis. It relies on changes in metabolic activity of tissues so it can detect pathology even before anatomic changes are apparent. Some RCCs can contain fat without calcification, and they can mimic benign renal angiomyolipomas on CT.[26] Thus, CT cannot conclusively distinguish between benign and malignant tumors.
Bone lesions typically associated with RCC are osteolytic, and they can be slow growing. 99mTc-methylene diphosphonate (Tc99m-MDP) bone scan can miss such lesions. FDG PET/CT relies on a different mechanism of detecting malignancies and it can overcome this problem. RCC can spread to the bone marrow, and Tc99m-MDP is only deposited on the bone surface. Seto et al. described a case where bone metastasis was missed by Tc99m-MDP bone scan but was detected by FDG PET/CT.[27] This is further supported by Wu et al., who found that FDG PET/CT has a higher sensitivity and better accuracy than Tc99m-MDP bone scan to detect bone metastases in patients with RCC.[28] Kang et al. stated that that FDG-PET is most sensitive for detecting metastases to bone.[29]
Hepatic metastasis of RCC can have low attenuation on magnetic resonance imaging (MRI).[30] In addition, MRI requires long examination time, and it is fairly expensive. Some RCC can contain calcifications, and MRI may not detect them.[31] Press et al. mentioned in their article that the overall diagnostic utility of ultrasonography (US) is questionable.[8] US cannot reliably stage renal cancer because solid renal tumors lack consistent sonographic patterns and US also suffers from low specificity.[8] Grant et al. wrote that RCC lesions typically have low FDG avidity.[32] Yet, this study has proved that FDG PET/CT can reliably detect the RCC metastasis. Kochhar et al. reported that FDG PET/CT imaging has a promising role in the imaging of renal lesions and can help prevent unnecessary biopsies and ensure optimal management of suspicious lesions.[25]
Rodríguez Martínez de Llano et al. mentioned that FDG PET/CT might replace conventional methods.[14] Park et al. also reported that FDG PET/CT had the potential to replace conventional methods in RCC management.[15] As the FDG PET/CT technology is improving, we can foresee its adoption as part of the routine protocol in restaging and metastasis detection in renal cell cancer cases. Fuccio et al. recommended the use of FDG-PET/CT in RCC restaging because they found that it is feasible.[11] FDG-PET/CT allows early diagnosis and staging before morphologic changes are evident. The tumor, nodes, and metastasis staging system is currently the most extensively used one, and it can also provide prognostic information.[33] Choosing the appropriate treatment depends on the stage of RCC. Subsequently, the results of the FDG-PET/CT exams guided the treatment plans and changed the management for the patients in this study. Thus, this exam contributes to the selection of the most suitable anti-cancer therapy for a patient.
Conclusion
From our experience, the FDG PET/CT studies exhibited 100% sensitivity and 100% specificity in metastasis detection of RCC. If metastasis is detected, it can change the treatment decision, and the patient can avoid unnecessary surgery. This imaging technique is very versatile and accurate. It can image the whole body to assess for metastasis. Because it produced excellent results, we recommend the incorporation of FDG PET/CT as a standard exam in restaging and metastasis detection of RCC. More studies involving multiple institutions need to be done to further confirm our findings.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Jeon HG, Jeong IG, Lee JH, Lee CJ, Kwak C, Kim HH, et al. Prognostic value of body mass index in Korean patients with renal cell carcinoma. J Urol. 2010;183:448–54. doi: 10.1016/j.juro.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Schöder H, Larson SM. Positron emission tomography for prostate, bladder, and renal cancer. Semin Nucl Med. 2004;34:274–92. doi: 10.1053/j.semnuclmed.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Cote ML, Colt JS, Schwartz KL, Wacholder S, Ruterbusch JJ, Davis F, et al. Cigarette smoking and renal cell carcinoma risk among black and white Americans: Effect modification by hypertension and obesity. Cancer Epidemiol Biomarkers Prev. 2012;21:770–9. doi: 10.1158/1055-9965.EPI-11-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scher HI, Motzer RJ. Bladder and renal cell carcinomas. In: Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, editors. Harrison's Principles of Internal Medicine. 16th ed. New York: McGraw-Hill; 2005. pp. 541–3. [Google Scholar]
- 5.Safaei A, Figlin R, Hoh CK, Silverman DH, Seltzer M, Phelps ME, et al. The usefulness of F-18 deoxyglucose whole-body positron emission tomography (PET) for re-staging of renal cell cancer. Clin Nephrol. 2002;57:56–62. doi: 10.5414/cnp57056. [DOI] [PubMed] [Google Scholar]
- 6.Khandani AH, Rathmell WK. Positron emission tomography in renal cell carcinoma: An imaging biomarker in development. Semin Nucl Med. 2012;42:221–30. doi: 10.1053/j.semnuclmed.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu NW, Khurana K, Sudarshan S, Pinto PA, Linehan WM, Bratslavsky G. Repeat partial nephrectomy on the solitary kidney: Surgical, functional and oncological outcomes. J Urol. 2010;183:1719–24. doi: 10.1016/j.juro.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Press GA, McClennan BL, Melson GL, Weyman PJ, Mauro MA, Lee JK. Papillary renal cell carcinoma: CT and sonographic evaluation. AJR Am J Roentgenol. 1984;143:1005–9. doi: 10.2214/ajr.143.5.1005. [DOI] [PubMed] [Google Scholar]
- 9.Li XS, Yao L, Gong K, Yu W, He Q, Zhou LQ, et al. Growth pattern of renal cell carcinoma (RCC) in patients with delayed surgical intervention. J Cancer Res Clin Oncol. 2012;138:269–74. doi: 10.1007/s00432-011-1083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majhail NS, Urbain JL, Albani JM, Kanvinde MH, Rice TW, Novick AC, et al. F-18 fluorodeoxyglucose positron emission tomography in the evaluation of distant metastases from renal cell carcinoma. J Clin Oncol. 2003;21:3995–4000. doi: 10.1200/JCO.2003.04.073. [DOI] [PubMed] [Google Scholar]
- 11.Fuccio C, Ceci F, Castellucci P, Spinapolice EG, Palumbo R, D'Ambrosio D, et al. Restaging clear cell renal carcinoma with 18F-FDG PET/CT. Clin Nucl Med. 2014;39:e320–4. doi: 10.1097/RLU.0000000000000382. [DOI] [PubMed] [Google Scholar]
- 12.Nakatani K, Nakamoto Y, Saga T, Higashi T, Togashi K. The potential clinical value of FDG-PET for recurrent renal cell carcinoma. Eur J Radiol. 2011;79:29–35. doi: 10.1016/j.ejrad.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 13.Kumar R, Shandal V, Shamim SA, Jeph S, Singh H, Malhotra A. Role of FDG PET-CT in recurrent renal cell carcinoma. Nucl Med Commun. 2010;31:844–50. doi: 10.1097/MNM.0b013e32833d6882. [DOI] [PubMed] [Google Scholar]
- 14.Rodríguez Martínez de Llano S, Jiménez-Vicioso A, Mahmood S, Carreras-Delgado JL. Clinical impact of (18) F-FDG PET in management of patients with renal cell carcinoma. Rev Esp Med Nucl. 2010;29:12–9. doi: 10.1016/j.remn.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Park JW, Jo MK, Lee HM. Significance of 18F-fluorodeoxyglucose positron-emission tomography/computed tomography for the postoperative surveillance of advanced renal cell carcinoma. BJU Int. 2009;103:615–9. doi: 10.1111/j.1464-410X.2008.08150.x. [DOI] [PubMed] [Google Scholar]
- 16.Dilhuydy MS, Durieux A, Pariente A, de Clermont H, Pasticier G, Monteil J, et al. PET scans for decision-making in metastatic renal cell carcinoma: A single-institution evaluation. Oncology. 2006;70:339–44. doi: 10.1159/000097946. [DOI] [PubMed] [Google Scholar]
- 17.Jadvar H, Kherbache HM, Pinski JK, Conti PS. Diagnostic role of [F-18]-FDG positron emission tomography in restaging renal cell carcinoma. Clin Nephrol. 2003;60:395–400. doi: 10.5414/cnp60395. [DOI] [PubMed] [Google Scholar]
- 18.Ramdave S, Thomas GW, Berlangieri SU, Bolton DM, Davis I, Danguy HT, et al. Clinical role of F-18 fluorodeoxyglucose positron emission tomography for detection and management of renal cell carcinoma. J Urol. 2001;166:825–30. [PubMed] [Google Scholar]
- 19.Fang X, Gupta N, Shen SS, Tamboli P, Charnsangavej C, Rashid A, et al. Intraluminal polypoid metastasis of renal cell carcinoma in gallbladder mimicking gallbladder polyp. Arch Pathol Lab Med. 2010;134:1003–9. doi: 10.5858/2009-0453-OA.1. [DOI] [PubMed] [Google Scholar]
- 20.Chung PH, Srinivasan R, Linehan WM, Pinto PA, Bratslavsky G. Renal cell carcinoma with metastases to the gallbladder: Four cases from the National Cancer Institute (NCI) and review of the literature. Urol Oncol. 2012;30:476–81. doi: 10.1016/j.urolonc.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patard JJ, Leray E, Rioux-Leclercq N, Cindolo L, Ficarra V, Zisman A, et al. Prognostic value of histologic subtypes in renal cell carcinoma: A multicenter experience. J Clin Oncol. 2005;23:2763–71. doi: 10.1200/JCO.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 22.Lee S, Jeon HG, Kwak C, Kim HH, Byun SS, Lee SE, et al. Gender-specific clinicopathological features and survival in patients with renal cell carcinoma (RCC) BJU Int. 2012;110:E28–33. doi: 10.1111/j.1464-410X.2011.10667.x. [DOI] [PubMed] [Google Scholar]
- 23.Powles T, Murray I, Brock C, Oliver T, Avril N. Molecular positron emission tomography and PET/CT imaging in urological malignancies. Eur Urol. 2007;51:1511–20. doi: 10.1016/j.eururo.2007.01.061. [DOI] [PubMed] [Google Scholar]
- 24.Aide N, Cappele O, Bottet P, Bensadoun H, Regeasse A, Comoz F, et al. Efficiency of [(18) F] FDG PET in characterising renal cancer and detecting distant metastases: A comparison with CT. Eur J Nucl Med Mol Imaging. 2003;30:1236–45. doi: 10.1007/s00259-003-1211-4. [DOI] [PubMed] [Google Scholar]
- 25.Kochhar R, Brown RK, Wong CO, Dunnick NR, Frey KA, Manoharan P. Role of FDG PET/CT in imaging of renal lesions. J Med Imaging Radiat Oncol. 2010;54:347–57. doi: 10.1111/j.1754-9485.2010.02181.x. [DOI] [PubMed] [Google Scholar]
- 26.Schuster TG, Ferguson MR, Baker DE, Schaldenbrand JD, Solomon MH. Papillary renal cell carcinoma containing fat without calcification mimicking angiomyolipoma on CT. AJR Am J Roentgenol. 2004;183:1402–4. doi: 10.2214/ajr.183.5.1831402. [DOI] [PubMed] [Google Scholar]
- 27.Seto E, Segall GM, Terris MK. Positron emission tomography detection of osseous metastases of renal cell carcinoma not identified on bone scan. Urology. 2000;55:286. doi: 10.1016/s0090-4295(99)00409-4. [DOI] [PubMed] [Google Scholar]
- 28.Wu HC, Yen RF, Shen YY, Kao CH, Lin CC, Lee CC. Comparing whole body 18F-2-deoxyglucose positron emission tomography and technetium-99m methylene diphosphate bone scan to detect bone metastases in patients with renal cell carcinomas - A preliminary report. J Cancer Res Clin Oncol. 2002;128:503–6. doi: 10.1007/s00432-002-0370-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang DE, White RL, Jr, Zuger JH, Sasser HC, Teigland CM. Clinical use of fluorodeoxyglucose F 18 positron emission tomography for detection of renal cell carcinoma. J Urol. 2004;171:1806–9. doi: 10.1097/01.ju.0000120241.50061.e4. [DOI] [PubMed] [Google Scholar]
- 30.Mueller-Lisse UG, Mueller-Lisse UL. Imaging of advanced renal cell carcinoma. World J Urol. 2010;28:253–61. doi: 10.1007/s00345-010-0557-z. [DOI] [PubMed] [Google Scholar]
- 31.Garin JM, Marco I, Salva A, Serrano F, Bondia JM, Pacheco M. CT and MRI in fat-containing papillary renal cell carcinoma. Br J Radiol. 2007;80:e193–5. doi: 10.1259/bjr/79274414. [DOI] [PubMed] [Google Scholar]
- 32.Grant FD, Fahey FH, Packard AB, Davis RT, Alavi A, Treves ST. Skeletal PET with 18F-fluoride: Applying new technology to an old tracer. J Nucl Med. 2008;49:68–78. doi: 10.2967/jnumed.106.037200. [DOI] [PubMed] [Google Scholar]
- 33.Lam JS, Shvarts O, Leppert JT, Figlin RA, Belldegrun AS. Renal cell carcinoma 2005: New frontiers in staging, prognostication and targeted molecular therapy. J Urol. 2005;173:1853–62. doi: 10.1097/01.ju.0000165693.68449.c3. [DOI] [PubMed] [Google Scholar]


