Abstract
Single-photon emission computed tomography-computed tomography (SPECT-CT) allows for physiological and anatomical co-registration in sentinel lymph node (SLN) mapping and offers additional benefits over conventional planar imaging. However, the clinical relevance when considering added costs and radiation burden of these reported benefits remains somewhat uncertain. This study aimed to evaluate the possible added value of SPECT-CT and intra-operative gamma-probe use over planar imaging alone in the South African setting. 80 patients with breast cancer or malignant melanoma underwent both planar and SPECT-CT imaging for SLN mapping. We assessed and compared the number of nodes detected on each study, false positive and negative findings, changes in surgical approach and or patient management. In all cases where a sentinel node was identified, SPECT-CT was more accurate anatomically. There was a significant change in surgical approach in 30 cases - breast cancer (n = 13; P 0.001) and malignant melanoma (n = 17; P 0.0002). In 4 cases a node not identified on planar imaging was seen on SPECT-CT. In 16 cases additional echelon nodes were identified. False positives were excluded by SPECT-CT in 12 cases. The addition of SPECT-CT and use of intra-operative gamma-probe to planar imaging offers important benefits in patients who present with breast cancer and melanoma. These benefits include increased nodal detection, elimination of false positives and negatives and improved anatomical localization that ultimately aids and expedites surgical management. This has been demonstrated in the context of industrialized country previously and has now also been confirmed in the setting of a emerging-market nation.
Keywords: Breat cancer, melanoma, sentinel lymph node, single-photon emission computed tomography-computed tomography
Introduction
The emerging-market nations may bear 70% of the world's cancer burden by 2030 – accurate staging in this setting becomes imperative as it impacts on surgical decision making.[1,2,3]
The sentinel lymph node (SLN) - the first lymph node that drains the primary tumour - is likely the first to receive metastatic seeding. Lymphatic mapping allows surgeons to locate and excise the sentinel node. However, it does not predict if the node has metastatic cells.[2]
By excising and histologically analysing sentinel node, it offers a minimally invasive method for detecting loco-regional spread and has superseded block dissection for nodal staging – it is associated with fewer debilitating complications;[4] it also allows the surgeon to proceed to block dissection in patients with positive malignant seeding.
Conventional scintigraphic imaging utilizes only anterior and lateral views to locate (and mark) the sentinel node. Single-photon emission computed tomography (SPECT) coupled to computed tomography (CT) allows for three-dimensional tomographic imaging with more accurate detailing of its anatomical.[4]
We aim to assess whether SPECT-CT and intra-operative gamma-probe use adds value in SLN localization and excision compared to conventional imaging alone.
Materials and Methods
Ethics
Ethics approval was granted and informed consent obtained in all prospective cases according to the declaration of Heklsinki of 1975 as revised in 2010.
Study design
Selection and description of participant
Inclusion criteria included all patients with proven breast cancer or malignant melanoma routinely referred for SLN mapping with a NxM0 stage.
Exclusion criteria included pregnancy, age below 18 years of age, or inability/refusal to give consent - where applicable. Furthermore, specific to the breast cancer cohort, patients with inflammatory breast cancer, a prior history of lymphoma and palpable axillary lymph nodes were also excluded.
Technical information
Injection technique
The radiotracers used included mostly 99mTc-Nanocoll, and when unavailable, 99mTc-Sentiscint injected sub-areolarly as a single injection in the quadrant containing the breast cancer, or sub-cutaneously and at four points around the malignant melanoma or its postsurgical scar.
Camera parameters
All studies were performed on Global Electronics' Centricity HAWKEYE 1 SPECT-CT gamma-camera (128 × 128 matrix, 60 frames, 25 s/frame). Filtered back projection reconstruction was used for image reconstruction.
Transmission scans
A transmission scan was performed for all planar imaging. Either 57Co source, a refillable 99mTechnetium refillable source or 99mTechnetium point source tracing the body contours were utilized. Activity of the 99mTechnetium source did not exceed 74 MBq.
Planar image acquisition
For the breast cancer cases, two planar acquisition sets were acquired at 30-45 min and 2 h post tracer injection, respectively. All anterior projections were obtained with a transmission study. For the melanoma cases, a dynamic acquisition was acquired between 30 and 60 s per frame over 20-30 min starting immediately after injection. For lower limb primary tumours, a hemi-body continuous scan at 10 cm/min was obtained. For primary tumours of the trunk, dynamic imaging was followed by planar images in the anterior, posterior, lateral and oblique projections (as deemed necessary). All planar images were acquired no later than 1 h post tracer injection.
Marking the skin
A surgical patient positioning was used for both planar anterior and any oblique projections. Using a 90° head configuration, a simultaneously anterior and lateral persistence scan was obtained to facilitate skin marking of the vertical and horizontal vectors. The skin was cleaned using aseptic method and covered in a transparent acrylic dressing. Location was confirmed using a Europrobe gamma-probe, which was also used in theatre.
Single-photon emission computed tomography-computed tomography acquisition
A low dose SPECT-CT was acquired immediately after identifying the SLN on planar images.
Data collection
Data collection and analysis included information on the injection technique, patient weight and height, stage of malignancy and histology - where clinically available.
Analysis and statistics
Analysis included interpretation of the planar images and SPECT images by two experienced nuclear physicians. A change in surgical approach was only deemed necessary when the study demonstrated unexpected nodal drainage or additional nodes to be excised - in accordance with standard surgical opinion and regardless of the eventual surgical outcome. A change in management involved those cases where the sentinel node was found to be positive for tumour, where nodes were located in a surgically inaccessible site, or not visualized - requiring alternative staging methods.
Planar images were evaluated and compared to SPECT data to determine whether it provided further useful information with regards to localization and number of nodes involved. The surgeon's opinion was obtained regarding any benefit of the use of SPECT and intra-operative gamma-probe as part of the standard imaging protocol. All data was captured on a standardized case report form and finding recorded using a commercial spreadsheet.
The Chi-square test was used to determine if there was a statistically significant change in surgical approach between conventional planar and SPECT-CT SLN mapping.
Results
Malignant melanoma
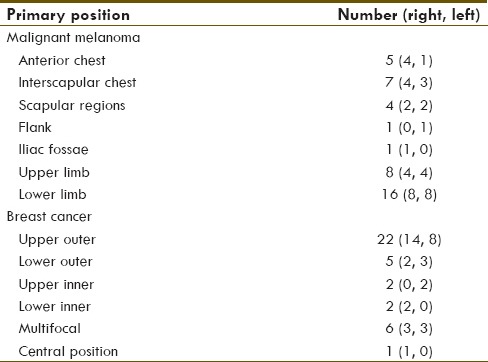
Melanoma cases comprised of 42 patients – 22 males (mean age of 50) an and 20 female (mean age of 52); one with carcinoma in situ, 6 with T1 disease, 9 with T2 disease, 10 with T3 disease and 4 with T4 disease according to the Breslow tumour staging (n = 30). Average body mass index (BMI) was 28.5 (n = 23). 16 nodes were operated the same day, while 26 were operated the day after imaging – neither method affected the overall outcome. Tumours were distributed as indicated in Table 1. All cases had a nuclear physician and gamma-probe in theatre and no blue dye was utilized. There was no discernible correlation between the anthropometry and the SLN detection - however, data for 19 cases was not available. In 4 cases where planar images could not demonstrate a SLN, a node was demonstrated on SPECT-CT. Using the combined protocol, there were therefore no failed cases. Where histology results were available (n = 35), 5 cases demonstrated positive histology; SPECT-CT also excluded two false positives on planar in this group - these additional nodes were shown to be higher echelon nodes, and proved negative for tumour on histology. In 4 cases SPECT-CT demonstrated additional lymph nodes over and above those detected on planar imaging. None, however, demonstrated positive histology.
Table 1.
Primary tumour distribution
Single-photon emission computed tomography-computed tomography excluded false positives in 4 cases - either as lymphatic tracer hold-up or artefacts. In all cases SPECT-CT provided improved spatial resolution and more accurate anatomical localization. In 17 cases SPECT-CT effected a change in surgical approach - in 1 case SPECT-CT identified an additional drainage territory over planar and in 1 case excluded one territory (the sentinel node proved to be positive for malignancy). In 1 case a popliteal node not identified on planar was demonstrated on SPECT-CT, where planar incorrectly identified the inguinal nodes as the sentinel. In 11 cases higher echelon nodes were identified on SPECT-CT not seen on planar. In three of the five histological positive nodes SPECT-CT effected a change in surgical procedure, which included excision of all other regional lymph nodes in the same basin.
Breast cancer
Breast cancer cases comprised of 38 patients - 37 females (mean age of 60 years) and one male (age of 79 years). 4 presented with T1a disease, 1 with T1b disease, 5 with T1c disease, 16 with T2 disease, 3 with T3 disease according to the AJCC staging criteria (n = 29). Average BMI was 29.9 (n = 13). 15 nodes were operated the same day, while 23 were operated the day after imaging - again, neither method affected the overall outcome. Primary tumour distribution is described in Table 1. All cases had a nuclear physician and gamma-probe in theatre and no blue dye was utilized. As with the melanoma patients there was no discernible correlation between anthropometry and SLN detection - data unavailable for 25 cases.
Three studies failed to detect a SLN on both planar and SPECT-CT imaging - no cause could be ascertained; one of these cases had positive histology at block dissection. No cases demonstrated detection by SPECT-CT only. Furthermore, none of the SPECT-CT images demonstrated more lymph nodes than planar images. SPECT-CT did, however, excluded false positive nodes (n = 11) in 8 cases. In four of the seven patients with positive histology, SPECT-CT resulted in a change in surgical procedure - surgery progressed to block nodal dissection. In 13 cases SPECT-CT effected a change in surgical approach - in 2 cases there was a change in drainage territory. In 4 cases, SPECT-CT excluded artefacts deemed to be nodes on planar images. In 5 cases additional higher echelon nodes were identified by SPECT-CT.
Chi-square analysis with respect to change in surgical approach
Overall, in all cases where a SLN was identified, SPECT-CT allowed for better visualization of the nodes with regards to exact anatomical localization when compared to planar imaging [Figures 1–4]. Barring the three failed studies, the surgeon operating found the SPECT-CT images more reliable to determine surgical approach and resulted in a statistically significant change in surgical approach in 30 cases, 13 in the breast cancer group [P 0.001; Table 2] and 17 in the melanoma group [P 0.0002; Table 3] - due to the aforementioned factors.
Figure 1.
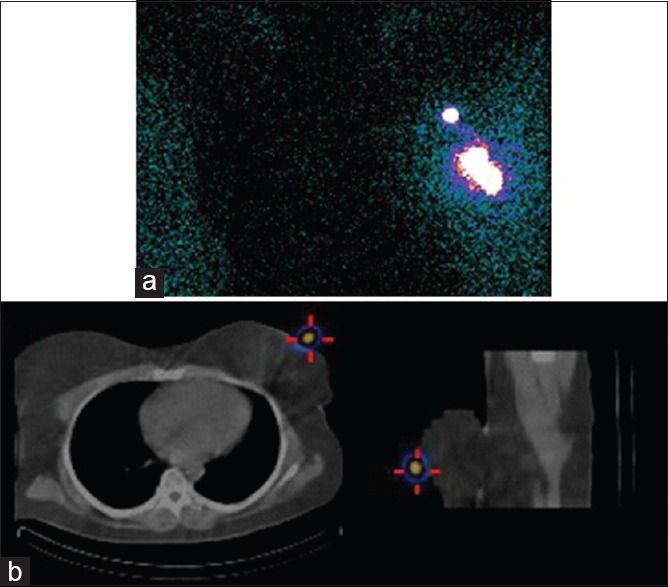
Patient with left sided breast cancer. Planar transmission scan demonstrates a focus deemed to be the sentinel lymph node (a), which is shown to be artifactual (superficial to the skin) on single-photon emission computed tomography-computed tomography (b)
Figure 4.
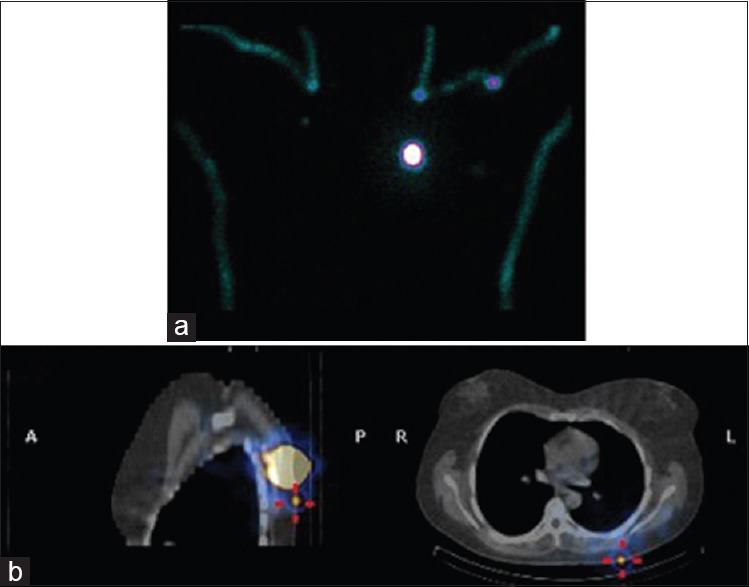
Patient with melanoma of the left scapular region. Shine through from the injection site on the silhouette images may be misleading. Furthermore no focus was demonstrated to suggest a sentinel lymph node (a). Single-photon emission computed tomography-computed tomography, however, demonstrated a superficial node in close proximity to the injection site (b)
Table 2.
Chi-square contingency table of malignant melanoma sample
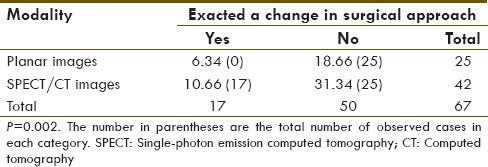
Table 3.
Chi-square contigency table of breast cancer sample
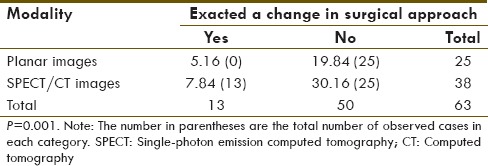
Figure 2.
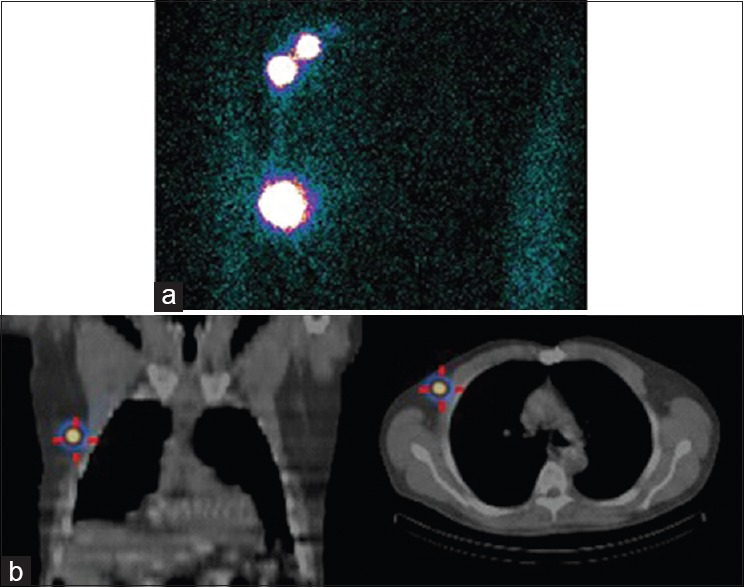
Patient with right-sided breast cancer. Two foci of tracer accumulation in noted on the transmission scan (a), however, single-photon emission computed tomography-computed tomography demonstrated that the inferior focus was in fact an area of tracer hold-up
Figure 3.
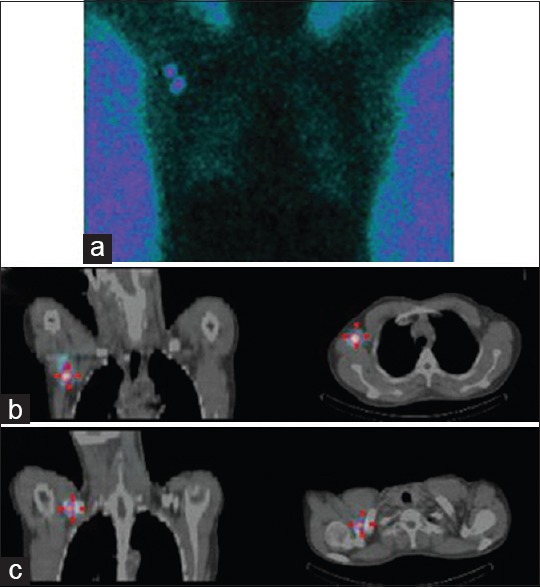
Patient with right-sided breast cancer. The planar transmission scan demonstrates a single focus in close proximity to the injection site (a). Single-photon emission computed tomography-computed tomography allowed for better anatomical localization of this node (b) also demonstrated a second higher-echelon node located in the right supraclavicular region (c)
Affect on patient management
The affect of SPECT-CT on patient management over planar findings could not be assessed, since all patients went to surgery regardless of the SLN mapping results. Patient management was affected in only three breast cancer cases, where no SLN was detected on either planar or SPECT-CT. These patients received axillary block dissection.
Intra-operative nodal localization
All except one node identified on SPECT-CT imaging was located intra-operatively using a gamma-probe - the node in question was deemed to superficial for separate excision and was excised along with the primary tumour.
Discussion
Globally breast cancer is the leading female malignancy, with a substantially higher incidence in the emerging economies compared to the industrialized nations - in all likelihood due to late presentation of the patient (poor referral infrastructure, economic constraints and poor public awareness campaigns).[5,6] In South Africa, breast cancer follows suite and is both the most common form of cancer in females as well as the leading cause of death of all female malignancies.[7]
Malignant melanoma rates are on the rise the world over - its rate is surpassed only by lung cancer in females, and it currently accounts for 1% of the world's cancer incidence. South Africa, with its sunny climate and poor personal ultraviolet protection measures, has one of the highest incidences of malignant melanoma in the world, comparable to Australia.[3,5,8]
Block dissection of the regional lymph nodes, whereby all the lymph nodes draining the tumour (regardless of metastatic involvement) are dissected, e.g. axilla or inguinal region does offer the opportunity for curative surgery especially in small tumours.[9] This approach, however, is fraught with complications for example, seroma, hematoma, lymphoedema of the affected limb drained by the excised nodes, neurological complications (e.g. numbness or paraesthesias), motion restriction due to scarring, axillary web syndrome, wound dehiscence (uncoupling) and infection.[1,10,11] In fact, it has been demonstrated that there is no improved survival benefit in elective whole nodal basin excision, with a concomitant increased level of morbidity.[12] It would therefore be preferable to avoid dissection of lymph nodes where there is clearly no metastatic involvement, by means of sentinel node mapping.
The SLN is the first node draining a tumour and it has been demonstrated to confer good patient prognosis when negative for tumour histologically. SLN mapping and excision offers a less invasive technique for nodal staging with fewer complications and is considered the only reliable method for identifying micrometastatic disease.[12,13]
Single-photon emission computed tomography-computed tomography has been found to be of additional value over and above conventional planar imaging in especially breast cancer and cutaneous malignant melanoma: It has been found to determine the exact anatomical location due to better anatomical resolution of the CT component; is more sensitive for finding lymph nodes on inconclusive or negative planar studies; detected additional nodes over and above those found on planar images and has helped to reduce the number of false positive findings.[4,14,15] We have confirmed this also in our setting with the present study.
While much of the effects of SPECT-CT are based on early stage cancers (as seen in the industrialized world), locally advanced disease may demonstrate unpredictable lymphatic spread, which reiterates the need to also confirm SPECT-CT's additive function in the emerging-market nations.
The utilization of an intra-operative gamma-probe has been shown to further expedite SLN biopsy - by allowing “real-time” tracking on the radio-marked SLN intra-operatively. Although most sentinel nodes can be identified during surgery with a hand-held probe, the addition of SPECT-CT prior to surgery, further complements and augments the ease of intra-operative gamma-probe use by allowing the surgeon to change his or her surgical approach prior to localizing the SLN.[14,16,17]
Despite the clear benefits demonstrated, others have found that SPECT-CT has a limited role in SLN mapping since planar and SPECT-CT seem to demonstrate similar sensitivities in most patients, SPECT-CT has additional costs and requires extra time with additional albeit low radiation dose.[13] It has been suggested that SPECT-CT should be limited to cases where findings on planar are negative, inconclusive, where unexpected drainage is demonstrated or where lower detection rate has been demonstrated, e.g. in obese patient, patients with scarring after extirpation of melanoma or in patients with melanoma of the trunk or head - where lymphatic drainage is often unpredictable.[13,18,19,20] It has also been common practice not to use SPECT-CT in melanoma of the limbs as the vast majority of these tumours drain to inguinal or axillary nodal basins.
We found that SPECT-CT was generally well received by the surgeons and expedited surgical approach in almost our entire sample. Our findings concur with those of Stoffels et al. who reported that SPECT-CT added value in melanoma localized to the limbs. This is due to the occurence of many metastatic nodes close to the injection site and in the case of lower limb melanoma, the SLN may be anywhere en route to the groin.[12] In fact SPECT-CT has demonstrated added value in up to 27% of patients with a leg melanoma.[19]
Furthermore, Kraft and Havel demonstrated that increased BMI has no influence over detection by either planar or SPECT-CT. The same study found and inverse correlation with detection by SPECT-CT and age as well as an increased detection rate in males when compared to female.[18,21] Our study, however, found no discernable link between BMI, age or gender and SLN detection.
Another benefit of SPECT-CT imaging includes improved identification of SLN located close to the injection site, which may otherwise go undetected on planar images. Our findings were similar.[22]
Conclusion
The addition of SPECT-CT and use of intra-operative gamma-probe to planar imaging offers important benefits in patients who present with breast cancer and melanoma. These benefits include increased nodal detection, elimination of false positives and negatives and improved anatomical localization that ultimately aids and expedites surgical management. This has been demonstrated in the context of industrialized nations previously and has now also been confirmed in the setting of a emerging-market nation.
Acknowledgements
This study formed part of a larger multi-center study initated by the International Atomic Energy Association.
We would like to extend special thanks to the technologists, other doctors and general staff in the Department of Nuclear Medicine at Steve Biko Academic Hospital for their assistance in this study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Davis PG, Serpell JW, Kelly JW, Paul E. Axillary lymph node dissection for malignant melanoma. ANZ J Surg. 2011;81:462–6. doi: 10.1111/j.1445-2197.2010.05491.x. [DOI] [PubMed] [Google Scholar]
- 2.Buscombe J, Paganelli G, Burak ZE, Waddington W, Maublant J, Prats E, et al. Sentinel node in breast cancer procedural guidelines. Eur J Nucl Med Mol Imaging. 2007;34:2154–9. doi: 10.1007/s00259-007-0614-z. [DOI] [PubMed] [Google Scholar]
- 3.Herbst M. In: Cancer Association of South Africa: Fact Sheet on Solar Radiation and Skin Cancer. van Rensburg SJ, editor. Johannesburg, South Africa: CANSA; 2013. pp. 1–19. [Google Scholar]
- 4.van der Ploeg IM, Valdés Olmos RA, Nieweg OE, Rutgers EJ, Kroon BB, Hoefnagel CA. The additional value of SPECT/CT in lymphatic mapping in breast cancer and melanoma. J Nucl Med. 2007;48:1756–60. doi: 10.2967/jnumed.107.043372. [DOI] [PubMed] [Google Scholar]
- 5.Center M, Siegel R, Jemal A, editors. Global Cancer Facts and Figures. 2nd ed. Atlanta, Georgia: American Cancer Society; 2008. [Google Scholar]
- 6.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 7.Lehohla P, editor. Mortality and Causes of Death in South Africa, 2010: Findings from Death Notification. Pretoria, South Africa: Statistics South Africa; 2010. [Google Scholar]
- 8.Bastuji-Garin S, Diepgen TL. Cutaneous malignant melanoma, sun exposure, and sunscreen use: Epidemiological evidence. Br J Dermatol. 2002;146(Suppl 61):24–30. doi: 10.1046/j.1365-2133.146.s61.9.x. [DOI] [PubMed] [Google Scholar]
- 9.Vordermark JS, Jones BM, Harrison DH. Surgical approaches to block dissection of the inguinal lymph nodes. Br J Plast Surg. 1985;38:321–5. doi: 10.1016/0007-1226(85)90235-8. [DOI] [PubMed] [Google Scholar]
- 10.Roses DF, Brooks AD, Harris MN, Shapiro RL, Mitnick J. Complications of level I and II axillary dissection in the treatment of carcinoma of the breast. Ann Surg. 1999;230:194–201. doi: 10.1097/00000658-199908000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starritt EC, Joseph D, McKinnon JG, Lo SK, de Wilt JH, Thompson JF. Lymphedema after complete axillary node dissection for melanoma: Assessment using a new, objective definition. Ann Surg. 2004;240:866–74. doi: 10.1097/01.sla.0000143271.32568.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoffels I, Boy C, Pöppel T, Kuhn J, Klötgen K, Dissemond J, et al. Association between sentinel lymph node excision with or without preoperative SPECT/CT and metastatic node detection and disease-free survival in melanoma. JAMA. 2012;308:1007–14. doi: 10.1001/2012.jama.11030. [DOI] [PubMed] [Google Scholar]
- 13.Kraft O, Havel M. Localisation of sentinel lymph nodes in patients with melanomas by planar lymphoscintigraphic and hybrid SPECT/CT imaging. Nucl Med Rev Cent East Eur. 2012;15:101–7. [PubMed] [Google Scholar]
- 14.Buck AK, Nekolla S, Ziegler S, Beer A, Krause BJ, Herrmann K, et al. SPECT/CT. J Nucl Med. 2008;49:1305–19. doi: 10.2967/jnumed.107.050195. [DOI] [PubMed] [Google Scholar]
- 15.Kraft O, Havel M. Sentinel lymph node identification in breat cancer-comparison of planar scintigraphy and SPECT-CT. Open Nucl Med J. 2012;4:5–13. [Google Scholar]
- 16.Mariani G, Erba P, Manca G, Villa G, Gipponi M, Boni G, et al. Radioguided sentinel lymph node biopsy in patients with malignant cutaneous melanoma: The nuclear medicine contribution. J Surg Oncol. 2004;85:141–51. doi: 10.1002/jso.20027. [DOI] [PubMed] [Google Scholar]
- 17.Yu S, Ma K, Wong K, Leung J, Leung L. Intraoperative gamma probe for sentinel node localisation: Evaluation study. J Hong Kong Coll Radiol. 2005;8:40–8. [Google Scholar]
- 18.Kraft O, Havel M. Sentinel lymph nodes and planar scintigraphy and SPECT/CT in various types of tumours. Estimation of some factors influencing detection success. Nucl Med Rev Cent East Eur. 2013;16:17–25. doi: 10.5603/NMR.2013.0004. [DOI] [PubMed] [Google Scholar]
- 19.Vermeeren L, van der Ploeg IM, Olmos RA, Meinhardt W, Klop WM, Kroon BB, et al. SPECT/CT for preoperative sentinel node localization. J Surg Oncol. 2010;101:184–90. doi: 10.1002/jso.21439. [DOI] [PubMed] [Google Scholar]
- 20.Even-Sapir E, Lerman H, Lievshitz G, Khafif A, Fliss DM, Schwartz A, et al. Lymphoscintigraphy for sentinel node mapping using a hybrid SPECT/CT system. J Nucl Med. 2003;44:1413–20. [PubMed] [Google Scholar]
- 21.Kraft O, Havel M. Detection of sentinel lymph nodes by SPECT-CT and planar scintigraphy: The influence of age, gender and BMI. J Biomed Graph Comput. 2012;2:11–22. [Google Scholar]
- 22.Uren RF. SPECT/CT Lymphoscintigraphy to locate the sentinel lymph node in patients with melanoma. Ann Surg Oncol. 2009;16:1459–60. doi: 10.1245/s10434-009-0463-z. [DOI] [PubMed] [Google Scholar]


