Abstract
Positron emission mammography (PEM), a relatively novel breast imaging modality, provides certain advantages over magnetic resonance imaging, including the ability to image biopsy samples. However, the radiation activity associated with PEM has remained a concern in clinical practice. We present a case of an invasive ductal carcinoma that was adequately imaged with a much lower than the standard 185 to 370 MBq activity of 18F-fluorodeoxyglucose. In addition, we demonstrate ultrasound-guided core-needle biopsy sample imaging with PEM to assess adequacy of sampling, a strategy that has previously only been documented with vacuum-assisted biopsy samples.
Keywords: Activity, biopsy, cancer, fluorodeoxyglucose, positron emission mammography
Introduction
Positron emission mammography (PEM) is being increasingly utilized in breast cancer imaging. The sensitivity of PEM is comparable to that of breast magnetic resonance imaging (MRI), and its specificity has been shown to be higher than breast MRI in presurgical planning.[1] PEM provides better spatial resolution and increased sensitivity in comparison to whole-body positron emission tomography (PET) imaging.[2,3] Another advantage PEM renders over whole-body PET is the ability to use a lower activity of 18F-fluorodeoxyglucose (18F-FDG). Currently, the recommended activity for PEM is 185 to 370 MBq. We present a case of a woman with invasive ductal carcinoma whose malignancy was imaged with PEM with a much lower than the conventionally used activity of 18F-FDG. In addition, we demonstrate a new application of PEM in confirming adequate sampling of ultrasound-guided core-needle biopsy samples.
Case Report
A 67-year-old female presented to an outside hospital with a right axillary mass [Figures 1a and 1b]. An ultrasound-guided biopsy confirmed invasive ductal carcinoma. A whole-body PET-computed tomography (CT) scan performed thereafter showed a 1.7-cm mass with an SUV maximum of 5.9 in the right axilla, consistent with the patient's known malignancy, and an adjacent FDG-avid right axillary lymph node [Figure 2].
Figure 1.
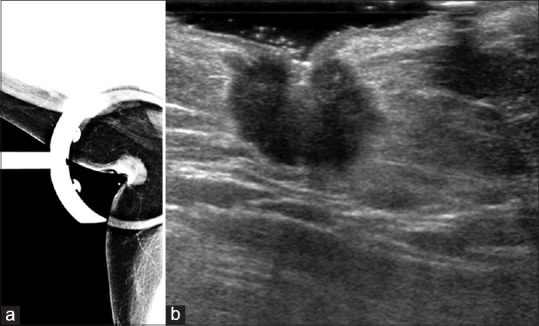
(a) Spot compression mammographic image of the right axilla of our patient shows a round hyperdense mass. (b) Ultrasound image of the right axilla shows an irregular, hypoechoic mass. On biopsy, this was found to be an invasive ductal carcinoma
Figure 2.
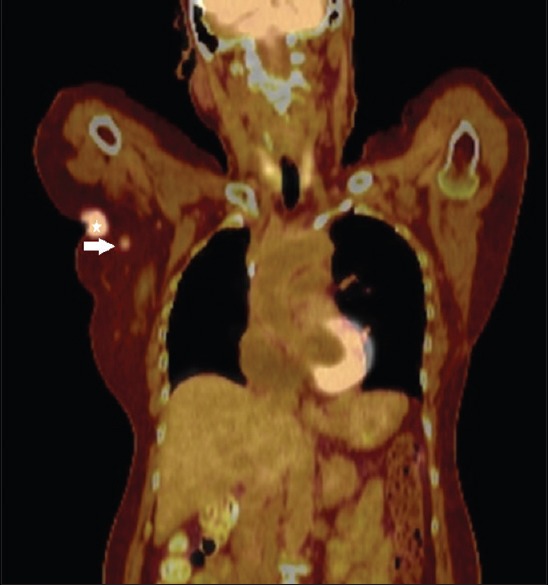
Fused coronal PET-CT image of our patient before the axillary lymph node biopsy shows a lobulated FDG-avid mass (star) in the right axilla, which represents the patient's known axillary invasive ductal carcinoma. An adjacent small FDG-avid round structure (arrow) was suspicious for metastatic axillary lymphadenopathy
A repeat whole body PET-CT scan was performed at our institution to assess extent of disease. The patient was given 362.6 MBq of 18F-FDG at 07:45 on the day of her whole body PET-CT. The scan was performed at approximately 09:18 and demonstrated stable findings in the right axilla. At 13:35, an ultrasound-guided core-needle biopsy of the patient's known FDG-avid right axillary lymph node was performed. PEM imaging (using PEM Flex Solo II, Naviscan) of the right axilla was then performed at 13:47 without administering more 18F-FDG. PEM imaging relied upon the remainder of the dose of 18F-FDG that the patient had received six hours earlier [Figure 3a]. These post-biopsy PEM images successfully and adequately displayed the patient's known right breast carcinoma utilizing much lower activity than conventional PEM imaging. In addition, these images showed that the FDG-avid right axillary lymph node had decreased in conspicuity after the biopsy in comparison to the pre-biopsy whole-body PET-CT, an indirect indication of successful biopsy sampling [Figure 3b]. Following PEM imaging of the right axilla, at approximately 14:50, the core biopsy samples were also examined with PEM imaging [Figures 4a and 4b], confirming activity within portions of the core biopsy samples.
Figure 3.
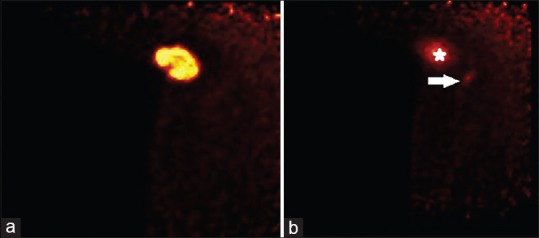
(a) PEM imaging of the right axillary tail shows the FDG-avid mass in the right axilla, which represents the patient's known invasive ductal carcinoma (IDC). Even with an approximate 37 MBq activity of 18F-FDG in the patient's system, the patient's carcinoma is well-visualized. (b) Another axillary tail postbiopsy PEM image of the right axilla shows the carcinoma (star) and the FDG-avid axillary lymph node (arrow). In comparison to the whole-body positron emission tomography-computed tomography image [Figure 2], the lymph node has changed in size and configuration, indirectly indicating correct biopsy sampling
Figure 4.
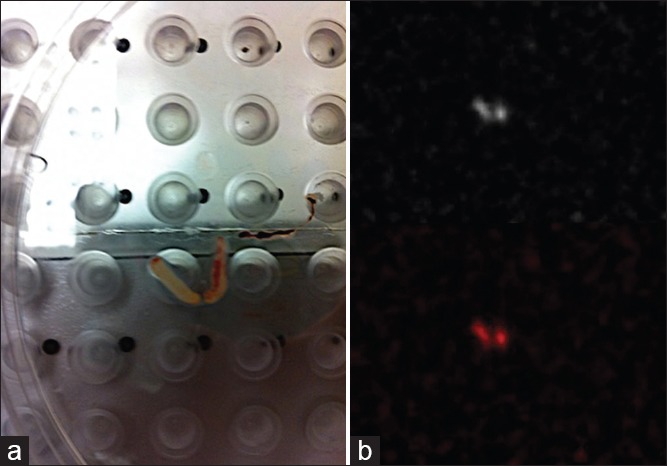
(a) Photograph of the ultrasound core needle biopsy specimen positioned on the detector plate of the PEM scanner. (b) Corresponding PEM image of the of the specimen reveals FDG activity within the core samples, confirming successful sampling of the lymph node
Discussion
Our case is of a woman with right axillary invasive ductal carcinoma whose PET-CT scan showed FDG-avid right axillary lymphadenopathy. Whole-body PET-CT imaging was performed approximately 90 minutes after 18F-FDG injection, followed by PEM imaging approximately 6 hours after injection. We additionally performed PEM imaging of the core-needle biopsy sample to assess for correct sampling.
The patient was injected with 362.6 MBq of 18F-FDG at 07:45. PEM images of the right axilla were acquired at 13:47 and of the biopsy samples at 14:50. 18F-FDG has a physical half-life of 110 min. 6 hours (3.3 half-lives) had elapsed between the initial administration of the radiotracer and the post-biopsy PEM images of the axilla leaving approximately 37 MBq of 18F-FDG activity available for PEM imaging. The time difference between the initial administration of 18F-FDG and PEM imaging of the core-needle biopsy sample was approximately 7 h or 3.8 half-lives, leaving approximately 25.9 MBq of 18F-FDG activity available for PEM imaging.
Our case illustrates that activity as low as 25.9 MBq 18F-FDG can be potentially utilized for PEM imaging, a level well below that of conventional recommendations of 185 to 370 MBq. Some recent studies have analyzed using lower levels of activity of 18F-FDG for PEM imaging. MacDonald and colleagues. studied different activities of 18F-FDG in phantom models in PEM imaging. They found that detection sensitivity was ≥90% for injected activities as low as 99.9 MBq for lesions ≥8 mm.[4] Mercier and colleagues compared the administration of 370 MBq and 185 MBq of 18F-FDG for PEM imaging and found no differences between the two activities in quality scores and BI-RADS characterization of lesions.[5] A study by Kalinyak and Schilling simulated activity reduction for PEM by reducing the number of coincident event counts used to reconstruct images.[6] They found no significant change in the sensitivity for lesion detection when the coincident event counts were reduced to represent an activity of 185 MBq.[6] Our case indicates an even lower level of activity may be sufficient for adequate PEM imaging.
A unique advantage that PEM offers over breast MRI is the ability to image not only the biopsy site but also the biopsy specimen to confirm adequate sampling. Our post-biopsy PEM images revealed that our targeted right axillary lymph node was less conspicuous and had changed in shape after the biopsy in comparison to the pre-biopsy PET-CT, indirectly suggesting correct sampling of the lymph node. In addition, PEM imaging of the core-needle biopsy samples confirmed FDG activity within portions of the samples, indicating that the correct area was sampled. This potential use of PEM to assess the adequacy of the biopsy samples has been previously described in the literature with PEM-guided biopsy samples using vacuum-assisted devices. However, to our knowledge, 18F-FDG activity in much smaller core-needle ultrasound biopsy samples has not been previously documented.
In summary, we discuss a case of utilization of lower levels of 18F-FDG activity in PEM imaging to reduce radiation dose, while still maintaining the ability to adequately visualize the patient's known breast cancer in vivo as well as assessing the adequacy of non-vacuum-assisted core-needle biopsy sampling in vitro. Since PEM imaging is being increasingly utilized, future studies are needed to confirm the validity of using these lower levels of radiotracer activity for PEM imaging.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Berg WA, Madsen KS, Schilling K, Tartar M, Pisano ED, Larsen LH, et al. Breast cancer: Comparative effectiveness of positron emission mammography and MR imaging in presurgical planning for the ipsilateral breast. Radiology. 2011;258:59–72. doi: 10.1148/radiol.10100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalles V, Zografos GC, Provatopoulou X, Koulocheri D, Gounaris A. The current status of positron emission mammography in breast cancer diagnosis. Breast Cancer. 2013;20:123–30. doi: 10.1007/s12282-012-0433-3. [DOI] [PubMed] [Google Scholar]
- 3.Rosen EL, Eubank WB, Mankoff DA. FDG PET, PET/CT, and breast cancer imaging. Radiographics. 2007;27(Suppl 1):S215–29. doi: 10.1148/rg.27si075517. [DOI] [PubMed] [Google Scholar]
- 4.Macdonald LR, Wang CL, Eissa M, Haseley D, Kelly MM, Liu F, et al. Positron emission mammography (PEM): Effect of activity concentration, object size, and object contrast on phantom lesion detection. Med Phys. 2012;39:6499–508. doi: 10.1118/1.4754651. [DOI] [PubMed] [Google Scholar]
- 5.Mercier G, Vassilakis N, Brennan J, DeVita F, Kachnic L. Clinical performance of low activity positron emission mammography. Oncology: Basic, translational and therapy. J Nucl Med. 2012;53:1231. [Google Scholar]
- 6.Kalinyak J, Schilling K. Radiotracer activity reduction in high resolution breast PET imaging. European Congress of Radiology. 2012. [Last accessed on 2014 Mar 30]. Available from: http://www.posterng.netkey.at/esr/viewing/index.php?module=viewing_poster and pi=112705 .


