Abstract
Background
We have delineated, across four prior studies, the role of positive dorsal medial prefrontal/anterior cingulate cortex (dmPFC/ACC)-amygdala circuit coupling during aversive processing in healthy individuals under stress. This translational circuit, termed the ‘aversive amplification circuit’, is thought to drive adaptive, harm-avoidant behavior in threatening environments. Here, in a natural progression of this prior work, we confirm that this circuit also plays a role in the pathological manifestation of anxiety disorders.
Methods
Forty-five unmedicated participants (N=22 generalized and social anxiety disorder/N=23 controls) recruited from Washington DC metropolitan area completed a simple emotion identification task during functional magnetic resonance imaging at the National Institutes of Health, Bethesda, MD, USA.
Findings
As predicted, a diagnosis by valence interaction was seen in whole-brain amygdala connectivity within the dmPFC/ACC clusters identified in our prior study; driven by significantly greater circuit coupling during fearful versus happy face processing in anxious, but not healthy, participants. Critically, and in accordance with contemporary theoretical approaches to psychiatry, circuit coupling correlated positively with self-reported anxious symptoms, providing evidence of a continuous circuit-subjective symptomatology relationship.
Interpretation
We track the functional role of a single neural circuit from its involvement in adaptive threat-biases under stress, to its chronic engagement in anxiety disorders in the absence of experimentally induced stress. Thus, we uniquely map a mood and anxiety related circuit across its adaptive and maladaptive stages. Clinically, this may provide a step towards a more mechanistic spectrum-based approach to anxiety disorder diagnosis and may ultimately lead to more targeted treatments.
Keywords: Anxiety, Aversive amplification, amygdala, dmPFC/DACC, connectivity
Introduction
Pathological anxiety is a large and growing global health problem1. Sufferers experience periods of crippling anxiety that adversely impact their daily living. One of the key contributing symptoms is a persistent and debilitating focus upon negative or potentially threatening life-experiences1. This negative affective bias can be experimentally quantified as elevated threat processing at the neural, psychological and behavioral levels1.
However, anxiety can also be an adaptive process that improves the ability to avoid harm. Indeed, negative affective biases towards threats are also seen in healthy individuals experiencing transient anxiety or stress1. It is plausible that the adaptive and maladaptive anxiety-driven negative affective biases are linked, perhaps falling on opposite ends of the same spectrum. Such a relationship would have implications for how we diagnose and treat these disorders, but evidence is at present lacking. This study therefore extends four of our prior studies mapping the circuit-based interactions between the dorsal medial prefrontal/anterior cingulate cortex (dmPFC/ACC) and the amygdala during adaptive threat processing in stressed healthy individuals into pathological anxiety.
Dorsal prefrontal-amygdala circuitry in anxiety
The role of the amygdala in threat processing is well known2–4, but regions of the brain do not of course respond in isolation, rather they constitute nodes in complex neural circuits5, 6. Our recent work has therefore begun to outline how interactions between the amygdala and higher cortical regions contribute towards threat processing biases. In particular, our work suggests that interactions between the dmPFC/ACC and amygdala constitute an ‘aversive-amplification’ circuit whereby increased positive coupling between these regions is associated with elevated threat processing under stress7–9. Note that this role, derived from translational animal research10, is thought to be distinct from a more commonly studied reciprocal, opposing inhibitory role11, 12 of adjacent ventral (and subgenual) regions of the prefrontal and cingulate cortex (discussed in more detail elsewhere13–15).
Specifically, we have shown that stress induced by a threat of shock in healthy individuals (a manipulation in which subjects are told they might potentially receive a shock and which reliably increases psychological, physiological, cognitive and neural concomitants of stress1) drives elevated attentional bias (on a face emotion identification task) to fearful faces16 as a function of increased positive functional connectivity between the dmPFC/ACC and amygdala (Figure 1)7. Secondly, we have replicated this finding utilizing a different technique; enhanced positive endogenous connectivity (i.e. oscillatory connectivity during ‘rest’ periods with no task) is seen between the dmPFC/ACC and the amygdala during prolonged periods of threat of shock in an adapted resting-state paradigm in healthy individuals9. Thirdly, we have shown that threat of shock leads to cognitive disturbances in working memory in healthy individuals1 that are also associated with increased coupling within this circuit17. Finally, we have reported that mimicking a pharmacological symptom of anxiety in healthy individuals - reduced serotonergic function – engages functional connectivity within this same circuit during the processing of fearful faces8. This fourth study in fact provides a putative mechanism by which selective serotonergic reuptake inhibitor (SSRI) medications may, via modulation of this circuit, alleviate anxiety8.
Figure 1.
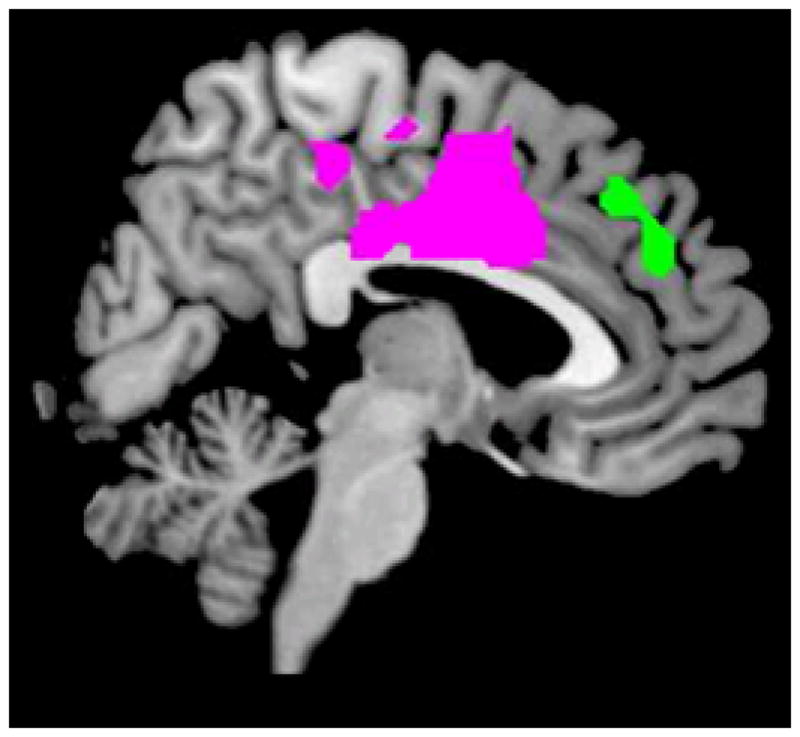
Regions of interest (ROIs) derived from our study utilizing threat of shock as a stress-induction in healthy individuals7. Both a posterior cluster encompassing dorsal anterior cingulate and medial prefrontal cortex (‘dorsal’ in violet) and a rostral cluster in the dorsal medial prefrontal cortex (‘rostral’ in green) showed increased positive connectivity with the amygdala during the processing of fearful faces under stress and were used to create ROIs for the present study. These ROIS are freely available to download from http://figshare.com/account/projects/1646
Thus, we have comprehensively mapped the workings of this circuit in healthy individuals. The primary goal of the present study, therefore, is to provide experimental evidence that this functionally-mapped circuit might play a key role in pathological anxiety disorders. Previous work focusing on threat processing in anxiety disorders has revealed abnormal activity in regions of the prefrontal / cingulate cortex (PFC) and the amygdala18–20. However studies to date have largely examined individual region activations (i.e. the change in the activity of a region in one condition vs. another), and not circuitry (i.e. the extent to which activity correlates between two regions in one condition vs. another). Correlation between regions is thought to reflect, at least in part, flow of information between these regions and can be seen in the absence of activation changes (for more information see21). Such between-region interactions thus provide more insight into the way these regions act as a circuit.
A continuous index?
It is increasingly recognized that psychiatric disorders are unlikely to fall within the categorical (healthy/unwell) diagnoses of current diagnostic criteria, but rather fall along a spectrum from more ‘normal’ to ‘impaired’ function (for more details see22). Our prior studies, in fact, also showed that activation of this circuit falls along a continuous dimension as a function of trait anxiety symptoms7, 9, with greater trait feelings of anxiety (a vulnerability factor for anxiety disorders) being associated with greater positive coupling within this circuit. This generates a secondary prediction: pathological anxiety symptoms will also fall along this continuum. That is to say, anxiety symptoms that are severe enough to interfere with daily living should be associated with even greater circuit engagement along the same dimensional index. Such a finding would, from a clinical perspective, help to reframe our understanding of anxiety disorders away from discreet diagnoses and towards more of a spectrum.
Methods
Participants
This single-site study was completed at the National Institutes of Health (NIH) Clinical Centre in Bethesda, MD, USA. Participants were recruited from the Washington DC metropolitan area for the study via flyers and advertisements placed in local newspapers. One line of recruitment sought subjects who had anxiety problems, whilst another line did not specify psychiatric issues. Following an initial phone screen, participants visited the NIH for comprehensive screening by a clinician: a physical exam, urine screen, and a Structured Clinical Interview for DSM-IV (SCID23). Exclusion criteria were: 1) contraindicated medical condition, 2) past/current psychiatric disorders other than anxiety disorders, and 3) use of psychoactive medications/illicit drugs as per urine screen. Subjects passing this screen were given the option to participate in the study either as a healthy control or a patient (depending upon diagnosis). Subjects completed measures of anxiety (STAI25,24,24), depression (BDI25) and IQ (WASI26). Five patients were excluded due to scan acquisition artefacts (e.g. caused by extreme movements or scanner malfunction) such that the final sample consisted of 45 unmedicated individuals of which 22 were suffering from a current anxiety disorder representing the naturalistic recruitment frequencies over a two year period (15 generalized anxiety disorder [GAD; of which 9 were comorbid with a secondary diagnosis of social anxiety disorder (SAD)]; and 7 SAD) and 23 were healthy. In the patients, the mean estimated illness duration was 16(8) years. Seven patients had undergone prior pharmacological treatment which was discontinued (>10 years prior, N=5; 6 months prior, N=1; 2 months prior, N=1). Unmedicated status was required to avoid potential drug-linked vascular confounds. Patients and controls were matched for demographic variables (table 1). Subjects provided written informed consent that was approved by the Combined Neuroscience Institutional Review Board of the National Institutes of Health.
Table 1. demographic and questionnaire measures.
All subjects right-handed except for 1 patient and 1 control who were left-handed.
| ANX (N= 22) | HC(N=23) | F | p | |
|---|---|---|---|---|
| Gender | 16 | Female | 14 | Female |
| Age | 28 (8) | 28 (6) | 0.04 | 0.9 |
| WASI | 118 (10) | 119 (10) | 0.2 | 0.6 |
| STAI state | 40 (12) | 25 (4) | 33 | <0.001 |
| STAI trait | 49 (12) | 26 (5) | 71 | <0.001 |
| BDI | 9 (9) | .7 (1) | 21 | <0.001 |
ANX=anxiety disorders; HC=healthy control; Age is in years; WASI= Wechsler Abbreviated Scale of Intelligence; STAI = state-trait anxiety inventory BDI=beck depression inventory; (standard deviation); F =f-test value
Task
Subjects used a button-box to identify whether faces16 were fearful or happy. The task consisted of 88 trials (44 fear and 44 happy) with 2000–4000ms jitter between trials. Each stimulus was presented for 990ms and 30s of fixation was presented at the start and end of the task. Note this is the same task adopted previously in healthy controls7 but without the concurrent threat of shock stress manipulation used in that study. Subjects were asked to respond as quickly as possible using a button box placed upon their abdomen in the scanner. The task was projected on a screen to the rear of the scanner, visible by means of a mirror attached to the head coil.
Functional Imaging
Using a 3T Siemens Skyra scanner one 207 volume acquisition epi sequence was acquired: flip-angle 70°; TR=2000ms;TE=30ms; FOV=100; slice-thickness=3mm; matrix=64×64 sagittal. The first 5 volumes from each run were discarded to allow for scanner equilibration. The structural sequence comprised an MPRAGE anatomical reference image: flip angle 9°; TR=1900ms; TE=2.1; inversion time=450; FOV=100; slice-thickness=0.9mm; matrix=256×256. Images were pre-processed and analyzed using ‘statistical parametric mapping’ (SPM) 8 (Wellcome Trust Centre for Neuroimaging, UK). SPM refers to the “conjoint use of the general linear model (GLM) and Gaussian random field (GRF) theory to analyze and make classical inferences about spatially extended data through statistical parametric maps (SPMs)” (see http://www.fil.ion.ucl.ac.uk/spm/doc/intro/ and expanded below). Analysis consisted of five well-established steps; 1) preprocessing to transform the blood-oxygen level dependent (BOLD) signal acquired during scanning into the same standardized space across time and subjects; 2) statistical activation analysis to generate BOLD signal activation estimates for each trial type (fearful vs. happy faces) for each subject (first-level event-related analysis using mass univariate general-linear models) 3) statistical connectivity analysis to estimate regions across the whole brain which significantly correlate with the BOLD activity seen in the amygdala during each trial type (a psycho-physiological interaction analysis); 4) group-level analysis in which summary estimates of activation or connectivity for each subject is compared across groups (second-level analysis using t-tests); 5) group-level continuous variable analysis in which summary estimates of activation or connectivity for each subject are correlated with individual difference measures (e.g. trait personality scales or mean reaction times). Preprocessing consisted of within-subject realignment, coregistration, segmentation, normalization and smoothing (with a gaussian kernel 8mm full width at half maximum). In an event-related analysis, a general-linear model was used to estimate the BOLD signal change associated with the onset-times of each face valence (fear, happy). Motion parameters created during the realignment phase were also included as ‘nuisance’ regressors in this model (to account for noise associated with subject motion). This was repeated for each subject and asks: what is the BOLD signal change associated with fearful and happy faces?
A generalized psycho-physiological interaction (gPPI) GLM was then created for each subject in the same manner as our prior studies7, 8 (note these were separate studies with separately recruited samples). Specifically, SPM8 code was used to generate the following regressors from the event-related model described above: 1) an eigenvariate summary of BOLD signal spatially localized within spatial confines of the amygdala seed used in our prior study7(an anatomical region of interest (ROI) defined by Automated Anatomical Labeling library27) across time; 2) separate ‘psychological’ regressors representing the onsets of each happy face and each fearful face; and 3) PPI interaction terms representing the interaction between the first two regressors. Next a GLM was created for each subject in which one regressor representing de-convolved BOLD signal was included alongside each psychological and PPI interaction terms for each event type. This model therefore asks: for each subject, which regions of the brain show a BOLD signal which correlates significantly with that of the amygdala during the events of interest (fear, happy)? This is the circuit-coupling measure we are interested in.
For each subject we then created a contrast representing regions across the whole brain which correlated more strongly with the amygdala during fearful face processing than happy face processing (fear versus happy contrast). These within-subject fear versus happy contrasts were then compared at the group level in a standard SPM8 two-sample (healthy versus anxious) t-test. This analysis provided us with an estimate of regions, across the whole brain, which showed greater correlation with the amygdala in anxious patients than healthy controls during fearful (relative to happy) face processing (i.e., a diagnosis by valence interaction). Similar analyses were completed for event-related activations of each trial vs. baseline.
In order to directly compare cross-study activation with our prior study we also created a priori regions of interest from the clusters in our first study7 (see figure 1) using the ‘get SPM cluster’ function of MARSBAR toolbox for SPM828. These clusters were generated at a threshold of p<0.001 uncorrected in our prior data from the within subject, whole-brain, threat by valence interaction map generated using the flexible factorial model in SPM8 (for more details refer to the original paper28 or download the ROIs from: http://figshare.com/account/projects/1646). This constituted the largest more dorsal and posterior peak dmPFC/dACC cluster (referred to as dorsal below) as well a more rostral dmPFC (referred to as rostral below). Activations falling within these regions in the group analyses of the present study can be said to overlap with the activation in our prior study.
We report Montreal Neurological Institute (MNI) standardized coordinates (denoted as x,y,z). For additional corroboration, we also extracted the activation and connectivity estimates (betas) from the peak-voxel (x,y,z=2,2,40) from our prior study. These extracted betas were then analyzed using general linear models in SPSS.
Continuous variable fMRI analyses were completed by separately correlating 1) STAI trait anxiety25 (measured on the day of testing), and 2) behavioral bias (fear minus happy reaction times) with the fear vs. happy connectivity estimates derived from the connectivity analysis. This analysis therefore asks, across the whole sample or within groups, which connectivity estimates (across the whole brain) correlate significantly with the bias or trait anxiety variables?
Upper and lower value boundaries for outliers were determined via the formula (3rd Quartile ± (2.2*(3rd Quartile – 1st Quartile)29 and extreme values fall within the bounds for outliers: −0.4 to 96.4 for trait anxiety; −2.6 to 4.8 for connectivity betas; hence no subjects were excluded. Interactions of interest were significant at p<0.05 family wise error rate (FWE) corrected for multiple comparisons across 1) the whole brain as well as 2) within our a priori regions of interest. Additional analyses are reported at p<0.001 uncorrected where they are of a priori relevance. Findings below these thresholds are denoted as non-significant.
Results
1) Traditional diagnosis analysis
Whole-brain connectivity of the diagnosis by valence interaction analysis revealed, as hypothesized, a whole-brain peak amygdala connectivity in the dmPFC/dACC (peak-x,y,z=4,−8,32, t=4.69, p(FWE-cluster-level-corrected)<0.03;Fig 2A/Table 2). Analyses of this connectivity using an ROI generated from the largest ROI cluster (dorsal) from our prior study (Fig 1) revealed significant overlap across studies (peak-x, y,z=4,−8,32, t=4.43, p(FWE-cluster-level-corrected)=0.001). The same pattern was seen at a non-significant threshold in an ROI generated from the rostral cluster (peak-x,y,z=0 26 48, t=3.09, p(uncorrected)<0.002).
Figure 2. Categorical analysis.
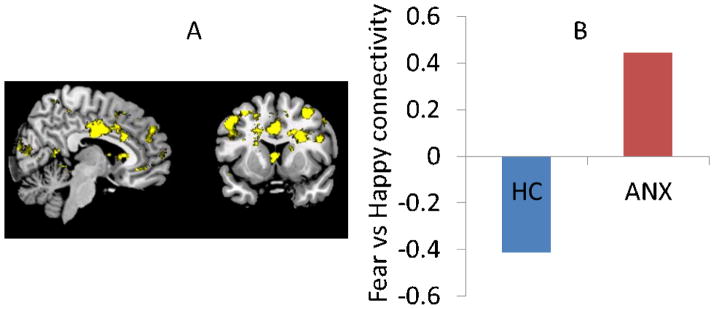
A) A whole-brain diagnosis by valence interaction in dmPFC-amygdala connectivity was seen driven by B) increased circuit activity during fearful vs. happy faces in patients (ANX= anxiety disorder; HC = healthy control; data extracted for illustration purposes from a priori peak x,y,z=2 2 40).
Table 2.
Connectivity analysis clusters Diagnosis x valance (p≤0.001)
| x,y,z | k | T | AAL |
|---|---|---|---|
| 4 −8 32 | 99 | 4.69 | Cingulum_Mid_R' |
| −4 −6 36 | 4.14 | Cingulum_Mid_L' | |
| 6 0 34 | 3.68 | Cingulum_Mid_R' | |
| 20 −10 34 | 127 | 4.65 | Cingulum_Mid_R' |
| 30 −4 40 | 4.64 | Precentral_R' | |
| 22 −6 44 | 4.62 | Precentral_R' | |
| −28 −14 26 | 46 | 4.58 | Insula_L' |
| −30 −4 32 | 3.93 | Precentral_L' | |
| −26 −22 26 | 3.83 | Caudate_L' | |
| −66 −26 2 | 35 | 4.46 | Temporal_Mid_L' |
| −16 44 −6 | 17 | 4.39 | Cingulum_Ant_L' |
| −14 −30 48 | 10 | 4.37 | Cingulum_Mid_L' |
| 34 −62 16 | 14 | 4.29 | Calcarine_R' |
| −26 16 52 | 38 | 4.19 | Frontal_Mid_L' |
| −18 22 46 | 3.43 | Frontal_Mid_L' | |
| −12 22 4 | 23 | 4.18 | Caudate_L' |
| −30 38 −4 | 12 | 4.08 | Frontal_Inf_Orb_L' |
| −52 | 37 | 4.06 | Cerebelum_4_5_L' |
| −36 −34 30 | 13 | 4.03 | Parietal_Inf_L' |
| 40 −30 6 | 4 | 4 | Temporal_Sup_R' |
| 28 10 28 | 13 | 3.96 | Frontal_Inf_Oper_R' |
| −26 0 26 | 3 | 3.96 | Caudate_L' |
| 26 −20 32 | 5 | 3.9 | Caudate_R' |
| 34 −4 38 | 2 | 3.88 | Precentral_R' |
| 20 12 34 | 3 | 3.84 | Cingulum_Mid_R' |
| −10 −26 44 | 4 | 3.82 | Cingulum_Mid_L' |
| 0 6 8 | 7 | 3.81 | Caudate_L' |
| 18 16 14 | 3 | 3.79 | Caudate_R' |
| −34 −66 6 | 3 | 3.69 | Occipital_Mid_L' |
| 0 12 34 | 22 | 3.69 | Cingulum_Mid_L' |
| −14 28 8 | 2 | 3.6 | Caudate_L' |
| −16 50 16 | 6 | 3.6 | Frontal_Sup_L' |
| 48 14 28 | 2 | 3.57 | Frontal_Inf_Oper_R' |
| 42 −14 −10 | 2 | .54 | Insula_R' |
| 26 −52 14 | 2 | 3.54 | Calcarine_R' |
| 36 −48 34 | 2 | 3.53 | Parietal_Inf_R' |
| −50 24 36 | 5 | 3.52 | Frontal_Mid_L' |
| −58 | 6 | 3.46 | Temporal_Sup_L' |
| 14 40 42 | 2 | 3.44 | Frontal_Sup_R' |
AAL=automated anatomical label27; k= cluster size;x,y,z= Montreal Neurological Institute (MNI) Coordinates
Breaking-down this interaction revealed a significant a priori fear versus happy activation in patients (dorsal: peak-x,y,z=14,4,36, t=4.0, p(uncorrected)=0.0008), but not controls (dorsal: p(uncorrected)>0.15). Betas extracted from the peak-x,y,z=2,2,40 voxel from our prior paper for illustration purposes showed that this valence by diagnosis interaction (F(1,43)=5.3, p=0.03; figure 2B) was driven by increased coupling to fear vs. happy in patients (t(21)=2.2,p=0.039), but not healthy controls (p=0.2).
Event-related analysis confirmed that the amygdala and dmPFC were significantly active in both conditions across all subjects (all trials versus baseline within ROIs: rostral x,y,z=−2,2,− 50, t=4.0 1,p(FWEvoxel)= 0.013 / dorsal x,y,z=2,8,56,t=3.88p(uncorrected)=0.0002 / amygdala x,y,z=28,−2,12, t=5.09 p(FWE-voxel)<0.001). However, breaking this down into trials and groups revealed no significant interaction with groups (rostral: valence by diagnosis: x,y,z=−2,4,30, t=1.7, p(uncorrected)=0.045/ dorsal: x,y,z=−2,2,28, t=1.9, p(uncorrected)=0.029 / amygdala valence by diagnosis p>0.4). Exploratory whole brain event-related activation interactions are presented in the supplement.
2) Continuous variable analysis
This fear vs. happy connectivity was seen along a continuum across the sample as a whole (whole-brain peak in the dmPFC (xyz=14, −8,52, T=5.09,p(FWE-cluster level)<0.001/dorsal ROI p(FWE-cluster level)<0.002) / rostral ROI p(uncorrected)=0.001)), but critically (as this is confounded by group effects) in the patient group alone (dorsal peak-x,y,z=−8,10,30, t=4.6,p(uncorrected)=0.00008 Fig 3A.). Moreover, no correlations were seen in depression ratings (BDI) across the whole group or within the patients alone (all p>0.2), indicating that this effect was specific to anxiety symptoms.
Figure 3. Continuous variable analysis.
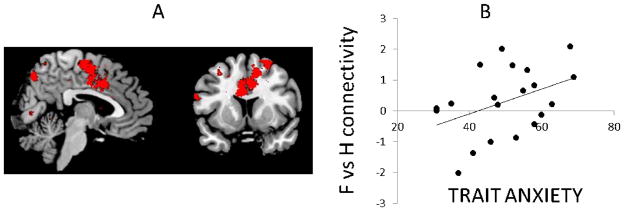
A) whole-brain positive correlation with trait anxiety scores: dmPFC-amygdala coupling falls along a continuum of self-reported anxiety symptoms within the patient group (image represents a group map of the regions in the brain which correlate with trait anxiety for the fear vs happy contrast) B) Data extracted from a priori peak x,y,z=2,2,40 in the patient group for illustration purposes.
Sub-diagnosis
SPSS GLMs of estimates extracted from the a priori peak voxel (xyz =2,2,40) revealed no significant differences on variables of interest across DSM sub-diagnoses (valence [fear, happy] by diagnosis [GAD; SAD; GAD/SAD; Healthy] interaction; F(3,41)=2.2,p=0.1,partial-eta2=0.1,95% confidence intervals=[.00;.29]); main-effect of diagnosis: p<0.2).
Restricting the primary analyses to a primary-GAD diagnosis (i.e., including GAD and comorbid GAD/SAD (N=15) but excluding SAD-only) replicated the whole-group effects (dorsal peak versus trait anxiety: x,y,z=4, −8,38,t=3.88,p(uncorrected)<0.001];[x,y,z=2,2,40 versus trait-anxiety;1- tailed:r(38)=0.3,p=0.04]).Thus, traditional sub-diagnoses are not sufficient to explain the neurobiological abnormality.
Behavior
Task accuracy was 81%, with a mean RT of 705ms for happy and 708ms for fearful faces. There was no main effect of valence in RT across the sample as a whole (fear versus happy RT t(44)=0.4,p=0.7) or a group by valence interaction in RT(p=0.14). However, supporting a brain-behaviour relationship, whole brain analysis across all subjects revealed a peak in the in the dorsal ACC driven by significant a negative relationship between fear vs. happy connectivity and fear versus happy reaction time (x,y,z=0,12,22,t=4, p(uncorrected)=0.0001). In other words, greater connectivity during the processing of fearful faces relative to happy faces was associated with faster responding to fearful relative to happy faces
Discussion
This study confirmed our hypothesis that engagement of the ‘aversive amplification circuit’ – recruited during stress in healthy individuals in our prior studies7, 9 - would be elevated in the absence of shock threat in pathological anxiety. Specifically we show elevated positive coupling within the dmPFC/ACC-amygdala circuit during fearful-face processing in generalized and social anxiety disorders. Moreover, we demonstrate that this elevated coupling follows a continuum of trait anxiety, with patients showing greatest coupling also presenting with the most severe symptomatology. Critically, this effect also overlaps the peaks highlighted in our prior study7. Thus, we reveal a circuit which contributes to both adaptive anxiety responses and, when chronically activated, to maladaptive responses; a prerequisite for more mechanistic, neurobiologically-rooted diagnosis and treatment of pathological anxiety.
From adaptive to maladaptive anxiety: a neural mechanism
We have previously termed this circuit an ‘aversive amplification’ circuit in accordance with translational rodent findings. Specifically, rodent work has shown the prelimbic prefrontal cortex to drive amygdala activity and lead to increased fear responding10. In humans, dorsal regions of the prefrontal (dmPFC) and anterior cingulate (dACC) cortex have been argued to represent the human functional homologues of this region30, 31. Prior work across a number of anxiety disorders has, for example, confirmed hyperactivity in the dmPFC/dACC and/or the amygdala in simple event-related studies5, 19, 20, 32, 33. Indeed, the studies by Milad30 and Mechias20 demonstrate a similar pattern to both our present and prior studies7 of a larger more posterior cluster and a smaller more rostral prefrontal cluster. In these studies, it is argued that the function of these regions is in fear-conditioning and conscious appraisal of threats, respectively, both functions which align well with our proposed circuit function. The present study, however, employs connectivity analysis to examine the coupling between this dorsal region and the amygdala in a circuit in pathological anxiety. This is important because coupling is thought to represent a distinct informational process relative to activation; reflecting, specifically, the flow of information and attentional processes21. It is not that these regions are any more or less efficient at processing information in the pathological condition; rather the extent to which they communicate is altered.
Our findings therefore allow us to map out a potential neural pathway for a key symptom which unites anxiety disorders, namely chronically elevated threat processing. The amygdala may detect threats, but the dACC/dmPFC may be a central node of a broader anxiety circuit, playing a key role in integrating threat information and orchestration response expression via synchronized activity with distant brain regions. Thus, this circuit is activated in stressful environments (such as shock anticipation7) to promote the adaptive34 detection of threatening stimuli (at the expense of non-threatening stimuli17). In healthy individuals under innocuous circumstances, mild threats (e.g., fearful faces) do not increase amygdala-dmPFC coupling, but in pathological anxiety this circuit becomes permanently ‘switched-on’, even in innocuous contexts, and contributes to a crippling focus upon negative life experiences. Of course this is not the only symptom characteristic of pathological anxiety, but it is a core feature which may unify both adaptive and pathological anxiety.
Clinical implications
Our ability to map a potential symptom pathway from adaptive to maladaptive states (and encompassing translational preclinical work) thus significantly improves the clinical potential of this circuit. Firstly, from a diagnostic perspective, we show that neural circuit engagement exists along a spectrum, as a function of self-reported symptoms, and irrespective of traditional DSM-prescribed generalized or social anxiety disorder diagnosis. Psychiatry is the only branch of medicine in which diagnosis is uniquely based upon self-reported symptoms rather than underlying mechanisms; the present findings provide the potential beginnings of a dimensional, mechanistic anxiety-index with diagnostic utility22, 35. Indeed, anxiety disorder subtypes are highly co-morbid36, 37 and our present data are consistent with the assumption that this is because –at least as far as generalized and social anxiety disorders go - the neural circuit underlying a core symptom of anxiety disorders, a bias towards threats, falls along a continuous diagnosis-independent spectrum. Such a spectrum could comprise a row of the “negative valence symptoms” category of the Research Domains Criteria (RDoC) matrix35, 38 which seeks to create biologically-informed psychiatric diagnoses. Future work with a much larger sample of individuals, a broader range of anxious traits, and across multiple sites will be the next step towards translating this into a clinically useful measure.
Secondly, our prior work with this circuit provides a mechanism by which we may be able to target treatments. The direction of correlation between symptom severity and circuit engagement suggests we should attempt to disrupt activity within this circuit. We have, in fact, shown this circuit to be inhibited by serotonin 8, such that serotonin reduction serves to increase activity within this circuit during the processing of fearful faces. Thus, SSRIs may restore inhibition of this circuit39, reducing responses to aversive stimuli. Such an understanding is key because, despite the ubiquity of SSRI medication, our understanding of their mechanism of action is almost entirely lacking39 leading to inefficient prescription. Perhaps this circuit will provide a means of recognizing patients who will show positive treatment response. In a field where a large proportion of patients fail to respond to their first treatment a small increase in success-rate would be beneficial.
Future work can therefore ask: what interventions, pharmacological or psychological, can serve to attenuate activity within this circuit during aversive processing? There is evidence, for instance, that cognitive-based treatments can target nodes within this circuit40. One of the biggest impediments to treatment development is the failure of animal screens to scale up to humans and a lack of naturalistic human screening markers1, 41. Since we have shown that it is possible to safely and reversibly activate this circuit in healthy individuals8, whilst at the same time linking it to clinical presentation, we may be able to use provocation of this circuit as a screen for more targeted assessment of candidate anxiolytics1, 42.
Additional observations
To demonstrate validity of our findings from multiple angles we obtained traditional sub-type diagnoses. This analysis replicated key effects independent of sub-diagnosis, but it should be noted that our naturalistic sample contained fewer subjects with a SAD diagnosis alone. Additional analyses indicated that this did not unduly impact the overall findings, but extra caution should be exercised in drawing conclusions about this particular subgroup given sample size.
It is important to note, moreover, that we are not arguing that this is the sole role of this circuit and, furthermore, that we are somewhat agnostic regarding naming the prefrontal region (e.g. dACC/dmPFC). Indeed, there is considerable variability in nomenclature, proposed function and activation patterns across these regions within the literature. What is critical is that we have seen an overlapping pattern across multiple studies examining anxiety-related processes using matched tasks. At this point, the specific name or function is arguably less important that the possibility that we are able to reveal a consistent, potentially clinically-relevant, signature from multiple related angles.
Conclusion
This study demonstrates engagement of the dmPFC-amygdala circuit during aversive processing in pathological anxiety. A detailed understanding of the relationship between neural circuitry and such core anxiety symptoms is, we argue, a prerequisite for more targeted diagnosis and treatments. We hope that this study constitutes a first step towards a more mechanistic and dimensional understanding of the pathology underlying anxiety disorders.
Supplementary Material
Systematic Review.
This study was part of a programmatic sequence of studies and takes its primary inspiration from these preceding studies. However, inspiration was also drawn from translational animal work 10,30, 31 and both original studies and review papers exploring activity within the studied regions in both patient and healthy populations2–5, 18–20, 32, 33. Further inspiration was drawn from data (again both original studies and reviews) exploring connectivity within this circuit13 and a related ventral circuit 11–15 in both patients and healthy controls. To the best of our knowledge, however, this is the first paper exploring connectivity within this circuit across social/generalized anxiety disorder and healthy controls. Papers were identified using pubmed/google scholar searches including combinations of the terms ‘anxiety’, ‘stress’, ‘anxiety disorders’, ‘GAD’, ‘social anxiety’, ‘PPI’, ‘fMRI’, ‘connectivity’, ‘amygdala’, ‘coupling’, ‘dACC’, ‘dmPFC’ around August 2013.
Interpretation.
The present data, together with our prior work, suggest that there may be a common mechanism, namely positive amygdala-dmpfc coupling during aversive processing, underlying both healthy stress responses and social and generalized anxiety disorders. Moreover this mechanism may track subjective anxiety symptoms such that greater recruitment of this circuit is associated with greater self-reported trait anxiety. Although early experimental work, this has two potential clinical implications. Firstly, this a step away from categorical diagnoses based upon symptoms and towards a more spectrum-based, mechanistic, understanding of anxiety pathology. Specifically, the present data provide experimental support for the idea that anxiety sub-types may share overlapping neurobiological abnormalities that fall along a spectrum from adaptive to pathological. Secondly, from a clinical perspective, this data may ultimately help target treatments. We have shown this circuit to be modulated by serotonin and as such we provide a potential mechanism by which such drugs enact their anxiolytic properties (which is still largely unknown). This may, in turn, provide a potential means of identifying individuals who will respond to such treatments (e.g. those who would be better suited to psychological or pharmacological intervention).
Acknowledgments
Funding: Intramural research program of National Institute of Mental Health
Role of the funding source: the study sponsor had no input in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication
This work was funded by the intramural program of the National Institutes of Health. OJR is supported by an individual Medical Research Council Career Development Award Fellowship. The authors declare that, except for income received from the primary employer, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest. Part of this data was included in an oral presentation by CG at the Society for Biological Psychiatry 2013 in San Francisco. OJR, LL, KV, PA, CG had access to data. OJR, CG designed research; LL, PA recruited and scheduled subjects; OJR, LL, KV, PA tested subjects; OJR, LL, PA processed data; OJR and CG analyzed data; CG, OJR, KV wrote paper. We thank Katherine O’Connell and Monique Ernst for helpful manuscript assistance.
Footnotes
Conflict of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Robinson OJ, Vytal K, Cornwell BR, Grillon C. THE IMPACT OF ANXIETY UPON COGNITION: PERSPECTIVES FROM HUMAN THREAT OF SHOCK STUDIES. Frontiers in Human Neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cools R, Calder A, Lawrence A, Clark L, Bullmore E, Robbins T. Individual differences in threat sensitivity predict serotonergic modulation of amygdala response to fearful faces. Psychopharmacology. 2005;180(4):670–9. doi: 10.1007/s00213-005-2215-5. [DOI] [PubMed] [Google Scholar]
- 3.Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM. Antidepressant Drug Treatment Modifies the Neural Processing of Nonconscious Threat Cues. Biological Psychiatry. 2006;59(9):816–20. doi: 10.1016/j.biopsych.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 4.Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nature Reviews Neuroscience. 2009;10(6):423–33. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- 5.Etkin A. Functional neuroanatomy of anxiety: a neural circuit perspective. Curr Top Behav Neurosci. 2010;2:251–77. doi: 10.1007/7854_2009_5. [DOI] [PubMed] [Google Scholar]
- 6.Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends in Cognitive Sciences. 2012;16(1):61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Robinson OJ, Charney DR, Overstreet C, Vytal K, Grillon C. The adaptive threat bias in anxiety: Amygdala–dorsomedial prefrontal cortex coupling and aversive amplification. Neuroimage. 2012;60(1):523–9. doi: 10.1016/j.neuroimage.2011.11.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson OJ, Overstreet C, Allen PS, Letkiewicz A, Vytal K, Pine DS, et al. The role of serotonin in the neurocircuitry of negative affective bias: Serotonergic modulation of the dorsal medial prefrontal-amygdala ‘aversive amplification’ circuit. Neuroimage. 2013;78(0):217–23. doi: 10.1016/j.neuroimage.2013.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vytal KE, Overstreet C, Charney DR, Robinson OJ, Grillon C. Sustained anxiety increases amygdala-dorsomedial prefrontal coupling: A mechanism for maintaining an anxious state. Journal of Psychiatry and Neuroscience. 2014;3;39(4):130145. doi: 10.1503/jpn.130145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable Roles of Prelimbic and Infralimbic Cortices, Ventral Hippocampus, and Basolateral Amygdala in the Expression and Extinction of Conditioned Fear. Neuropsychopharmacology. 2011;36(2):529–38. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8(6):828–34. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 12.Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, et al. The structural and functional connectivity of the amygdala: From normal emotion to pathological anxiety. Behavioural Brain Research. 2011;223(2):403–10. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Passamonti L, Crockett MJ, Apergis-Schoute AM, Clark L, Rowe JB, Calder AJ, et al. Effects of Acute Tryptophan Depletion on Prefrontal-Amygdala Connectivity While Viewing Facial Signals of Aggression. Biological Psychiatry. 2012;71(1):36–43. doi: 10.1016/j.biopsych.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motzkin JC, Philippi CL, Wolf RC, Baskaya MK, Koenigs M. Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biological Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.02.014. (Advanced Online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson OJ, Letkiewicz AM, Overstreet C, Ernst M, Grillon C. The effect of induced anxiety on cognition: threat of shock enhances aversive processing in healthy individuals. Cogn Affect Behav Neurosci. 2011;11:217–27. doi: 10.3758/s13415-011-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vytal K, Charney D, Overstreet C, Robinson OJ, Grillon C. The Role of Dorsomedial Prefrontal Cortex circuitry in Anxiety-Related Cognitive Impairment In Submission. 2013 [Google Scholar]
- 18.Rauch SL, Shin LM, Wright CI. Neuroimaging Studies of Amygdala Function in Anxiety Disorders. Annals of the New York Academy of Sciences. 2003;985(1):389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- 19.Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, et al. A Functional Magnetic Resonance Imaging Study of Amygdala and Medial Prefrontal Cortex Responses to Overtly Presented Fearful Faces in Posttraumatic Stress Disorder. Arch Gen Psychiatry. 2005;62(3):273–81. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 20.Mechias M-L, Etkin A, Kalisch R. A meta-analysis of instructed fear studies: Implications for conscious appraisal of threat. Neuroimage. 2010;49(2):1760–8. doi: 10.1016/j.neuroimage.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 21.Salinas E, Sejnowski TJ. Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci. 2001;2(8):539–50. doi: 10.1038/35086012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kupfer DJ, Regier DA. Neuroscience, Clinical Evidence, and the Future of Psychiatric Classification in DSM-5. American Journal of Psychiatry. 2011;168(7):672–4. doi: 10.1176/appi.ajp.2011.11020219. [DOI] [PubMed] [Google Scholar]
- 23.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders—Patient edition (SCID-I/P, 11/2002 Revision) New York: New York State Psychiatric Institute; 2002. [Google Scholar]
- 24.Spielberger CD, Gorsuch RL, Lushene RE. The state-trait anxiety inventory. Palo Alto, Calif: Consulting Psychologists Press Inc; 1970. [Google Scholar]
- 25.Beck AT, Steer RA. BDI, Beck depression inventory: manual. Psychological Corporation; New York: 1987. [Google Scholar]
- 26.Wechsler D. Wechsler abbreviated scale of intelligence. Psychological Corporation; 1999. [Google Scholar]
- 27.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. Neuroimage. 2002;15(1):273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 28.Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. Neuroimage. 2002;16(2) [Google Scholar]
- 29.Hoaglin DC, Iglewicz B. Fine-Tuning Some Resistant Rules for Outlier Labeling. Journal of the American Statistical Association. 1987;82(400):1147–9. [Google Scholar]
- 30.Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A Role for the Human Dorsal Anterior Cingulate Cortex in Fear Expression. Biological Psychiatry. 2007;62(10):1191–4. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 31.Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Current Opinion in Neurobiology. 2006;16(6):723–7. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Graham BM, Milad MR. The Study of Fear Extinction: Implications for Anxiety Disorders. Am J Psychiatry. 2011;168(12):1255–65. doi: 10.1176/appi.ajp.2011.11040557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin LM, Bush G, Milad MR, Lasko NB, Brohawn KH, Hughes KC, et al. Exaggerated Activation of Dorsal Anterior Cingulate Cortex During Cognitive Interference: A Monozygotic Twin Study of Posttraumatic Stress Disorder. Am J Psychiatry. 2011;168(9):979–85. doi: 10.1176/appi.ajp.2011.09121812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat Rev Neurosci. 2013;14(7):488–501. doi: 10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research Domain Criteria (RDoC): Toward a New Classification Framework for Research on Mental Disorders. American Journal of Psychiatry. 2010;167(7):748–51. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 36.Kessler RC, Avenevoli S, McLaughlin KA, Green JG, Lakoma MD, Petukhova M, et al. Lifetime comorbidity of DSM-IV disorders in the US National Comorbidity Survey Replication Adolescent Supplement (NCS-A) Psychological Medicine. 2012;42(09):1997–2010. doi: 10.1017/S0033291712000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goisman RM, Goldenberg I, Vasile RG, Keller MB. Comorbidity of anxiety disorders in a multicenter anxiety study. Comprehensive Psychiatry. 1995;36(4):303–11. doi: 10.1016/s0010-440x(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 38.Cuthbert B, Insel T. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Medicine. 2013;11(1):126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCabe C, Mishor Z, Filippini N, Cowen PJ, Taylor MJ, Harmer CJ. SSRI administration reduces resting state functional connectivity in dorso-medial prefrontal cortex. Molecular psychiatry. 2011;16(6):592–4. doi: 10.1038/mp.2010.138. [DOI] [PubMed] [Google Scholar]
- 40.Straube T, Glauer M, Dilger S, Mentzel H-J, Miltner WH. Effects of cognitive-behavioral therapy on brain activation in specific phobia. Neuroimage. 2006;29(1):125–35. doi: 10.1016/j.neuroimage.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 41.Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13(10):1161–9. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harmer CJ, Cowen PJ, Goodwin GM. Efficacy markers in depression. Journal of Psychopharmacology. 2011;25(9):1148–58. doi: 10.1177/0269881110367722. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


