Abstract
Background
Clinical pharmacists who review medication orders can reduce potentially inappropriate medications (PIMs) in hospitalized elderly patients, but this approach may be inefficient for settings with high clinical volume.
Design
Pilot intervention.
Setting
Academic, tertiary care hospital.
Participants
Hospitalized geriatric patients, age 65 or older, admitted to General Medicine, Orthopedics, and Urology Services during a 3 week period in 2011 and who wereadministered at least one medication from a list of 240 PIMs.
Intervention
A computerized PIMS dashboard flagged patients with at least one administered PIM or a high calculated anticholinergic score. Additionally, the dashboard displayed 48-hour cumulative narcotic and benzodiazepine administration. Patients were ranked to reflect the estimated risk of an adverse event using logical combinations of data (e.g. use of multiple sedatives in a non-monitored location). In a pilot implementation, a clinical pharmacist reviewed the flagged patient records and delivered an immediate point-of-care intervention for the treating physician.
Measurements
Clinician response to pharmacist intervention.
Results
Of797 patients admitted over a three-week period, the PIMS dashboard flagged 179 patients (22%) and 485 patient-medication pairs for review by the clinical pharmacist. Seventy-one patient records with 139patient-medication pairs required additional manual review of the electronic medical record. Twenty-two patients receiving 40 inappropriate medication orders were judged to warrant an intervention, which was delivered by personal communication via phone or text message. Clinicians enacted 31 of 40 (78%) pharmacist recommendations.
Conclusion
An electronic PIMs dashboard provided an efficient mechanism for clinical pharmacists to rapidly screen the medication regimens of hospitalized elderly and deliver a timely point-of-care intervention when indicated.
Keywords: medication safety, vulnerable elderly, pharmacy intervention, clinical informatics, acute care, geriatrics
Background
Patients over age 65 are at higher risk of experiencing an adverse drug event (ADE), particularly if they are prescribed medications associated with risk of delirium, falls, cognitive decline, sedation, and other adverse reactions.1-7 Potentially inappropriate medications (PIMs) in elderly are identified on multiple published lists, including the highly cited Beers criteria, Screening Tool of Older Persons' Prescriptions (STOPP), and scales of anticholinergic load.8-11,13,14,31 These publications are developed based on evidence review and expert guidance and list medications, medication doses or frequencies, or drug combinations which are thought to cause more harm than benefit in vulnerable elderly. Example drug classes include benzodiazepines, centrally and peripherally acting anticholinergics, antipsychotics, non-steroidal anti-inflammatory drugs (NSAIDs), opioids, antihypertensives, and other cardiac medications. Despite guidelines cautioning against their use, prescribing of PIMs is common in the hospital setting15-19
As previously published by our group and others, automated interventions to reduce the use of PIMs are variably effective.3,20,22 Evaluations of clinical decision support systems to encourage substitution or discontinuation of PIMsdemonstrate that physicians often ignore or override automated alerts due to perceived lack of clinical relevance (where the alert does not incorporate clinical context) or “alert fatigue.” 3,20-22 Some authors have pointed out geriatric medication decisions are difficult and not always amenable to resolution via a computerized prompt.23-25 The revised 2007 ACOVE (Assessing Care of Vulnerable Elders) indicators from the American Geriatrics Society (AGS) places emphasis on patient education, drug regimen review, prescribing indicated medications, and medication monitoring.26 Active review by a clinical pharmacist has shown significant improvement in the appropriateness of prescribing, as well as trends toward improved satisfaction and reduced emergency department visits and death at 1 year.27 However, untargeted approaches that rely entirely on clinical pharmacists have high labor costs and may be difficult to sustain.28
We developed tools and procedures to conduct real-time geriatric medication risk (GMR) surveillance as a complement to clinical pharmacy review and decision support targeting the ordering provider. This approach extends a model for pharmacy services successfully applied to the prescription of aminoglycosides, vancomycin, insulin, and medication management during acute kidney injury.29 We utilized a hospital clinical information system to perform an automated review of medication orders, flag cases, and synthesize the relevant information about those cases into a clinical dashboard that could be reviewed by a clinical pharmacist. This pilot study is designed to test the feasibility, clinician responsiveness, and acceptability of a PIMs dashboard, coupled with an intervention by a clinical pharmacist, in reducing the use of PIMs among hospitalized elders.
Methods
Setting and Population
Vanderbilt University Hospital (VUH) is a 658-bed teaching hospital which serves as a principal referral center for physicians and patients throughout the Southeast. Patients over 65 years old and admitted to the General Medicine, Orthopedics, and Urology services were targeted by the pilot. The study was approved by the institutional IRB as a quality improvement intervention.
Description of Usual Care
Patient information at VUH is entered and stored in an electronic health record (EHR), StarPanel. All physician orders are entered electronically in Horizon Expert Orders (HEO), the hospital's computerized order entry system. HEO currently provides decision support for the medication ordering process, suggesting for example, lower doses of certain medications when prescribed to the elderly or to patients with impaired kidney function. However, apart from providing on-screen text messages (which may be overlooked or ignored), the system relies on physicians to be personally attentive to the medication's geriatric risk profile. There is currentlyno automated support to flag PIMs for further review, nor is there routine clinical pharmacist involvement on all medical units to assist physicians with more appropriate prescribing.
Pilot Intervention and Study Procedures
We developed an electronic PIMs dashboard (Figure 1) which identifies, in real-time, hospitalized patients who are age 65 or older and who have been prescribed at least one PIM. PIMs were defined as medications included on either the Beers criteria, STOP lists, or includedwithin a published anticholinergic risk scale, cross-referenced with medications that are included on the pilot institution's hospital formulary8,10,11. The dashboard displays each patient's age, gender, location, height, weight, estimated kidney function, flagged PIMs, anticholinergic score 10, total opioid equivalent use, benzodiazepine use, name of the inpatient servicewith origin of the order or order set, and a link to the summary view of the EHR. Additionally, the dashboardused a highernumber of PIMs and use of any benzodiazepine to sort the patient list by those potentially at highest risk, and draw the pharmacist's attention to those who may need an intervention more promptly.
Figure 1. Screenshot of electronic PIM dashboard directing pharmacists to patients at highest risk of adverse drug events (ADEs).
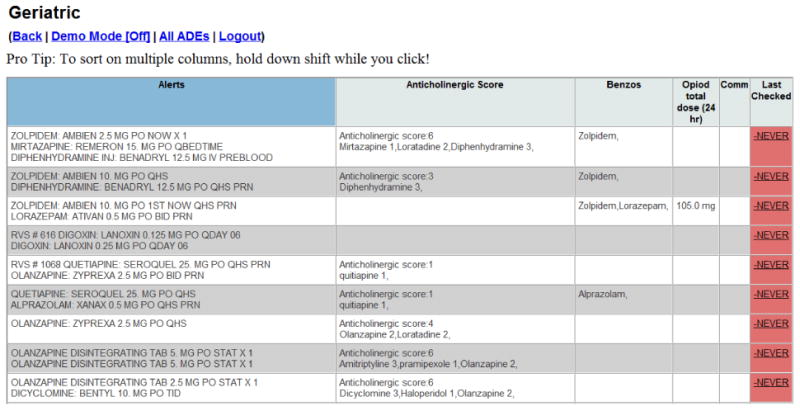
To briefly summarize the dashboard functions, a patient is added to the dashboard if any of the PIMs listed as “triggers” are currently prescribed subject to a minimum daily dose requirement (Appendix 1). Additionally, regardless of the presence of PIMs, patients are added if the calculated anticholinergic score across all administered medications is greater than or equal to 3. The alerts column shows all ordered priority drugs that are still active or have been discontinued less than 48 hours before. The benzodiazepine column (“Benzos”) shows benzodiazepines that are currently activeorders or have been discontinued less than 48 hours before. The opioid column on the dashboard shows the morphine daily dose calculated as the sum of administered opioids over the previous 24 hours converted to morphine equivalents. All doses, ordered and administered listed in Appendix 1 are in milligrams.
To demonstrate feasibility and potential efficacy, the project team designed a pilot implementation with a clinical pharmacist intervention protocol. The key pilot metrics included the proportion of patients displayed on the dashboard which were judged by the reviewing clinical pharmacist to require intervention and the proportion of interventions which led to the expected change in prescribing. The pilot was conducted between May 24th and June 30th 2011 on 6 geriatric-rich services: 4 medical and 2 surgical subspecialties, orthopedics and urology. A geriatric clinical pharmacist (DH) examined all patients on the dashboard on 21 work days within the time frame and selected patients featured on the dashboard for full EHR review. The interventionincludedthree stages: first, the pharmacist examined a detailed view of patient records appearing on the dashboard, which displayed details of PIMs (orders, administrations, and laboratory values such as drug levels and creatinine) ordered by time. The pharmacist subsequently selected patient records for a more detailed second-stage examination of the full electronic health record including the review of recent narrative progress notes by clinicians and nurses. Implicit criteriawere used to determine whether a clinical pharmacistwas indicated and relied on the pharmacist's judgment of the patient's level of risk. The clinical context taken into account at this stage included cumulative medication exposure history, dosing, overlapping indications, chronicity, renal function, laboratory indications of medication toxicity, and location. For example, the pharmacist reported examining in detail the use of benzodiazepines in non-monitored locations or multiple sedating medications with apparently overlapping indications. In the final stage, the pharmacist reviewed the full EHR for additional clinical detail including progress notes and other narratives describing comorbidities, evidence for patient frailty, baseline function, and acute hospital course. Once patients were selected for an intervention, consultations were designed to brief, consisting of a personal communication by phone or text paging to deliver a recommendation to reduce medication risk. Recommended interventions included drug discontinuations, dosing changes, increased monitoring, formulary substitutions and provider and staff education.
Analysis
Quantitative analysis of the pilot was descriptive including the number and proportion of patients selected for each stage by electronic and implicit pharmacist criteria.
Results
Of all patients 65 and older admitted during the 3-week study period, 797 patients were screened by the PIMs dashboard based on the electronic criteria involving service and age. Of screened patients, 179 patients prescribed 485 PIMs were flagged by the dashboard algorithms for clinical review by the pharmacist. Patients meeting full review criteria were aged 72 ± 7 years. Nine (12.7%) had a diagnosis of dementia and 5 (7%) were admitted from nursing homes. The majority of flagged patients (86%) had more than one PIM. Following clinical review using the dashboard's highlighted prescribing and laboratory data, the clinical pharmacist selected 71 patients prescribed 134 PIMs for a more detailed chart review. Approximately half (49%) of reviewed patient-drug pairs were present on admission and the remainder were initiated in the hospital. Based on the dashboard flags and chart review, the clinical pharmacist subsequently selected 37 patient-medication pairs involving 22 patients cared for by 12 providers for the intervention (Figure 1). Among the 37 medications, 31 had been flagged by the dashboard algorithms and 6 others were identified for intervention due to two additional concerns: impaired kidney function and drug-drug interactions. The clinical pharmacist estimated spending2 hours per day (approximately 40 hours in total) to review the dashboard for intervention candidates and contact individual providers.
Figure 1.
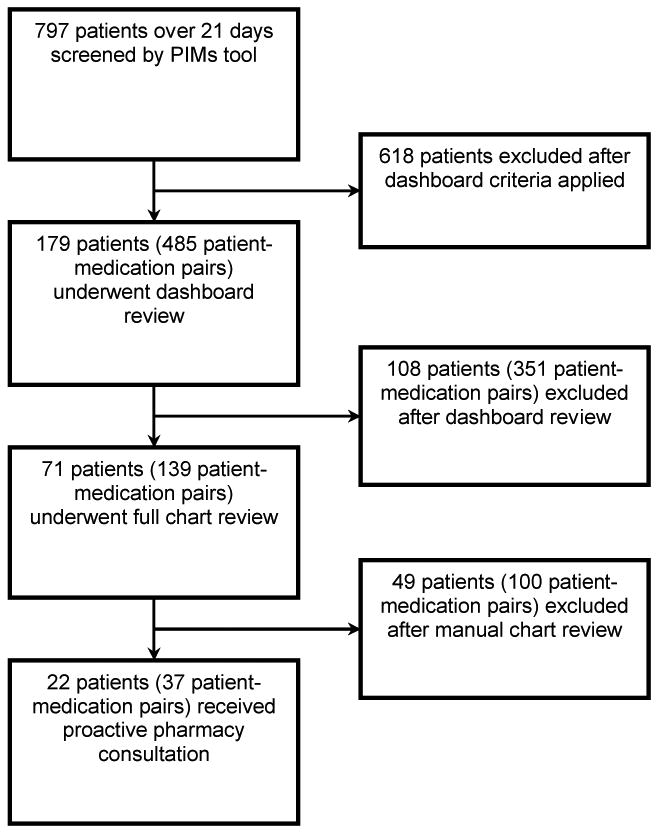
Flow diagram depicting screening by Potentially Inappropriate Medications (PIMs) tool and by pharmacist. After electronic criteria were applied, the pharmacist determined whether proactive consultation was indicated based on diagnoses, duration of PIM use, dose, renal function, and concomitant medications.
Pharmacist Intervention
The pharmacist issued 37recommendations to revise prescriptions and/or change the clinical monitoring strategy. The most common recommendations were to discontinue medication (n=11), change dose or frequency (n=13), substitute with an alternative (n=6), or increase clinical monitoring (n=2). Recommendations were accepted by ordering clinicians in 78% of cases. The most common instance of not accepting the pharmacist's recommendation was in the case of drug substitution, where only 1 of 6 recommended changes was accepted.
Of the patients not selected for full review, the pharmacist felt that the medications were appropriately selected and dosed for an acute hospital admission. Of the drugs not selected for intervention, the majority (83%) had an appropriate indication or dosing at baseline in the judgment of the reviewing pharmacist. Additional reasons cited by the pharmacist were that the patient was on the drug chronically at home (34%) without noted problem, or was receiving a minimal dose or tapered course that was not felt to impact the risk for an adverse event.
Of the 134 PIMs selected by the pharmacist for review, 58 (43%) were stopped at discharge, representing 9 (6.7%) of chronic outpatient medications stopped while the patient was in the hospital and 49 (36%) inpatient medications discontinued prior to discharge. The majority (59%) of patients were discharged to home, 37% were discharged to nursing home or rehabilitation and 4% expired.
Discussion
An electronic PIMs dashboard identified a large population of elderly inpatients prescribed PIMs and facilitated a focused pharmacy review and subsequent proactive interventionfor patients susceptibleto an adverse drug event. With the dashboard, the pharmacist could quickly find patient or drug characteristics that increased risk including overlapping sedating medications, cumulative dosing of opiates, or interacting medications. A fully manual approach would have required abstracting or reviewing data from a much larger set of charts. Alternatively, the pharmacist would need to be physically present on every rounding team. The majority of patients flagged by the dashboard did not require a proactive intervention after review of dashboard or chart data by the pharmacist. If efficacy is validated in controlled studies, this approach could save considerable time, and leverage the limited numbers of clinical pharmacy staff with geriatric expertise.
A large number of prescribing guidelines and principles for the care of vulnerable older adults are published, yet there is little guidance on how to efficiently translate these recommendations to clinical practice. Extensive clinical knowledge is required to effectively screen patients' medication regimens for highest-risk PIMs, and this skill is concentrated among clinicians and pharmacists who have expertise in caring for a geriatric population. The use of automated methods to leverage the efforts of skilled clinicians or ancillary services to improve patient care is a promising approach to bridge this implementation gap.
Other rigorously evaluated pharmacy interventions to reduce errors or inappropriate prescribing have not yet proven to be effective at improving patient outcomes.30 Though we did not assess clinical outcomes in this pilot study, we did successfully establish the technical feasibility, implementation logistics, and intermediate effect on prescribing of the technology. More efficient and targeted use of clinical pharmacy services to assist with hospital care of vulnerable elderly could impact patient outcomes associated with PIM use, including delirium, physical activity, falls, and restraint use. Additional studies could also be conducted to adapt this intervention model to health care settings with less robust EHRs.
The study is limited by the reliance on a single pharmacist with a strong clinical background to apply implicit criteria for selecting patients for an intervention. With training, the dashboard tool could be used by any clinical pharmacist, although clinical judgment and final recommendations may vary between pharmacists. Acceptance of a clinical pharmacist's recommendation by physicians in private practice, or different subspecialties might vary substantially from what was presented in this report, and presents an additional research and implementation challenge for further dissemination of this approach. However, we believe this intervention model will be accepted to complement rounding pharmacists and therapeutic drug monitoring team.
In conclusion, an electronic PIMs dashboard provided an efficient mechanism for clinical pharmacists to rapidly screen the medication regimens of hospitalized elderly and deliver a timely point-of-care intervention when indicated.
Supplementary Material
Table 1. Characteristics of Medications selected for Full Pharmacy Review and Intervention.
| Drug Category | Selected for Full Pharmacy Review, N (%) (N=139) |
Selected for Intervention, N (%) (N=37) |
|---|---|---|
| Benzodiazepines | 18 (13%) | 1 (3%) |
| Non-benzodiazepine sedating medications | 12 (9%) | 3 (8%) |
| NSAIDs | 18 (13%) | 8 (22%) |
| Antipsychotics | 9 (7%) | 4 (11%) |
| Anticholinergic | 47 (34%) | 4 (11%) |
| Digoxin | 10 (7%) | 2 (5%) |
| Other | 25 (18%) | 15 (41%) |
NSAIDs = Non-steroidal Anti-inflammatory Drugs
Acknowledgments
We would like to acknowledge the Department of Pharmacy Services including David Gregory, Pharm D, and Bob Lobo, Pharm D for their helpful insight in developing the live intervention, as well as Lemuel Russell Waitman, Ph.D, for his assistance in the initial phases of development of the PIMs dashboard.
Funding Sources: This workwassupported in part by grantsfrom the DW Reynolds Foundation, HRSA Geriatric Education Centers Grant #1D31HP08823-01-00, and Vanderbilt CTSA Grant UL1 RR024975 from NCRR/NIH
Footnotes
Conflict of Interest: Marketa Marvanova: Occasional educational lectures (Honoraria); Pharmacy consultant for American Academy of Neurology publication Continuum:Lifelong learning in neurology (Consultant); Occasionally, last in 2012 (Expert Testimony)
James Powers: Geriatric Education Centers (HRSA); Occasional educational lectures; Health Spring Pharmacy Advisory Committee
Author Contributions: Dr. Peterson designed the study, developed the pharmacy screening tool, interpreted the data, and prepared the manuscript. Drs. Kripalani andMarvanova participated in designing the study, interpreting the data, and preparing the manuscript. Ms. Danciu participated in developing the pharmacy screening tool, assisted in collecting the data, and preparing the manuscript.Dr. Harrell participated in designing the study, delivering the intervention, and preparing the manuscript. Ms. Rodriguez assisted in collecting the dataand preparing the manuscript. Dr. Powersparticipated in designing the study, developing the pharmacy screening tool, interpreting the data, and preparing the manuscript. Dr. Salanitro participated in interpreting the data and preparing the manuscript. All authors approved the final manuscript.
Sponsor's Role: None
References
- 1.Fick DM, Mion LC, Beers MH, et al. Health outcomes associated with potentially inappropriate medication use in older adults. Res Nurs Health. 2008;31:42–51. doi: 10.1002/nur.20232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallagher PF, Barry PJ, Ryan C, et al. Inappropriate prescribing in an acutely ill population of elderly patients as determined by Beers' Criteria. Age Ageing. 2008;37:96–101. doi: 10.1093/ageing/afm116. [DOI] [PubMed] [Google Scholar]
- 3.Peterson JF, Kuperman GJ, Shek C, et al. Guided prescription of psychotropic medications for geriatric inpatients. Arch Intern Med. 2005;165:802–807. doi: 10.1001/archinte.165.7.802. [DOI] [PubMed] [Google Scholar]
- 4.Chew ML, Mulsant BH, Pollock BG, et al. Anticholinergic activity of 107 medications commonly used by older adults. J Am Geriatr Soc. 2008;56:1333–1341. doi: 10.1111/j.1532-5415.2008.01737.x. [DOI] [PubMed] [Google Scholar]
- 5.Rothberg MB, Pekow PS, Liu F, et al. Potentially inappropriate medication use in hospitalized elders. J Hospl Med. 2008;3:91–102. doi: 10.1002/jhm.290. [DOI] [PubMed] [Google Scholar]
- 6.Page RL, Ruscin JM. The risk of adverse drug events and hospital-related morbidity and mortality among older adults with potentially inappropriate medication use. Am J Geriatr Pharmacol. 2006;4:297–305. doi: 10.1016/j.amjopharm.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Laroche1 ML, Charmes JP, Bouthier F, et al. Inappropriate medications in the elderly. Clin Pharmacol Ther. 2009;85:94–97. doi: 10.1038/clpt.2008.214. [DOI] [PubMed] [Google Scholar]
- 8.American Geriatrics Society 2012 Beers Criteria Expert panel. American Geriatrics Society updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616–631. doi: 10.1111/j.1532-5415.2012.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carnahan RM, Lund BC, Perry PJ, et al. The anticholinergic drug scale as a measure of drug related anticholinergic burden: Association with anticholinergic activity. J Clin Pharmacol. 2006:1481–1486. doi: 10.1177/0091270006292126. [DOI] [PubMed] [Google Scholar]
- 10.Rudolph JL, Salow MJ, Angelini ML, et al. The anticholinergic scale and the anticholinergic adverse effects in older persons. Arch Intern Med. 2008;168:508–513. doi: 10.1001/archinternmed.2007.106. [DOI] [PubMed] [Google Scholar]
- 11.Gallaher P, O'Mahony D. STOPP (screening tool of older persons' potentially inappropriate prescriptions): Aapplication to acutely ill elderly patients and comparison with Beers' criteria. Age Ageing. 2008;37:673–679. doi: 10.1093/ageing/afn197. [DOI] [PubMed] [Google Scholar]
- 12.Barry PJ, Gallagher P, Ryan C, et al. START (screening tool to alert doctors to the right treatment)—an evidence-based screening tool to detect prescribing omissions in elderly patients. Age Ageing. 2007;36:632–8. doi: 10.1093/ageing/afm118. [DOI] [PubMed] [Google Scholar]
- 13.Stefanaci RG, Cavallaro E, Beers MH, et al. Developing explicit positive Beers criteria for preferred central nervous system medications in older adults. Consult Pharm. 2009;24:601–610. doi: 10.4140/tcp.n.2009.601. [DOI] [PubMed] [Google Scholar]
- 14.Hedva BL, Marcus E, Christen C. Beyond the Beers criteria: a comparative overview of explicit criteria. Ann Pharmacother. 2010;44:1968–1975. doi: 10.1345/aph.1P426. [DOI] [PubMed] [Google Scholar]
- 15.Hajjar ER, Joseph T, Hanlon JT, et al. Unnecessary drug use in frail older people at hospital discharge. J Am Geriatr Soc. 2005;53:1518–1523. doi: 10.1111/j.1532-5415.2005.53523.x. [DOI] [PubMed] [Google Scholar]
- 16.Akazawa M, Imai H, Igarashi A, et al. Potentially inappropriate medication use in elderly Japanese patients. Am J Geriatr Pharmacother. 2010;8:146–160. doi: 10.1016/j.amjopharm.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Onder G, Landi F, Cesari M, et al. Inappropriate medication use among hospitalized older adults in Italy: Results from the Italian group of pharmacoepidemiology in the elderly. Eur J Clin Pharmacol. 2003;59:157–162. doi: 10.1007/s00228-003-0600-8. [DOI] [PubMed] [Google Scholar]
- 18.Hanlon JT, Artz MB, Pieper CF, et al. Inappropriate medication use among frail elderly inpatients. Ann Pharmacother. 2004;38:9–14. doi: 10.1345/aph.1D313. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton H, Gallagher P, Ryan C, et al. Potentially inappropriate medications defined by STOPP criteria and the risk of adverse drug events in older hospitalized patients. Arch Intern Med. 2011;171:1013–1019. doi: 10.1001/archinternmed.2011.215. [DOI] [PubMed] [Google Scholar]
- 20.Smith DH, Perrin N, Feldstein A, et al. The impact of prescribing safety alerts for elderly persons in an electronic medical record: An interrupted time series evaluation. Arch Intern Med. 2006;166:1098–1104. doi: 10.1001/archinte.166.10.1098. [DOI] [PubMed] [Google Scholar]
- 21.Tamblyn R, Huang A, Taylor L, et al. A randomized trial of the effectiveness of on-demand versus computer-triggered drug decision support in primary care. J Am Med Inform Assoc. 2008;15:430–438. doi: 10.1197/jamia.M2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peterson JF, Rosenbaum BP, Waitman LR, et al. Physicians' response to guided geriatric dosing: initial results from a randomized trial. Stud Health Technol Inform. 2007;12(Pt 2):1037–1040. [PubMed] [Google Scholar]
- 23.Mattison MLP, Afonso KA, Ngo LH, et al. Preventing potentially inappropriate medication use in hospitalized older patients with a computerized provider order entry warning system. Arch Intern Med. 2010;170:1331–1336. doi: 10.1001/archinternmed.2010.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmader KE, Hanlon JT, Pieper CF, et al. Effects of geriatric evaluation and management on adverse drug reactions and suboptimal prescribing in the frail elderly. Am J Med. 2004;116:394–401. doi: 10.1016/j.amjmed.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 25.Gallagher PF, O'Connor MN, O'Mahony D. Prevention of potentially inappropriate prescribing for elderly patients: A randomized controlled trial using STOPP/START criteria. Clin Pharmacol Ther. 2011;89:845–854. doi: 10.1038/clpt.2011.44. [DOI] [PubMed] [Google Scholar]
- 26.Shrank WH, Polinski JM, Avorn J. Quality indicators for medication use in vulnerable elders. J Am Geriatr Soc. 2007;55:s2, S373–S382. doi: 10.1111/j.1532-5415.2007.01345.x. [DOI] [PubMed] [Google Scholar]
- 27.Spinewine A, Swine C, Dhillon S, et al. Effect of a collaborative approach on the quality of prescribing for geriatric inpatients: A randomized, controlled trial. J Am Geriatr Soc. 2007;55:658–665. doi: 10.1111/j.1532-5415.2007.01132.x. [DOI] [PubMed] [Google Scholar]
- 28.Kaboli PJ, Hoth AB, McClimon BJ, et al. Clinical pharmacists and inpatient medical care: A systematic review. Arch Intern Med. 2006;166:955–964. doi: 10.1001/archinte.166.9.955. [DOI] [PubMed] [Google Scholar]
- 29.McCoy AB, Cox ZL, Neal EB, et al. Real-time pharmacy surveillance and clinical decision support to reduce adverse drug events in acute kidney injury: A randomized, controlled trial. Appl Clin Inform. 2012;3:221–238. doi: 10.4338/ACI-2012-03-RA-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kripalani S, Roumie CL, Dalal AK, et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge. Ann Intern Med. 2012;157:1–10. doi: 10.7326/0003-4819-157-1-201207030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryan C, O'Mahony D, Byrne S. Application of STOPP and START criteria: Interrater reliability among pharmacists. Ann Pharmacother. 2009;43:1239–1244. doi: 10.1345/aph.1M157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


