Abstract
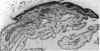
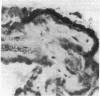
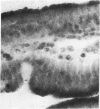
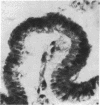
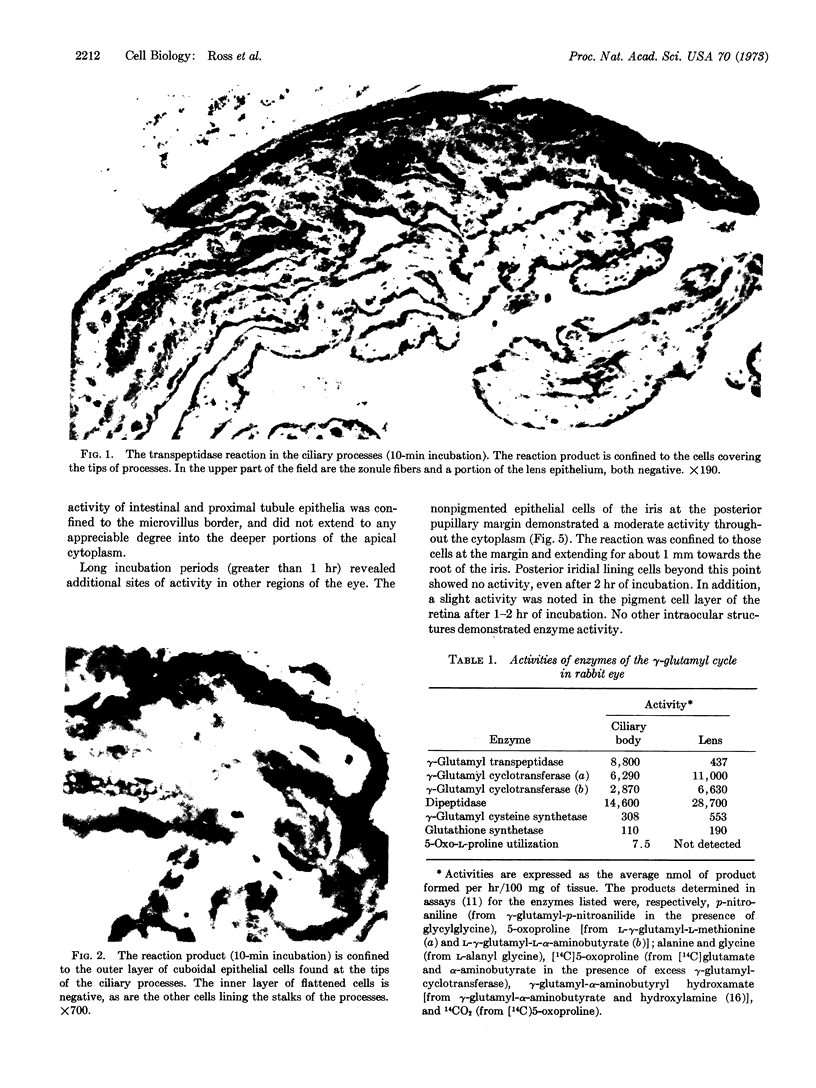
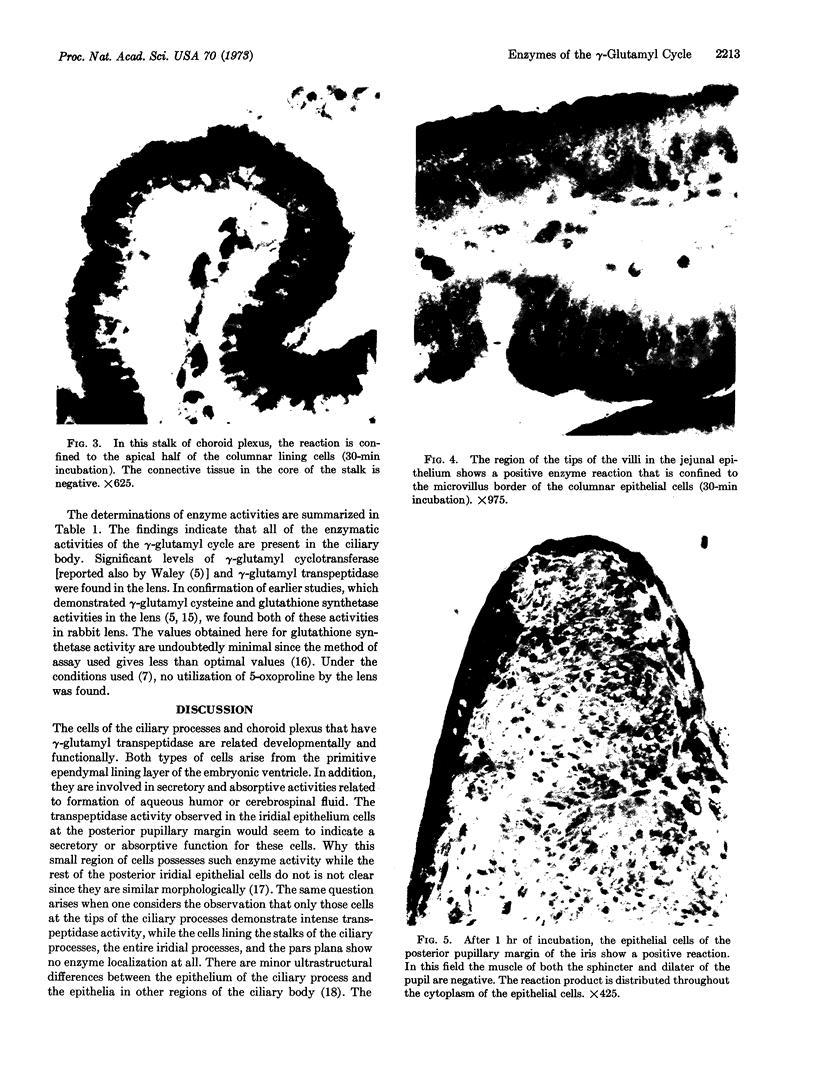
The enzymes of the γ-glutamyl cycle have been found in rabbit ciliary body and, except for 5-oxoprolinase, also in the ocular lens. Histochemical studies show that γ-glutamyl transpeptidase is localized mainly in the basal portions of the epithelial cells of the ciliary body; the findings are similar to those observed in the chloroid plexuses. The histochemical staining reaction in the ciliary epithelium is more intense than in the chloroid plexus, intestine, and kidney. γ-Glutamyl transpeptidase staining activity in the epithelium of the intestinal and renal proximal convoluted tubules is confined to the microvillus border. Moderate transpeptidase activity was found in the cytoplasm of nonpigmented epithelial cells of the iris at the posterior pupillary margin. The histochemical and enzyme activity studies are consistent with the thesis that the γ-glutamyl cycle functions in transport of amino acids across the blood-aqueous humor barrier.
Keywords: eye, rabbit, aminoacid transport, glutathione, ciliary epithelium
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERT Z., ORLOWSKI M., SZEWCZUK A. Histochemical demonstration of gamma-glutamyl transpeptidase. Nature. 1961 Aug 19;191:767–768. doi: 10.1038/191767a0. [DOI] [PubMed] [Google Scholar]
- BELLOWS J. G., SHOCH D. E. Alloxan diabetes and the lens. Am J Ophthalmol. 1950 Oct;33(10):1555–1564. doi: 10.1016/0002-9394(50)91216-5. [DOI] [PubMed] [Google Scholar]
- GLENNER G. G., FOLK J. E. Glutamyl peptidases in rat and guinea pig kidney slices. Nature. 1961 Oct 28;192:338–340. doi: 10.1038/192338a0. [DOI] [PubMed] [Google Scholar]
- Harding J. J. Free and protein-bound glutathione in normal and cataractous human lenses. Biochem J. 1970 May;117(5):957–960. doi: 10.1042/bj1170957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KINSEY V. E., MERRIAM F. C. Studies on the crystalline lens. II. Synthesis of glutathione in the normal and cataractous rabbit lens. AMA Arch Ophthalmol. 1950 Sep;44(3):370–380. [PubMed] [Google Scholar]
- Kozart D. M. Light and electron microscopic study of regional morphologic differences in the processes of the ciliary body in the rabbit. Invest Ophthalmol. 1968 Feb;7(1):15–33. [PubMed] [Google Scholar]
- McMILLAN P. J., RYERSON S. J., MORTENSEN R. A. The metabolism of lens glutathione studies with glycine-C14. Arch Biochem Biophys. 1959 Mar;81(1):119–123. doi: 10.1016/0003-9861(59)90181-x. [DOI] [PubMed] [Google Scholar]
- Meister A. On the enzymology of amino acid transport. Science. 1973 Apr 6;180(4081):33–39. doi: 10.1126/science.180.4081.33. [DOI] [PubMed] [Google Scholar]
- Mooz E. D., Meister A. Tripeptide (glutathione) synthetase. Purification, properties, and mechanism of action. Biochemistry. 1967 Jun;6(6):1722–1734. doi: 10.1021/bi00858a022. [DOI] [PubMed] [Google Scholar]
- Orlowski M., Meister A. The gamma-glutamyl cycle: a possible transport system for amino acids. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1248–1255. doi: 10.1073/pnas.67.3.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIRIE A., VAN HEYNINGEN R., BOAG J. W. Changes in lens during the formation of x-ray cataract in rabbits. Biochem J. 1953 Jul;54(4):682–688. doi: 10.1042/bj0540682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REDDY D. V., KINSEY V. E. Studies on the crystalline lens. IX. Quantitative analysis of free amino acids and related compounds. Invest Ophthalmol. 1962 Oct;1:635–641. [PubMed] [Google Scholar]
- Rathbun W. B. Gamma-glutamyl-cysteine synthetase from bovine lens. I. Purification and properties. Arch Biochem Biophys. 1967 Oct;122(1):62–72. doi: 10.1016/0003-9861(67)90124-5. [DOI] [PubMed] [Google Scholar]
- Reddy D. V. Distribution of free amino acids and related compounds in ocular fluids, lens, and plasma of various mammalian species. Invest Ophthalmol. 1967 Oct;6(5):478–483. [PubMed] [Google Scholar]
- Reddy D. V., Klethi J., Kinsey V. E. Studies on the crystalline lens. XII. Turnover of glycine and glutamic acid in glutathione and ophthalmic acid in the rabbit. Invest Ophthalmol. 1966 Dec;5(6):594–600. [PubMed] [Google Scholar]
- Tate S. S., Ross L. L., Meister A. The -glutamyl cycle in the choroid plexus: its possible function in amino acid transport. Proc Natl Acad Sci U S A. 1973 May;70(5):1447–1449. doi: 10.1073/pnas.70.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Werf P., Orlowski M., Meister A. Enzymatic conversion of 5-oxo-L-proline (L-pyrrolidone carboxylate) to L-glutamate coupled with cleavage of adenosine triphosphate to adenosine diphosphate, a reaction in the -glutamyl cycle. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2982–2985. doi: 10.1073/pnas.68.12.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Werf P., Stephani R. A., Orlowski M., Meister A. Inhibition of 5-oxoprolinase by 2-imidazolidone-4-carboxylic acid. Proc Natl Acad Sci U S A. 1973 Mar;70(3):759–761. doi: 10.1073/pnas.70.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]