Abstract
Objective. CagA+/vacAs1+/vacAm1+ Helicobacter pylori upregulates the expression of tumor necrosis factor receptor–associated factor 1 (TRAF1), tumor necrosis factor receptor superfamily member 9 (4-1BB), and B-cell lymphoma-extra large (Bcl-xL) in human gastric epithelial cells. We investigated the correlation between cagA/vacAs1/vacAm1 and TRAF1/4-1BB/Bcl-xL expression in gastric mucosal tissue of patients with gastric disorders. Methods. We collected gastric mucosa samples from 35 chronic, nonatrophic gastritis (CG) patients, 41 atrophic gastritis patients, 44 intestinal metaplasia with atypical hyperplasia (IM) patients, and 28 gastric carcinoma (Ca) patients. The expression of TRAF1, 4-1BB, and Bcl-xL was determined using western blotting. The expression of cagA, vacAs1, and vacAm1 in H. pylori was examined with polymerase chain reaction. Results. The expression of TRAF1, 4-1BB, and Bcl-xL was significantly upregulated in IM and Ca patients (P < 0.05 compared with CG). There were more cases of cagA+/vacAs1+/vacAm1+ H. pylori infection in samples with elevated TRAF1, 4-1BB, or Bcl-xL expression (P < 0.05). Additionally, there were a remarkably large number of samples with upregulated TRAF1/4-1BB/Bcl-xL expression in cases of cagA+/vacAs1+/vacAm1+ H. pylori infection (44 cases, 67.7%; P < 0.05). Conclusions. The pathogenesis of IM and Ca may be promoted by cagA+/vacAs1+/vacAm1+ H. pylori, possibly via upregulated TRAF1, 4-1BB, and Bcl-xL in gastric mucosal tissue.
1. Introduction
Helicobacter pylori, a gram-negative bacterium present in nearly 50% of the global population [1, 2], is one of the main causes of peptic ulcer disease [3]. In addition, H. pylori infection is related to gastric carcinoma, possibly inducing chronic gastritis, and progresses to the premalignant stages of atrophic gastritis, intestinal metaplasia, and, eventually, gastric carcinoma [4–6]. However, knowledge of the pathogenesis of H. pylori infection and gastric diseases is incomplete.
H. pylori strains that express virulence genes such as cagA and vacA are linked to increased risk of gastric cancer [7]. Previously, we performed a comparative genomic study of gastric epithelial cells cocultured with H. pylori and found that the expression of tumor necrosis factor receptor–associated factor 1 (TRAF1), 4-1BB (also known as CD137), and B-cell lymphoma-extra large (Bcl-xL) was significantly upregulated in human gastric epithelial GES-1 cells infected with H. pylori expressing the virulence genotype cagA+/vacAs1+/vacAm1+ [8]. TRAF1 expression in human gastric mucosa is related to the H. pylori virulence genotype cagA+/vacAs1+/vacAm1+ [9], whereas the correlation of 4-1BB and Bcl-xL gene expression with cagA, vacAs1, and vacAm1 toxicity at different stages of gastric disease have not been studied.
In the present study, we investigated TRAF1, 4-1BB, and Bcl-xL expression in gastric mucosa derived from H. pylori–infected patients with chronic nonatrophic gastritis (CG), atrophic gastritis (AG), intestinal metaplasia with atypical hyperplasia (IM), and gastric carcinoma (Ca). We also determined the association between H. pylori virulence factors (cagA, vacAs1, vacAm1) and TRAF1/4-1BB/Bcl-xL expression at the different stages of gastric disease. Our findings may provide valuable insight into the pathogenesis and progression of chronic gastritis into gastric carcinoma.
2. Materials and Methods
2.1. Reagents
DNA marker and 2x Tag Master Mix were purchased from Beijing CoWin Biotech (Beijing, China). Polyvinyl difluoride (PVDF) membrane was obtained from Millipore (Billerica, MA, USA). PageRuler Prestained Protein Ladder was obtained from Thermo Fisher Scientific (Waltham, MA, USA). Primary antibodies against TRAF1 and Bcl-xL were purchased from Cell Signaling Technology (Beverly, MA, USA). Mouse monoclonal anti–glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody was obtained from Millipore. Anti–4-1BB antibody was obtained from Abcam (Cambridge, MA, USA). Goat anti-rabbit and anti-mouse immunoglobulin G (IgG) horseradish peroxidase- (HRP-) conjugated secondary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Unless otherwise stated, all other reagents were provided by the Central South University Cancer Research Institute, Changsha, China.
2.2. Sample Collection
In total, 148 gastric antrum samples were collected from patients with different gastric mucosal diseases at the Gastroscope Room, The Third Xiangya Hospital of Central South University, Changsha, China, from January 2013 to December 2013. H. pylori infection was determined via rapid urease test, C13 urea breath test, and histological examination. All recruited subjects were H. pylori positive; H. pylori–positive results were confirmed by at least two of the above tests. Among the patients, 35 had CG, 41 had AG, 44 had IM, and 28 had Ca. Subjects were recruited after they had signed an informed consent. Ethical approval for the study was granted by The Third Xiangya Hospital of Central South University.
2.3. Western Blotting
Total protein was extracted from the tissue, and protein concentrations were measured using a bicinchoninic acid protein assay kit according to the manufacturer's instructions (Beijing CoWin Biotech). Equal amounts of protein extracts were separated using sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to PVDF membranes. The PVDF membranes were blocked before they were incubated with primary antibodies at 4°C overnight. The primary antibodies used were anti-TRAF1 (1 : 500), anti–4-1BB (1 : 500), anti–Bcl-xL (1 : 1000), and anti-GAPDH (1 : 5000). After washing, the membranes were incubated with goat anti-rabbit or anti-mouse IgG HRP-labeled secondary antibodies (1 : 5000). Immunobands were visualized using an enhanced chemiluminescence kit according to the manufacturer's instructions (Beijing CoWin Biotech). The densitometric values of the immunobands were used for statistical analysis. GAPDH was used as the internal control. Data were quantified from at least three independent experiments.
2.4. Polymerase Chain Reaction
Total DNA was extracted from H. pylori–infected gastric mucosal tissue using a General AllGen Kit for genomic DNA extraction according to the manufacturer's instructions (Beijing CoWin Biotech). The purity and concentration of the extracted DNA was measured using an ultraviolet spectrometer with an optical density (OD) 260/OD280 ratio. The primers for the H. pylori virulence factors were designed as described previously [10]. The forward and reverse primers and PCR product length are listed in Table 1. The PCR conditions were as follows: initial denaturation at 95°C for 5 min; denaturation at 95°C for 30 s; annealing at different temperatures (57°C (cagA), 52°C (vacAs1), 56°C (vacAm1)) for 30 s; and extension at 72°C for 40 s for a total of 39-40 cycles, followed by a final extension at 72°C for 7 min [11].
Table 1.
Primers and PCR conditions.
| Gene | Primers (forward, reverse) | Annealing temperature (°C) | Amplified product length (bp) |
|---|---|---|---|
| cagA | 5′-GATAACAGGCAAGCTTTTGAGG-3′ | 57 | 349 |
| 5′-CTGCAAAAGATTGTTTGGCAGA-3′ | |||
|
| |||
| vacAs1 | 5′-ATGGAAATACAACAAACACAC-3′ | 52 | 259 |
| 5′-CTGCTTGAATGCGCCAAAC-3′ | |||
|
| |||
| vacAm1 | 5′-CAATCTGTCCAATCAAGCGAG-3′ | 56 | 570 |
| 5′-GCGTCTAAATAATTCCAAGG-3′ | |||
2.5. Statistical Analysis
Data were analyzed using SPSS 17.0 software (IBM, New York, NY, USA). Measurement data are presented as the means ± standard deviation. Comparison among measurement data in different groups was conducted with diverse mean comparison of analysis of variance. A chi-square test was used for comparing enumeration data from different groups. Data correlation was analyzed using Spearman's analysis. P < 0.05 was considered significantly different.
3. Results
3.1. TRAF1, 4-1BB, and Bcl-xL Expression in Gastric Mucosal Tissue
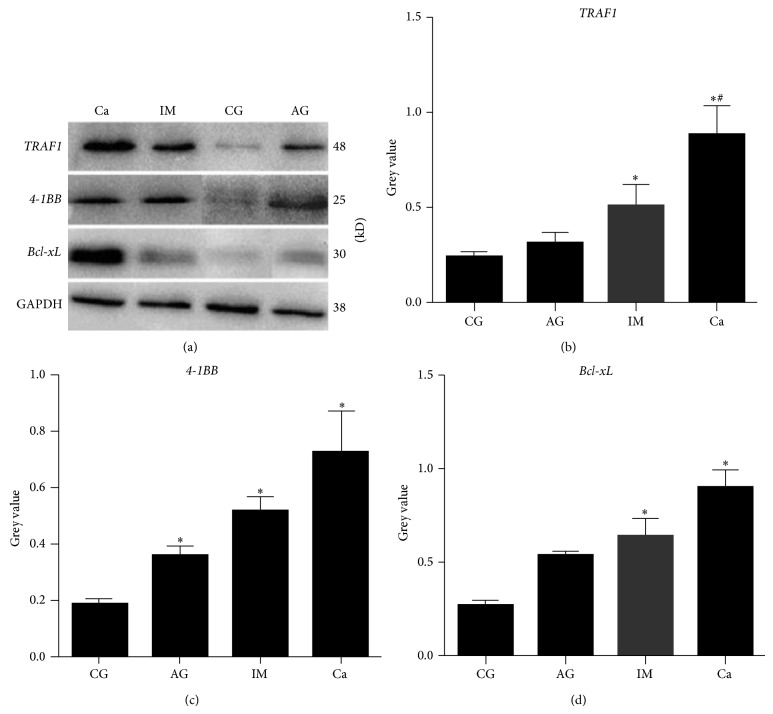
First, we examined TRAF1, 4-1BB, and Bcl-xL protein expression in gastric mucosal tissue from subjects with CG, AG, IM, and Ca using western blotting. As seen in Figure 1, TRAF1, 4-1BB, and Bcl-xL levels were significantly upregulated in IM and Ca gastric mucosa as compared with CG gastric mucosa (P < 0.05). The expression of 4-1BB was also elevated in AG gastric mucosa compared to CG gastric mucosa (P < 0.05). Moreover, compared to AG gastric mucosa, there was an obvious increase in TRAF1 protein expression in Ca gastric mucosa (P < 0.05). These findings show that the expression of TRAF1, 4-1BB, and Bcl-xL was upregulated, especially in the gastric mucosa of IM and Ca patients in comparison to the other groups. Spearman's analysis indicated that the expression of TRAF1, 4-1BB, and Bcl-xL was positively correlated. The TRAF1 and 4-1BB, TRAF1 and Bcl-xL, and 4-1BB and Bcl-xL correlation coefficient were 0.678, 0.702, and 0.694, respectively (P < 0.05 for all three groups).
Figure 1.
Western blotting examination of TRAF1, 4-1BB, and Bcl-xL protein expression in gastric mucosal tissue from subjects with CG, AG, IM, or Ca. (a) Representative western blots. Relative expression of (b) TRAF1, (c) 4-1BB, and (d) Bcl-xL was quantified against GAPDH. Data were quantified from three independent experiments. * P < 0.05 compared with CG; # P < 0.05 compared with AG.
3.2. Analysis of Virulence Factors in H. pylori Extracted from Gastric Mucosa
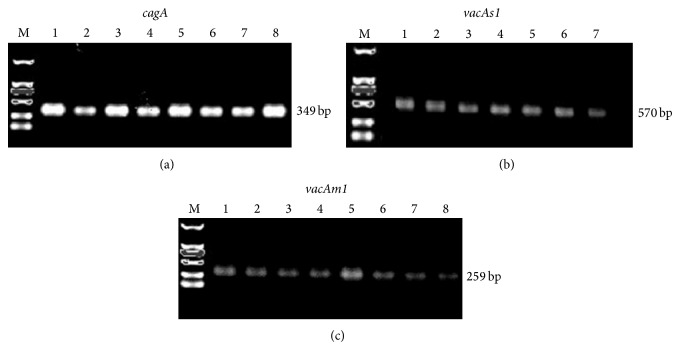
We analyzed H. pylori virulence factors expression, that is, cagA, vacAs1, and vacAm1, in H. pylori extracted from the different gastric mucosal tissues. Representative positive expression of the virulence factors is presented in Figure 2. To clarify the association between the H. pylori virulence factors and TRAF1, 4-1BB, and Bcl-xL expression, the number of samples positive or negative for cagA, vacAs1, and vacAm1 was stratified according to TRAF1, 4-1BB, and Bcl-xL expression (Table 2). According to the results of western blot, the gastric cancer and intestinal metaplasia with atypical hyperplasia groups were classified as the TRAF1, 4-1BB, and Bcl-xL high expression group, while the gastritis and atrophic gastritis groups were classified as the TRAF1, 4-1BB, and Bcl-xL low expression group. The number and percentage of cagA-, vacAs1-, and vacAm1-positive cases were greatly upregulated in samples with high TRAF1, 4-1BB, or Bcl-xL expression (P < 0.05 compared with cases negative for cagA, vacAs1, vacAm1). In addition, the number of cagA+/vacAs1+/vacAm1+ cases in samples with elevated TRAF1, 4-1BB, or Bcl-xL expression was increased dramatically (P < 0.05 compared with unaltered TRAF1, 4-1BB, or Bcl-xL expression).
Figure 2.
PCR amplification of (a) cagA, (b) vacAs1, and (c) vacAm1 expression in H. pylori extracted from gastric mucosal tissue. Lane M, DNA marker; numbered lanes, samples with positive expression of the target genes. Base pair value indicates fragment of interest.
Table 2.
Samples positive or negative for cagA, vacAs1, or vacAm1 stratified according to TRAF1, 4-1BB, and Bcl-xL expression.
| Group | TRAF1 | 4-1BB | Bcl-xL | |||
|---|---|---|---|---|---|---|
| ↑ | — | ↑ | — | ↑ | — | |
| cagA+/vacAs1+/vacAm1+ | 50 (64.9)* | 15 (21.1) | 50 (66.7)* | 15 (20.5) | 47 (59.4)* | 18 (26.1) |
| cagA+/vacAs1+/vacAm1− | 6 (7.8) | 10 (14.1) | 7 (9.3) | 9 (12.3) | 4 (5.1) | 12 (17.4) |
| cagA+/vacAs1−/vacAm1+ | 3 (3.9) | 12 (16.9) | 3 (4.0) | 12 (16.4) | 7 (8.9) | 8 (11.6) |
| cagA+/vacAs1−/vacAm1− | 3 (3.9) | 10 (14.1) | 3 (4.0) | 10 (13.7) | 3 (3.8) | 10 (14.5) |
| cagA−/vacAs1+/vacAm1+ | 3 (3.9) | 6 (8.5) | 5 (6.7) | 4 (5.5) | 5 (6.3) | 4 (5.8) |
| cagA−/vacAs1+/vacAm1− | 5 (6.5) | 8 (11.3) | 1 (1.3) | 12 (16.4) | 3 (3.8) | 10 (14.5) |
| cagA−/vacAs1−/vacAm1+ | 2 (2.6) | 5 (7.0) | 3 (4.0) | 4 (5.5) | 4 (5.1) | 3 (4.3) |
| cagA−/vacAs1−/vacAm1− | 5 (6.5) | 5 (7.0) | 3 (4.0) | 7 (9.6) | 6 (7.6) | 4 (5.8) |
| cagA+ | 62 (80.5) | 47 (66.2) | 63 (84.0) | 46 (63.0) | 61 (77.2) | 48 (69.6) |
| cagA− | 15 (19.5) | 24 (33.8) | 12 (16.0) | 27 (37.0) | 18 (22.8) | 21 (30.4) |
| vacAs1+ | 64 (83.1) | 39 (54.9) | 63 (84.0) | 40 (54.8) | 59 (74.7) | 44 (63.8) |
| vacAs1− | 13 (16.9) | 32 (45.1) | 12 (16.0) | 33 (45.2) | 20 (25.3) | 25 (36.2) |
| vacAm1+ | 58 (75.3) | 38 (53.5) | 61 (81.3) | 35 (47.9) | 63 (79.7) | 33 (47.8) |
| vacAm1− | 19 (24.7) | 33 (46.5) | 14 (18.7) | 38 (52.1) | 16 (20.3) | 36 (52.2) |
|
| ||||||
| Total | 77 (100.0) | 71 (100.0) | 75 (100.0) | 73 (100.0) | 79 (100.0) | 69 (100.0) |
↑, upregulated; —, unaltered. * P < 0.05 compared with unaltered group. Data are presented as the number of cases and percentage (%).
3.3. Correlation between cagA/vacAs1/vacAm1 and TRAF1/4-1BB/Bcl-xL Expression
To explore the potential association between cagA/vacAs1/vacAm1 and TRAF1/4-1BB/Bcl-xL expression, we analyzed the number and percentage of samples with positive or negative cagA/vacAs1/vacAm1 expression and differential TRAF1/4-1BB/Bcl-xL expression. A remarkably large number of samples with upregulated TRAF1/4-1BB/Bcl-xL expression were cagA+/vacAs1+/vacAm1+ (44 cases, 67.7%; P < 0.05; Table 3). From these findings, we believe that cagA+/vacAs1+/vacAm1+ H. pylori may promote the IM and Ca pathogenesis, possibly by upregulating TRAF1, 4-1BB, and Bcl-xL expression in the gastric mucosal tissue.
Table 3.
Correlation between expression of cagA, vacAs1, and vacAm1 and TRAF1, 4-1BB, and Bcl-xL.
| Group | T+/4+/B+ | T+/4+/B− | T+/4−/B+ | T+/4−/B− | T−/4+/B+ | T−/4+/B− | T−/4−/B+ | T−/4−/B− | Total |
|---|---|---|---|---|---|---|---|---|---|
| cagA+/vacAs1+/vacAm1+ | 44 (67.7)* | 4 (6.2) | 1 (1.5) | 1 (1.5) | 0 (0.0) | 2 (3.1) | 2 (3.1) | 11 (16.9) | 65 (100.0) |
| cagA+/vacAs1+/vacAm1− | 2 (12.5) | 2 (12.5) | 0 (0.0) | 2 (12.5) | 2 (12.5) | 1 (6.3) | 0 (0.0) | 7 (43.8) | 16 (100.0) |
| cagA+/vacAs1−/vacAm1+ | 1 (6.7) | 0 (0.0) | 2 (13.3) | 0 (0.0) | 2 (13.3) | 0 (0.0) | 2 (13.3) | 8 (53.3) | 15 (100.0) |
| cagA+/vacAs1−/vacAm1− | 1 (7.7) | 0 (0.0) | 1 (7.7) | 1 (7.7) | 0 (0.0) | 2 (15.4) | 1 (7.7) | 7 (53.8) | 13 (100.0) |
| cagA−/vacAs1+/vacAm1+ | 2 (22.2) | 0 (0.0) | 1 (11.1) | 0 (0.0) | 2 (22.2) | 1 (11.1) | 0 (0.0) | 3 (33.3) | 9 (100.0) |
| cagA−/vacAs1+/vacAm1− | 1 (7.7) | 0 (0.0) | 2 (15.4) | 2 (15.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 8 (61.5) | 13 (100.0) |
| cagA−/vacAs1−/vacAm1+ | 1 (14.3) | 0 (0.0) | 0 (0.0) | 1 (14.3) | 2 (28.6) | 0 (0.0) | 1 (14.3) | 2 (28.6) | 7 (100.0) |
| cagA−/vacAs1−/vacAm1− | 3 (30.0) | 0 (0.0) | 1 (10.0) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 2 (20.0) | 3 (30.0) | 10 (100.0) |
* P < 0.05 compared with other groups. T, TRAF1; 4, 4-1BB; B, Bcl-xL. Data are presented as the number of cases and percentage (%).
4. Discussion
Previously, we found that TRAF1, 4-1BB, and Bcl-xL were significantly upregulated in GES-1 cells infected with H. pylori expressing the virulence genotype cagA+/vacAs1+/vacAm1+ [8]. Here, we show that expression of TRAF1, 4-1BB, and Bcl-xL was significantly upregulated in IM and Ca patients compared with CG patients. Moreover, compared to samples in which TRAF1, 4-1BB, or Bcl-xL expression was unaltered, there were more cagA+/vacAs1+/vacAm1+ H. pylori–infected cases in samples with elevated TRAF1, 4-1BB, or Bcl-xL expression.
TRAF1 belongs to a group of structurally similar adapter proteins (TRAFs), but differs from other TRAFs because it lacks the conserved N-terminal RING domain found in other TRAF family proteins [12]. TRAF1 plays a critical role in regulating apoptosis by indirectly modulating the transcription factor nuclear factor-κB inducible gene expression [12]. In a previous study, we found that TRAF1 was upregulated in several gastric cancer cell lines, including BGC823, SGC7901, and MGC803 [13]. Moreover, TRAF1 expression in human gastric mucosa is related to the H. pylori virulence genotype cagA+/vacAs1+/vacAm1+ [9]. In accordance with these findings, we demonstrate here that TRAF1 was highly expressed in the gastric mucosa of IM and Ca patients and that TRAF1 upregulation correlated positively with the H. pylori cagA+/vacAs1+/vacAm1+ virulence genotype.
Bcl-xL and 4-1BB are two key regulators in the TRAF1 signaling pathway. TRAF1 has a prosurvival effect in CD8 T cells via the 4-1BB–mediated upregulation of Bcl-xL [14]. Bcl-xL, an antiapoptotic member of the Bcl-2 family, is involved in modulating the angiogenic phenotype of human tumor cells [15, 16]. Via cross-talk with P-glycoprotein, Bcl-xL acts as an antiapoptotic factor in H. pylori–related gastric carcinogenesis [17]. Hence, we investigated 4-1BB and Bcl-xL expression in the gastric mucosa from different gastric diseases. 4-1BB expression was greatly upregulated in AG, IM, and Ca gastric mucosa, and Bcl-xL levels were increased, especially in IM and Ca gastric mucosa. Consistent with our findings, Yang et al. reported that Bcl-xL expression was relatively low in CG and AG patients, but was markedly increased in Ca patients [18]. As TRAF1, 4-1BB, and Bcl-xL are correlated positively, TRAF1 may trigger 4-1BB–mediated Bcl-xL activity. TRAF1–4-1BB–Bcl-xL signaling pathway upregulation may play a critical role in the development gastritis into gastric cancer.
A significantly high prevalence of East Asian cagA-positive H. pylori infection has been reported in gastric cancer patients (84.6%), suggesting that cagA-positive H. pylori infection and gastric cancer are closely associated [19]. Furthermore, it has been suggested that the vacAs1+/vacAm1+ genotype is associated with gastric cancer [20]. Importantly, a high proportion of subjects with upregulated TRAF1, 4-1BB, and Bcl-xL expression in the present study were infected with cagA+/vacAs1+/vacAm1+ H. pylori. In contrast, Matsumoto et al. showed that H. pylori vacA reduced Bcl-xL expression in gastric adenocarcinoma cell lines and led to apoptosis [21]. This discrepancy might be due to the differences between in vitro and in vivo conditions. Additionally, we cannot discount the possibility that differing virulence genotypes (cagA+/vacAs1+/vacAm1+, vacA+) may have different effects. Future studies should continue to investigate the effects of the cagA+/vacAs1+/vacAm1+ virulence genotype on TRAF1, 4-1BB, and Bcl-xL expression in cultured gastric cancer cells.
In summary, our study implies that cagA+/vacAs1+/vacAm1+ H. pylori infection might promote gastritis progression to gastric cancer, possibly by upregulating TRAF1, 4-1BB, and Bcl-xL expression in gastric mucosal tissue. It is possible that cagA+/vacAs1+/vacAm1+ H. pylori upregulates TRAF1 activation, which triggers 4-1BB–mediated Bcl-xL activation, thereby exerting an antiapoptotic effect and contributing to the pathogenesis of gastric cancer. Nevertheless, the underlying mechanism involved in this process requires further clarification.
Acknowledgments
This study was supported by grants from the Natural Science Foundation of Hunan Province, China (Grant no. 14JJ2036), the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (Grant batch. 48), the Major Special Project of Ministry of Science and Technology, China (Grant no. 2012ZX09304003-005), the Department of Science and Technology of Hunan Province (Grant no. 2012FJ4311), and the National Science Foundation of China (Grant no. 81171597). The authors would like to thank Medjaden Bioscience Limited for assisting in the preparation of this paper.
Conflict of Interests
The authors declare that they have no conflict of interests.
Authors' Contribution
Fen Wang conceived the study and drafted the paper. Zhiying Liu carried out the molecular genetic studies and drafted the paper. Guangkui Bu participated in drafting the paper and performed the statistical analysis. Xiayu Li, Nanfang Qu, and Jin Peng participated in the sample collection and western blotting. Canxia Xu and Shourong Shen participated in the design of the study. Yi Yuan conceived the study and helped in drafting the paper. All the authors read and approved the final paper. Fen Wang and Xiang Wu have an equal contribution to this study.
References
- 1.McColl K. E. L. Helicobacter pylori infection. The New England Journal of Medicine. 2010;362(17):1597–1604. doi: 10.1056/nejmcp1001110. [DOI] [PubMed] [Google Scholar]
- 2.Suerbaum S., Michetti P. Helicobacter pylori infection. The New England Journal of Medicine. 2002;347(15):1175–1186. doi: 10.1056/nejmra020542. [DOI] [PubMed] [Google Scholar]
- 3.Malnick S. D., Melzer E., Attali M., Duek G., Yahav J. Helicobacter pylori: friend or foe? World Journal of Gastroenterology. 2014;20(27):8979–8985. doi: 10.3748/wjg.v20.i27.8979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process—first American Cancer Society Award lecture on cancer epidemiology and prevention. Cancer Research. 1992;52(24):6735–6740. [PubMed] [Google Scholar]
- 5.Lu B., Li M. Helicobacter pylori eradication for preventing gastric cancer. World Journal of Gastroenterology. 2014;20(19):5660–5665. doi: 10.3748/wjg.v20.i19.5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L., Zou X., Liu Y.-F., Sheng G.-Y. Association between helicobacter pylori infection and chronic idiopathic neutropenia. Journal of Huazhong University of Science and Technology—Medical Science. 2013;33(3):353–356. doi: 10.1007/s11596-013-1123-x. [DOI] [PubMed] [Google Scholar]
- 7.Ki M.-R., Hwang M., Kim A.-Y., et al. Role of vacuolating cytotoxin VacA and cytotoxin-associated antigen CagA of Helicobacter pylori in the progression of gastric cancer. Molecular and Cellular Biochemistry. 2014;396(1-2):23–32. doi: 10.1007/s11010-014-2138-8. [DOI] [PubMed] [Google Scholar]
- 8.Wang F., Luo L.-D., Pan J.-H., et al. Comparative genomic study of gastric epithelial cells co-cultured with Helicobacter pylori. World Journal of Gastroenterology. 2012;18(48):7212–7224. doi: 10.3748/wjg.v18.i48.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang F., Bu G., Feng Q., et al. The expression level of TRAF1 in human gastric mucosa is related to virulence genotypes of Helicobacter pylori . Scandinavian Journal of Gastroenterology. 2014;49(8):925–932. doi: 10.3109/00365521.2014.919015. [DOI] [PubMed] [Google Scholar]
- 10.Yamaoka Y., Kodama T., Gutierrez O., Kim J. G., Kashima K., Graham D. Y. Relationship between Helicobacter pylori iceA, cagA, and vacA status and clinical outcome: studies in four different countries. Journal of Clinical Microbiology. 1999;37(7):2274–2279. doi: 10.1128/jcm.37.7.2274-2279.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arévalo-Galvis A., Trespalacios-Rangel A. A., Otero W., Mercado-Reyes M. M., Poutou-Piñales R. A. Prevalence of cagA, vacA, babA2 and iceA genes in H. pylori strains isolated from Colombian patients with functional dyspepsia. Polish Journal of Microbiology. 2012;61(1):33–40. [PubMed] [Google Scholar]
- 12.Leo E., Deveraux Q. L., Buchholtz C., et al. TRAF1 is a substrate of caspases activated during tumor necrosis factor receptor-alpha-induced apoptosis. The Journal of Biological Chemistry. 2001;276(11):8087–8093. doi: 10.1074/jbc.m009450200. [DOI] [PubMed] [Google Scholar]
- 13.Wang F., Yang Y., Feng Q., et al. Construction of RNAi targeting TRAF1 gene and effect of TRAF1 on gastric cancer cells. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2012;37(9):876–882. doi: 10.3969/j.issn.1672-7347.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Sabbagh L., Pulle G., Liu Y., Tsitsikov E. N., Watts T. H. ERK-dependent Bim modulation downstream of the 4-1BB-TRAF1 signaling axis is a critical mediator of CD8 T cell survival in vivo. Journal of Immunology. 2008;180(12):8093–8101. doi: 10.4049/jimmunol.180.12.8093. [DOI] [PubMed] [Google Scholar]
- 15.Giorgini S., Trisciuoglio D., Gabellini C., et al. Modulation of bcl-xL in tumor cells regulates angiogenesis through CXCL8 expression. Molecular Cancer Research. 2007;5(8):761–771. doi: 10.1158/1541-7786.MCR-07-0088. [DOI] [PubMed] [Google Scholar]
- 16.Adams J. M., Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26(9):1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rocco A., Compare D., Liguori E., et al. MDR1-P-glycoprotein behaves as an oncofetal protein that promotes cell survival in gastric cancer cells. Laboratory Investigation. 2012;92(10):1407–1418. doi: 10.1038/labinvest.2012.100. [DOI] [PubMed] [Google Scholar]
- 18.Yang L., Levi E., Zhu S., Du J., Majumdar A. P. N. Cancer stem cells biomarkers in gastric carcinogenesis. Journal of Gastrointestinal Cancer. 2013;44(4):428–435. doi: 10.1007/s12029-013-9534-2. [DOI] [PubMed] [Google Scholar]
- 19.Satomi S., Yamakawa A., Matsunaga S., et al. Relationship between the diversity of the cagA gene of Helicobacter pylori and gastric cancer in Okinawa, Japan. Journal of Gastroenterology. 2006;41(7):668–673. doi: 10.1007/s00535-006-1838-6. [DOI] [PubMed] [Google Scholar]
- 20.Kidd M., Lastovica A. J., Atherton J. C., Louw J. A. Heterogeneity in the Helicobacter pylorivacA and cagA genes: association with gastroduodenal disease in South Africa? Gut. 1999;45(4):499–502. doi: 10.1136/gut.45.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsumoto A., Isomoto H., Nakayama M., et al. Helicobacter pylori VacA reduces the cellular expression of STAT3 and pro-survival Bcl-2 family proteins, Bcl-2 and Bcl-X L, leading to apoptosis in gastric epithelial cells. Digestive Diseases and Sciences. 2011;56(4):999–1006. doi: 10.1007/s10620-010-1420-1. [DOI] [PubMed] [Google Scholar]