Abstract
During nitrogen starvation, a nonselective bulk degradation of cytosolic proteins and organelles including ribosomes, termed macro-autophagy (hereafter termed autophagy), is induced. The precise mechanism of RNA degradation by this pathway has not been yet elucidated. In this issue of the The EMBO Journal, Huang et al characterize an autophagy-dependent RNA catabolism in yeast and identify the enzymes responsible for the degradation process.
See also: H Huang et al (January 2015)
To maintain cellular survival under various stress conditions, such as nutrient limitation, cells need to achieve a balance between protein synthesis and protein degradation. One of the pathways that ensures this cellular homeostasis is autophagy. Autophagy is characterized by the formation of autophagosomes, double-membrane-layered vesicles that are delivered to the vacuole and fuse with it. The released single-membrane-layered autophagic bodies are then lysed to degrade and recycle their contents, thereby contributing to the maintenance of cell survival under stress (Abada & Elazar, 2014).
While autophagy has long been considered nonselective, in recent years selective variants of autophagy that allow adaptation to changes in environmental conditions have been identified. Under nonlimited growth conditions for example, the cytoplasm-to-vacuole targeting (Cvt) pathway is responsible for the selective transport of aminopeptidase 1 (Ape1) to the vacuole. Under stress conditions, selective uptake of whole organelles such as mitochondria (mitophagy) or ribosomes (ribophagy), or parts of the nucleus (piecemeal microautophagy of the nucleus/PMN), is initiated. These selective autophagic processes depend on the core autophagic machinery (Nakatogawa et al, 2009), but some additional factors, such as Atg32 (Kanki et al, 2009; Okamoto et al, 2009), Atg11 (Kim et al, 2001) and Atg19 (Shintani et al, 2002), are responsible for their selective nature. As selective autophagy is directed to specific organelles, cytosolic constituents that might harm the cell when transported to the vacuole are excluded. However, upon nitrogen starvation, which leads to nonselective autophagy, abundant constituents such as ribosomes, comprising about half of the cellular proteins in yeast (Beau et al, 2008), are trapped within the autophagosome and undergo vacuolar degradation.
Unlike the substantial body of knowledge on the degradation of proteins, carbohydrates and lipids by specific enzymes inside the vacuole, the mechanism responsible for vacuolar degradation of RNA has not been extensively studied. A well-known complex for RNA degradation is the exosome. However, this network of RNA exonucleases and endonucleases is localized in the cytosol and the nucleus and operates mainly under normal growth conditions (Schneider & Tollervey, 2013). RNautophagy, a new type of selective autophagy in mammals that is responsible for lysosomal RNA degradation, was recently reported (Fujiwara et al, 2013). In this issue of The EMBO Journal, Huang et al (2015) provide new mechanistic insights into the autophagy-dependent bulk RNA degradation that takes place under nitrogen starvation in yeast. By performing whole-cell metabolome analysis of the yeast, these authors found that starvation leads to time-dependent changes in the intracellular concentrations of RNA metabolites in wild-type but not in autophagy-depleted cells. Upon nitrogen starvation, cytosolic RNA is sequestered into autophagosomes and transported into the vacuole. Importantly, Huang et al (2015) identify the enzymes responsible for the degradation of RNA within the vacuole (Fig1). Like most vacuole-mediated degradation processes, RNA degradation is dependent on Pep4 and its role as a master activator of vacuolar enzymes and on the protease activity of Prb1 to lyse the autophagic body (Fig1A). The released RNA is then processed by the ribonuclease Rny1, which is the sole vacuolar RNase identified in yeast so far (Fig1B). Rny1 catalyzes the cleavage of single-strand RNA producing mono-or oligonucleotides with a terminal 3′-phosphate group. A starvation-induced increase in 3′-NMP (nucleotide monophosphate) is therefore observed in wild-type but not in autophagy-deficient or rny1Δ cells. This was accompanied by an accumulation of RNA within the vacuole of the rny1Δ cells. Furthermore, the authors identify Pho8, which is known for its broad substrate specificity, as the phosphatase responsible for processing of the 3′-NMPs into the corresponding nucleosides (Fig1C). Fluorescence microscopy reveals that Rny1 and Pho8 localize inside the vacuole under starvation and that the expression, especially of Rny1, is robustly induced. The processing of 3′-NMPs seems to be specific, as Phm8, a nucleotidase previously reported to be responsible for RNA degradation under starvation in yeast (Xu et al, 2013), is not essential under these conditions. The generated nucleosides are further catabolized in the cytoplasm by the purine nucleoside phosphorylase Pnp1 and the pyrimidine nucleoside hydrolase Urh1 (Fig1D). Since the resulting bases, in particular uracil and xanthine, cannot be utilized as nitrogen sources, they are excreted from the cell within 2 h of starvation.
Figure 1. Autophagy-dependent RNA degradation under nitrogen starvation.
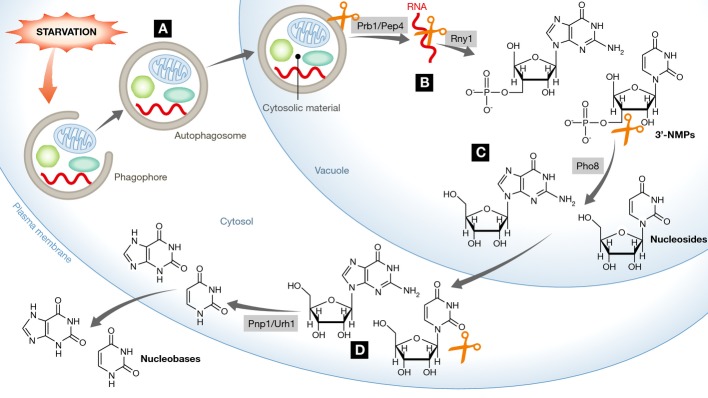
Upon nitrogen starvation, RNA is engulfed by a phagophore and transported into the vacuole by the resulting autophagosome (A). The released RNA is then processed by Rny1 to nucleotides (3′-NMPs) (B), which are further catabolized by Pho8 to form nucleosides (C). The generated nucleosides are transported from the vacuole to the cytosol, where they are processed by Pnp1 and Urh1 to nucleobases, which are then excreted from the cell (D).#
A critical conclusion of their study is the authors' hypothesis that the vacuolar degradation of RNA is mediated by nonselective autophagy. By measuring the nucleoside levels in different deletion strains, they showed that the observed degradation is not dependent on factors such as Nvj1, which is essential for PMN, or Atg19 (Cvt). Moreover, although the nucleoside level in atg11Δ cells decreased, it remained intermediate between those of wild-type and atg7Δ cells, thus excluding Atg11 as an essential protein for this process. In an earlier study, it was suggested that upon nitrogen starvation in yeast, mature ribosomes are selectively degraded by ribophagy in a Bre5-and Ubp3-dependent manner (Kraft et al, 2008). Huang et al (2015) report, however, that deletion of these factors leads to only a slight delay in RNA degradation. These differences might be a result of the use of different yeast strains and conditions but also indicate that several different autophagic processes might participate in ribosome and RNA degradation. Clearly, additional studies are needed to resolve this issue.
The study by Huang et al (2015) provides new insights into the autophagic degradation of RNA, while also developing an approach that might serve in the future as a complementary way to determine autophagic activity. Nevertheless, several questions remain to be answered. Is the vacuolar degradation essential for cell survival under nitrogen starvation? Are there additional proteins that contribute to autophagic RNA degradation? What proteins contribute to the excretion of uracil and xanthine from the cell? How is the maintenance of cellular metabolic balance and homeostasis ensured? Is the degradation regulated by the amount of available RNA types, or is a specific type of RNA preferentially degraded? Future research on selectivity and/or nonselectivity can be expected to shed further light on the process of RNA degradation under starvation.
Footnotes
Correction added on 14 January 2015, after first online publication: in the structure of 3'-NMPs, the phosphate group was moved to the correct 3' position.
References
- Abada A, Elazar Z. Getting ready for building: signaling and autophagosome biogenesis. EMBO Rep. 2014;15:839–852. doi: 10.15252/embr.201439076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beau I, Esclatine A, Codogno P. Lost to translation: when autophagy targets mature ribosomes. Trends Cell Biol. 2008;18:311–314. doi: 10.1016/j.tcb.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Furuta A, Kikuchi H, Aizawa S, Hatanaka Y, Konya C, Uchida K, Yoshimura A, Tamai Y, Wada K, Kabuta T. Discovery of a novel type of autophagy targeting RNA. Autophagy. 2013;9:403–409. doi: 10.4161/auto.23002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Kawamata T, Horie T, Tsugawa H, Nakayama Y, Ohsumi Y, Fukusaki E. Bulk RNA degradation by nitrogen starvation-induced autophagy in yeast. EMBO J. 2015;34:154–168. doi: 10.15252/embj.201489083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanki T, Wang K, Cao Y, Baba M, Klionsky DJ. Atg32 is a mitochondrial protein that confers selectivity during mitophagy. Dev Cell. 2009;17:98–109. doi: 10.1016/j.devcel.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kamada Y, Stromhaug PE, Guan J, Hefner-Gravink A, Baba M, Scott SV, Ohsumi Y, Dunn WA, Klionsky DJ. Cvt9/Gsa9 functions in sequestering selective cytosolic cargo destined for the vacuole. J Cell Biol. 2001;153:381–396. doi: 10.1083/jcb.153.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft C, Deplazes A, Sohrmann M, Peter M. Mature ribosomes are selectively degraded upon starvation by an autophagy pathway requiring the Ubp3p/Bre5p ubiquitin protease. Nat Cell Biol. 2008;10:602–610. doi: 10.1038/ncb1723. [DOI] [PubMed] [Google Scholar]
- Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol. 2009;10:458–467. doi: 10.1038/nrm2708. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Kondo-Okamoto N, Ohsumi Y. Mitochondria-anchored receptor Atg32 mediates degradation of mitochondria via selective autophagy. Dev Cell. 2009;17:87–97. doi: 10.1016/j.devcel.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Schneider C, Tollervey D. Threading the barrel of the RNA exosome. Trends Biochem Sci. 2013;38:485–493. doi: 10.1016/j.tibs.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani T, Huang WP, Stromhaug PE, Klionsky DJ. Mechanism of cargo selection in the cytoplasm to vacuole targeting pathway. Dev Cell. 2002;3:825–837. doi: 10.1016/s1534-5807(02)00373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YF, Létisse F, Absalan F, Lu W, Kuznetsova E, Brown G, Caudy AA, Yakunin AF, Broach JR, Rabinowitz JD. Nucleotide degradation and ribose salvage in yeast. Mol Syst Biol. 2013;9:665. doi: 10.1038/msb.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]


