Abstract
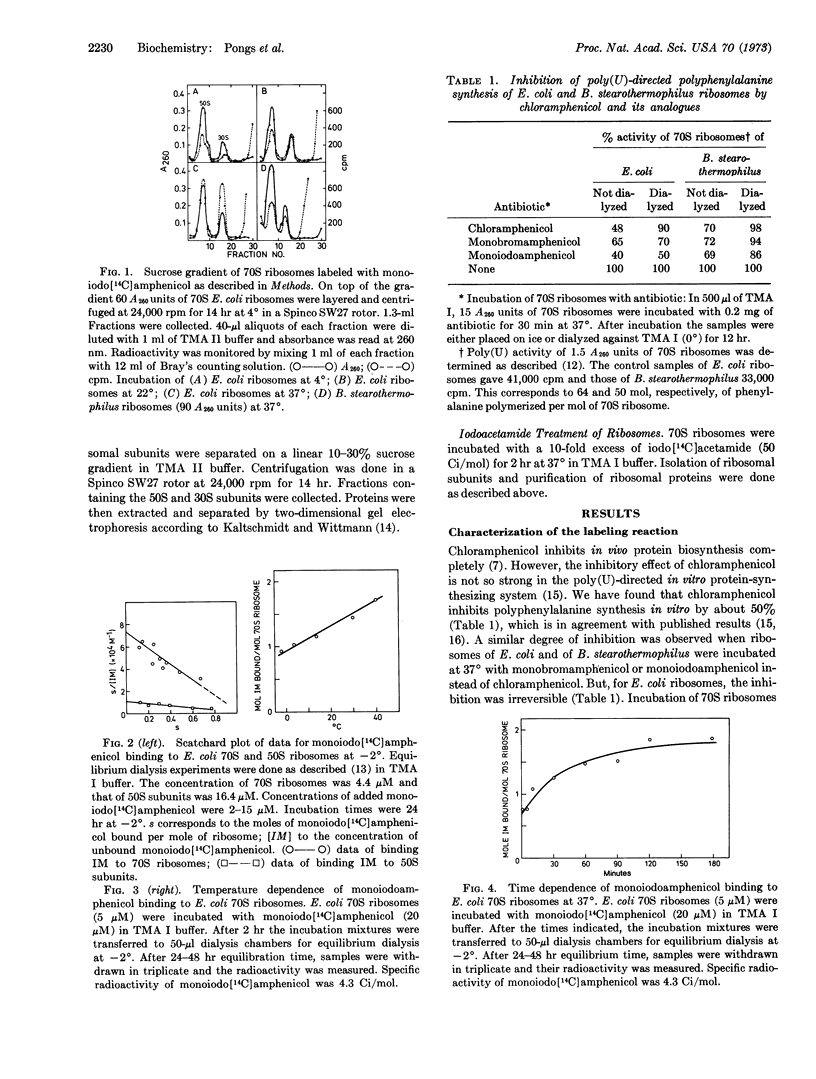
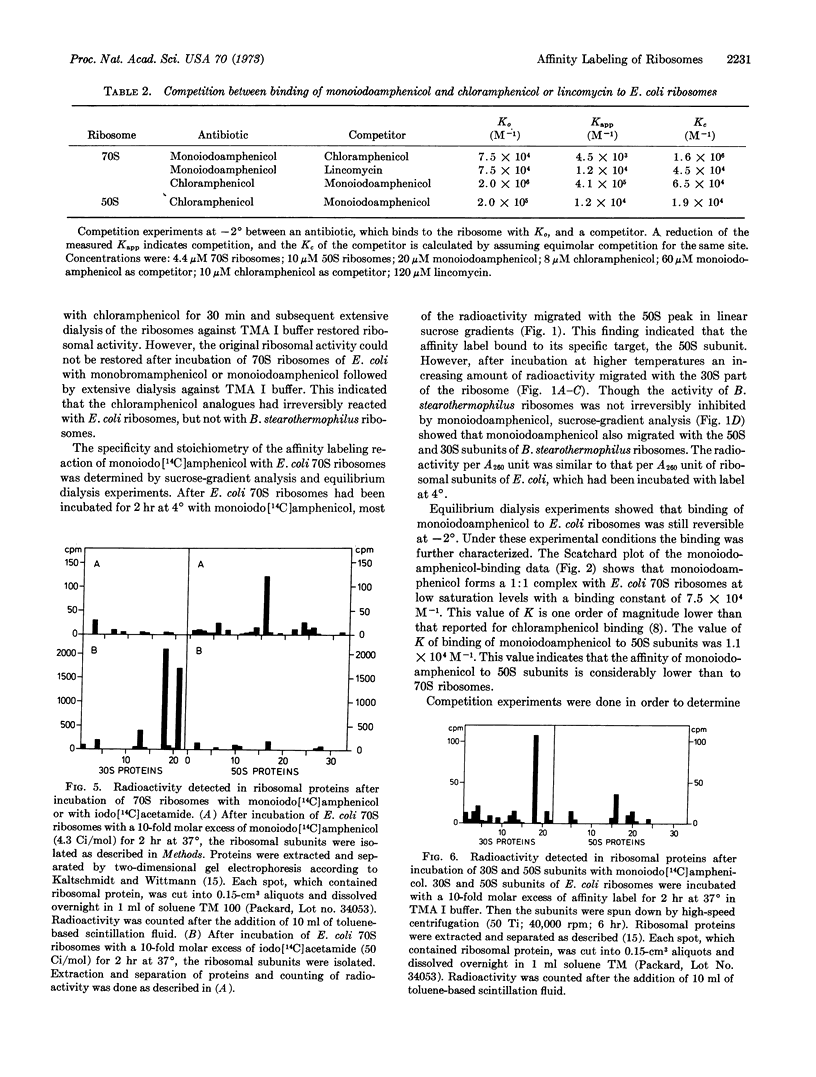
Monoiodoamphenicol, a synthetic analogue of chloramphenicol, has been shown by competition experiments with chloramphenicol and lincomycin to bind at the same site of 70S ribosomes as chloramphenicol. At — 2° it forms a 1:1 complex with 70S ribosomes having a value of K (7.5 × 104 M-1) that is one order of magnitude lower than that of chloramphenicol. At 37°, monoiodoamphenicol irreversibly inhibits the protein-synthesizing activity of E. coli ribosomes. It is shown that the analogue reacted preferentially with protein L16 of E. coli 70S ribosomes, and we therefore conclude that protein L16 belongs to the chloramphenicol-binding site of E. coli ribosomes.
Since the chemically reactive group of monoiodoamphenicol resembles iodoacetamide, the reaction of E. coli 70S ribosomes with monoiodoamphenicol was compared to that with iodoacetamide. Iodoacetamide did not react with protein L16, but it predominantly reacted with proteins S18 of the 30S subunit. Furthermore, monoiodoamphenicol was reacted with E. coli ribosomal subunits. Isolated 50S subunits bound monoiodoamphenicol by about one order of magnitude less than 70S ribosomes. Again, protein L16 reacted with the affinity label. Monoiodoamphenicol reacted with protein S18 in isolated 30S subunits; it also bound to 70S ribosomes of Bacillus stearothermophilus, however, it did not bind irreversibly to these 70S ribosomes.
Keywords: antibiotics, ribosomal proteins, two-dimensional gel electrophoresis, equilibrium dialysis
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bald R., Erdmann V. A., Pongs O. Irreversible binding of chloramphenicol analogues to E. coli ribosomes. FEBS Lett. 1972 Dec 1;28(2):149–152. doi: 10.1016/0014-5793(72)80698-7. [DOI] [PubMed] [Google Scholar]
- Birge E. A., Kurland C. G. Altered ribosomal protein in streptomycin-dependent Escherichia coli. Science. 1969 Dec 5;166(3910):1282–1284. doi: 10.1126/science.166.3910.1282. [DOI] [PubMed] [Google Scholar]
- Czernilofsky A. P., Kuechler E. Affinity label for the tRNA binding site on the Escherichia coli ribosome. Biochim Biophys Acta. 1972 Jul 31;272(4):667–671. doi: 10.1016/0005-2787(72)90526-6. [DOI] [PubMed] [Google Scholar]
- Erdmann V. A., Fahnestock S., Higo K., Nomura M. Role of 5S RNA in the functions of 50S ribosomal subunits. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2932–2936. doi: 10.1073/pnas.68.12.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahnestock S., Erdmann V., Nomura M. Reconstitution of 50S ribosomal subunits from protein-free ribonucleic acid. Biochemistry. 1973 Jan 16;12(2):220–224. doi: 10.1021/bi00726a007. [DOI] [PubMed] [Google Scholar]
- Fernandez-Munoz R., Monro R. E., Torres-Pinedo R., Vazquez D. Substrate- and antibiotic-binding sites at the peptidyl-transferase centre of Escherichia coli ribosomes. Studies on the chloramphenicol. lincomycin and erythromycin sites. Eur J Biochem. 1971 Nov 11;23(1):185–193. doi: 10.1111/j.1432-1033.1971.tb01607.x. [DOI] [PubMed] [Google Scholar]
- Funatsu G., Wittmann H. G. Ribosomal proteins. 33. Location of amino-acid replacements in protein S12 isolated from Escherichia coli mutants resistant to streptomycin. J Mol Biol. 1972 Jul 28;68(3):547–550. doi: 10.1016/0022-2836(72)90108-8. [DOI] [PubMed] [Google Scholar]
- KUCAN Z., LIPMANN F. DIFFERENCES IN CHLORAMPHENICOL SENSITIVITY OF CELL-FREE AMINO ACID POLYMERIZATION SYSTEMS. J Biol Chem. 1964 Feb;239:516–520. [PubMed] [Google Scholar]
- Kaltschmidt E., Wittmann H. G. Ribosomal proteins. XII. Number of proteins in small and large ribosomal subunits of Escherichia coli as determined by two-dimensional gel electrophoresis. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1276–1282. doi: 10.1073/pnas.67.3.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIRENBERG M. W., MATTHAEI J. H. The dependence of cell-free protein synthesis in E. coli upon naturally occurring or synthetic polyribonucleotides. Proc Natl Acad Sci U S A. 1961 Oct 15;47:1588–1602. doi: 10.1073/pnas.47.10.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki M., Mizushima S., Nomura M. Identification and functional characterization of the protein controlled by the streptomycin-resistant locus in E. coli. Nature. 1969 Apr 26;222(5191):333–339. doi: 10.1038/222333a0. [DOI] [PubMed] [Google Scholar]
- Pellegrini M., Oen H., Cantor C. R. Covalent attachment of a peptidyl-transfer RNA analog to the 50S subunit of Escherichia coli ribosomes. Proc Natl Acad Sci U S A. 1972 Apr;69(4):837–841. doi: 10.1073/pnas.69.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongs O., Bald R., Reinwald E. On the structure of yeast tRNA Phe . Complementary-oligonucleotide binding studies. Eur J Biochem. 1973 Jan 3;32(1):117–125. doi: 10.1111/j.1432-1033.1973.tb02586.x. [DOI] [PubMed] [Google Scholar]
- Randall-Hazelbauer L. L., Kuland C. G. Identification of three 30S proteins contributing to the ribosomal A site. Mol Gen Genet. 1972;115(3):234–242. doi: 10.1007/BF00268887. [DOI] [PubMed] [Google Scholar]
- Traub P., Nomura M. Structure and function of E. coli ribosomes. V. Reconstitution of functionally active 30S ribosomal particles from RNA and proteins. Proc Natl Acad Sci U S A. 1968 Mar;59(3):777–784. doi: 10.1073/pnas.59.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub P. Structure, function and in vitro reconstitution of escherichia coli ribosomes. Curr Top Microbiol Immunol. 1970;52:1–93. doi: 10.1007/978-3-642-95130-5_1. [DOI] [PubMed] [Google Scholar]
- WOLFE A. D., HAHN F. E. MODE OF ACTION OF CHLORAMPHENICOL. IX. EFFECTS OF CHLORAMPHENICOL UPON A RIBOSOMAL AMINO ACID POLYMERIZATION SYSTEM AND ITS BINDING TO BACTERIAL RIBOSOME. Biochim Biophys Acta. 1965 Jan 11;95:146–155. doi: 10.1016/0005-2787(65)90219-4. [DOI] [PubMed] [Google Scholar]
- Weisblum B., Davies J. Antibiotic inhibitors of the bacterial ribosome. Bacteriol Rev. 1968 Dec;32(4 Pt 2):493–528. [PMC free article] [PubMed] [Google Scholar]


