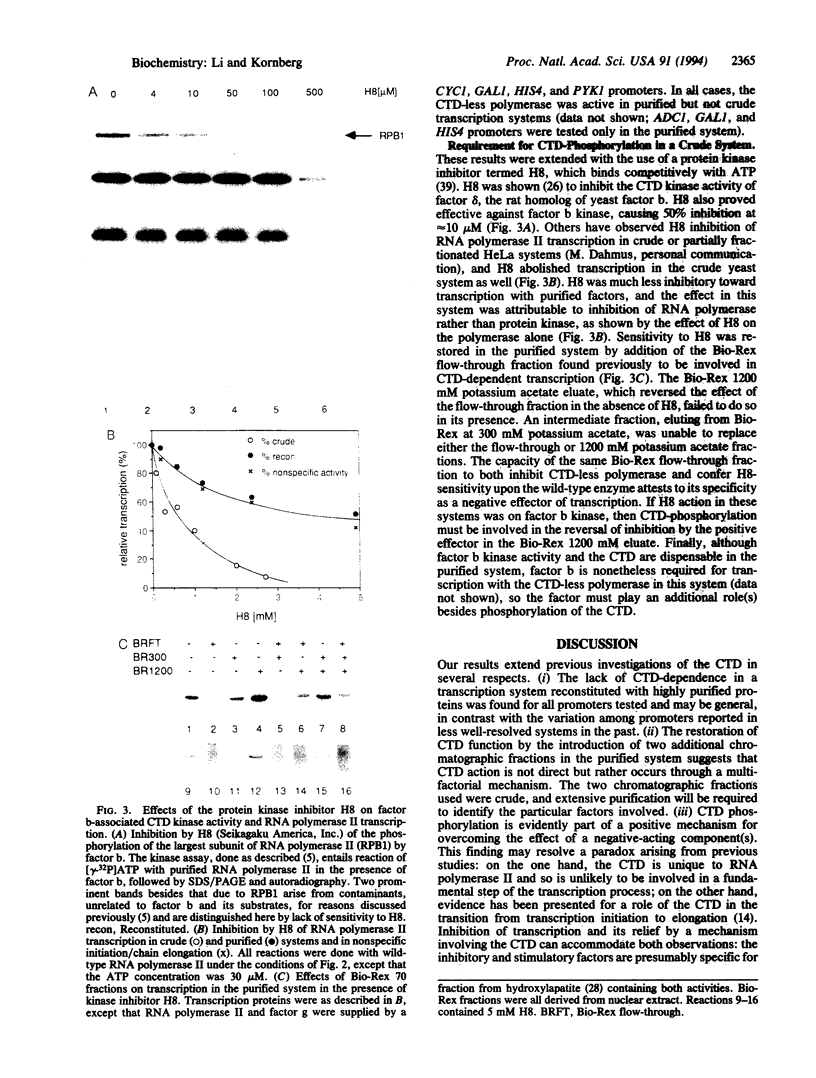
Abstract
RNA polymerase II lacking a C-terminal domain (CTD) was active in transcription with purified proteins from yeast but failed to support transcription in a yeast extract. CTD dependence could be reconstituted in the purified system by addition of two fractions from the extract. An inhibitory fraction abolished transcription by both wild-type and CTD-less RNA polymerases; a stimulatory fraction restored activity of the wild-type polymerase but had a much lesser effect on the CTD-less enzyme. Parallel results were obtained with the use of a kinase inhibitor that prevents phosphorylation of the CTD by RNA polymerase II initiation factor b. The kinase inhibitor abolished transcription by wild-type polymerase in yeast extract but had no significant effect in the purified system. The requirement for both the CTD and kinase action for transcription in an extract indicates that CTD phosphorylation is involved in opposing the negative effector in the extract. Factor b must play a role(s) in addition to phosphorylation of the CTD because it was still required for transcription with polymerase lacking a CTD in the purified system.
Full text
PDF
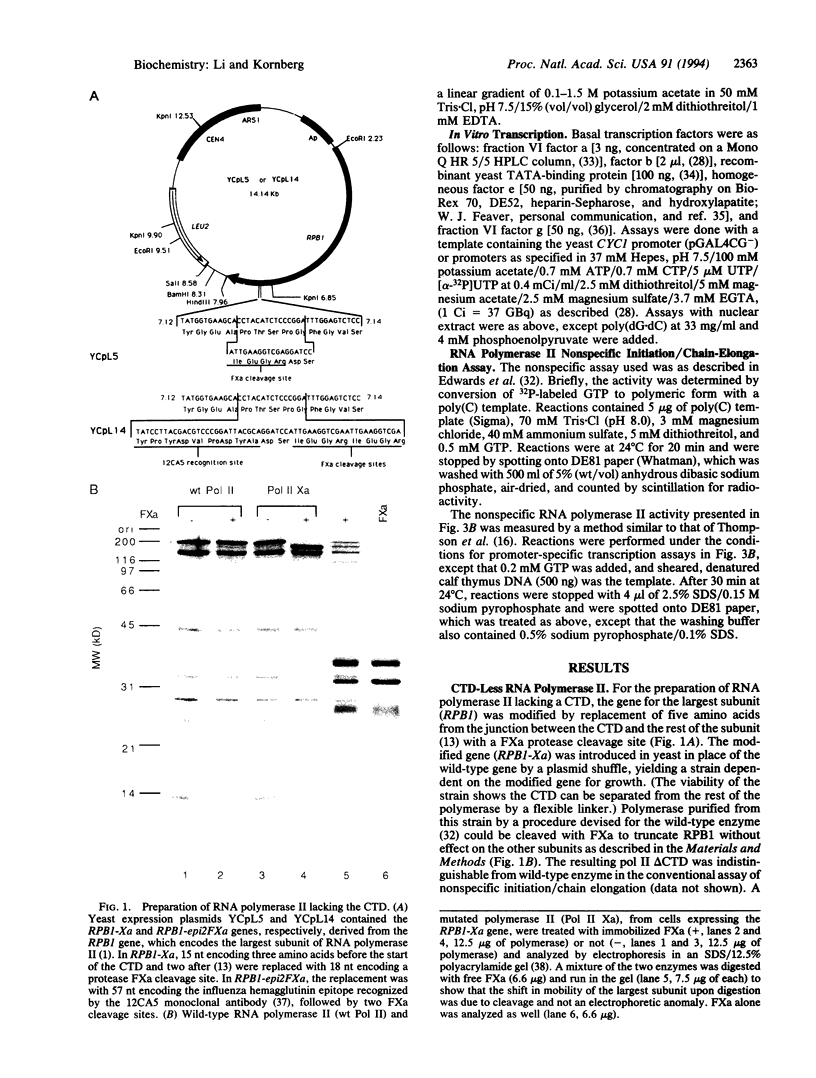
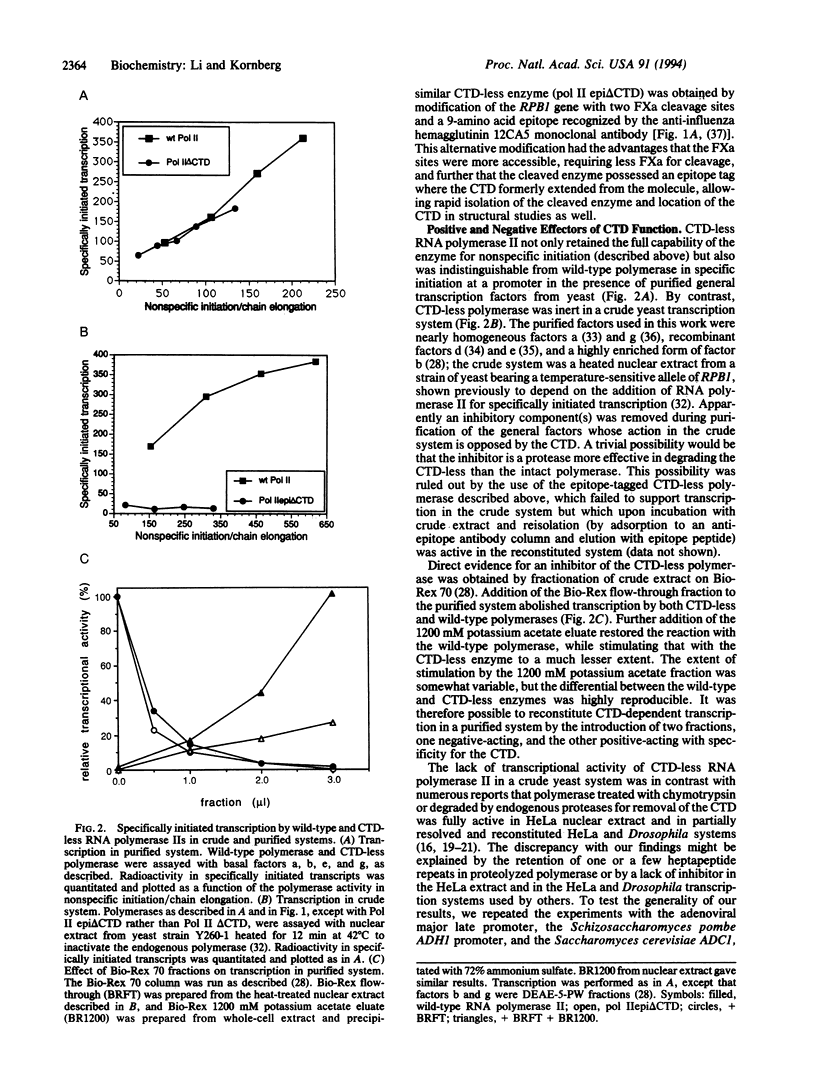

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison L. A., Ingles C. J. Mutations in RNA polymerase II enhance or suppress mutations in GAL4. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2794–2798. doi: 10.1073/pnas.86.8.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker D. M., Guarente L. High-efficiency transformation of yeast by electroporation. Methods Enzymol. 1991;194:182–187. doi: 10.1016/0076-6879(91)94015-5. [DOI] [PubMed] [Google Scholar]
- Buermeyer A. B., Thompson N. E., Strasheim L. A., Burgess R. R., Farnham P. J. The HIP1 initiator element plays a role in determining the in vitro requirement of the dihydrofolate reductase gene promoter for the C-terminal domain of RNA polymerase II. Mol Cell Biol. 1992 May;12(5):2250–2259. doi: 10.1128/mcb.12.5.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buratowski S., Sharp P. A. Transcription initiation complexes and upstream activation with RNA polymerase II lacking the C-terminal domain of the largest subunit. Mol Cell Biol. 1990 Oct;10(10):5562–5564. doi: 10.1128/mcb.10.10.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadena D. L., Dahmus M. E. Messenger RNA synthesis in mammalian cells is catalyzed by the phosphorylated form of RNA polymerase II. J Biol Chem. 1987 Sep 15;262(26):12468–12474. [PubMed] [Google Scholar]
- Chao D. M., Young R. A. Tailored tails and transcription initiation: the carboxyl terminal domain of RNA polymerase II. Gene Expr. 1991 Apr;1(1):1–4. [PMC free article] [PubMed] [Google Scholar]
- Conaway J. W., Bradsher J. N., Conaway R. C. Mechanism of assembly of the RNA polymerase II preinitiation complex. Transcription factors delta and epsilon promote stable binding of the transcription apparatus to the initiator element. J Biol Chem. 1992 May 15;267(14):10142–10148. [PubMed] [Google Scholar]
- Dahmus M. E., Kedinger C. Transcription of adenovirus-2 major late promoter inhibited by monoclonal antibody directed against RNA polymerases IIO and IIA. J Biol Chem. 1983 Feb 25;258(4):2303–2307. [PubMed] [Google Scholar]
- Edwards A. M., Kane C. M., Young R. A., Kornberg R. D. Two dissociable subunits of yeast RNA polymerase II stimulate the initiation of transcription at a promoter in vitro. J Biol Chem. 1991 Jan 5;266(1):71–75. [PubMed] [Google Scholar]
- Feaver W. J., Gileadi O., Kornberg R. D. Purification and characterization of yeast RNA polymerase II transcription factor b. J Biol Chem. 1991 Oct 5;266(28):19000–19005. [PubMed] [Google Scholar]
- Feaver W. J., Gileadi O., Li Y., Kornberg R. D. CTD kinase associated with yeast RNA polymerase II initiation factor b. Cell. 1991 Dec 20;67(6):1223–1230. doi: 10.1016/0092-8674(91)90298-d. [DOI] [PubMed] [Google Scholar]
- Fischer L., Gerard M., Chalut C., Lutz Y., Humbert S., Kanno M., Chambon P., Egly J. M. Cloning of the 62-kilodalton component of basic transcription factor BTF2. Science. 1992 Sep 4;257(5075):1392–1395. doi: 10.1126/science.1529339. [DOI] [PubMed] [Google Scholar]
- Gileadi O., Feaver W. J., Kornberg R. D. Cloning of a subunit of yeast RNA polymerase II transcription factor b and CTD kinase. Science. 1992 Sep 4;257(5075):1389–1392. doi: 10.1126/science.1445600. [DOI] [PubMed] [Google Scholar]
- Henry N. L., Sayre M. H., Kornberg R. D. Purification and characterization of yeast RNA polymerase II general initiation factor g. J Biol Chem. 1992 Nov 15;267(32):23388–23392. [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Kelleher R. J., 3rd, Flanagan P. M., Chasman D. I., Ponticelli A. S., Struhl K., Kornberg R. D. Yeast and human TFIIDs are interchangeable for the response to acidic transcriptional activators in vitro. Genes Dev. 1992 Feb;6(2):296–303. doi: 10.1101/gad.6.2.296. [DOI] [PubMed] [Google Scholar]
- Kim W. Y., Dahmus M. E. The major late promoter of adenovirus-2 is accurately transcribed by RNA polymerases IIO, IIA, and IIB. J Biol Chem. 1989 Feb 25;264(6):3169–3176. [PubMed] [Google Scholar]
- Koleske A. J., Buratowski S., Nonet M., Young R. A. A novel transcription factor reveals a functional link between the RNA polymerase II CTD and TFIID. Cell. 1992 May 29;69(5):883–894. doi: 10.1016/0092-8674(92)90298-q. [DOI] [PubMed] [Google Scholar]
- Kolodziej P. A., Young R. A. Epitope tagging and protein surveillance. Methods Enzymol. 1991;194:508–519. doi: 10.1016/0076-6879(91)94038-e. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laybourn P. J., Dahmus M. E. Transcription-dependent structural changes in the C-terminal domain of mammalian RNA polymerase subunit IIa/o. J Biol Chem. 1989 Apr 25;264(12):6693–6698. [PubMed] [Google Scholar]
- Liao S. M., Taylor I. C., Kingston R. E., Young R. A. RNA polymerase II carboxy-terminal domain contributes to the response to multiple acidic activators in vitro. Genes Dev. 1991 Dec;5(12B):2431–2440. doi: 10.1101/gad.5.12b.2431. [DOI] [PubMed] [Google Scholar]
- Lu H., Zawel L., Fisher L., Egly J. M., Reinberg D. Human general transcription factor IIH phosphorylates the C-terminal domain of RNA polymerase II. Nature. 1992 Aug 20;358(6388):641–645. doi: 10.1038/358641a0. [DOI] [PubMed] [Google Scholar]
- Moyle M., Lee J. S., Anderson W. F., Ingles C. J. The C-terminal domain of the largest subunit of RNA polymerase II and transcription initiation. Mol Cell Biol. 1989 Dec;9(12):5750–5753. doi: 10.1128/mcb.9.12.5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet M. L., Young R. A. Intragenic and extragenic suppressors of mutations in the heptapeptide repeat domain of Saccharomyces cerevisiae RNA polymerase II. Genetics. 1989 Dec;123(4):715–724. doi: 10.1093/genetics/123.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet M., Sweetser D., Young R. A. Functional redundancy and structural polymorphism in the large subunit of RNA polymerase II. Cell. 1987 Sep 11;50(6):909–915. doi: 10.1016/0092-8674(87)90517-4. [DOI] [PubMed] [Google Scholar]
- Peterson C. L., Kruger W., Herskowitz I. A functional interaction between the C-terminal domain of RNA polymerase II and the negative regulator SIN1. Cell. 1991 Mar 22;64(6):1135–1143. doi: 10.1016/0092-8674(91)90268-4. [DOI] [PubMed] [Google Scholar]
- Rose M. D., Broach J. R. Cloning genes by complementation in yeast. Methods Enzymol. 1991;194:195–230. doi: 10.1016/0076-6879(91)94017-7. [DOI] [PubMed] [Google Scholar]
- Sayre M. H., Tschochner H., Kornberg R. D. Purification and properties of Saccharomyces cerevisiae RNA polymerase II general initiation factor a. J Biol Chem. 1992 Nov 15;267(32):23383–23387. [PubMed] [Google Scholar]
- Sayre M. H., Tschochner H., Kornberg R. D. Reconstitution of transcription with five purified initiation factors and RNA polymerase II from Saccharomyces cerevisiae. J Biol Chem. 1992 Nov 15;267(32):23376–23382. [PubMed] [Google Scholar]
- Serizawa H., Conaway J. W., Conaway R. C. Phosphorylation of C-terminal domain of RNA polymerase II is not required in basal transcription. Nature. 1993 May 27;363(6427):371–374. doi: 10.1038/363371a0. [DOI] [PubMed] [Google Scholar]
- Serizawa H., Conaway R. C., Conaway J. W. A carboxyl-terminal-domain kinase associated with RNA polymerase II transcription factor delta from rat liver. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7476–7480. doi: 10.1073/pnas.89.16.7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M. The heptad repeat in the largest subunit of RNA polymerase II binds by intercalating into DNA. Nature. 1990 Apr 5;344(6266):562–565. doi: 10.1038/344562a0. [DOI] [PubMed] [Google Scholar]
- Thompson N. E., Steinberg T. H., Aronson D. B., Burgess R. R. Inhibition of in vivo and in vitro transcription by monoclonal antibodies prepared against wheat germ RNA polymerase II that react with the heptapeptide repeat of eukaryotic RNA polymerase II. J Biol Chem. 1989 Jul 5;264(19):11511–11520. [PubMed] [Google Scholar]
- Tschochner H., Sayre M. H., Flanagan P. M., Feaver W. J., Kornberg R. D. Yeast RNA polymerase II initiation factor e: isolation and identification as the functional counterpart of human transcription factor IIB. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11292–11296. doi: 10.1073/pnas.89.23.11292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usheva A., Maldonado E., Goldring A., Lu H., Houbavi C., Reinberg D., Aloni Y. Specific interaction between the nonphosphorylated form of RNA polymerase II and the TATA-binding protein. Cell. 1992 May 29;69(5):871–881. doi: 10.1016/0092-8674(92)90297-p. [DOI] [PubMed] [Google Scholar]
- Young R. A. RNA polymerase II. Annu Rev Biochem. 1991;60:689–715. doi: 10.1146/annurev.bi.60.070191.003353. [DOI] [PubMed] [Google Scholar]
- Zehring W. A., Greenleaf A. L. The carboxyl-terminal repeat domain of RNA polymerase II is not required for transcription factor Sp1 to function in vitro. J Biol Chem. 1990 May 25;265(15):8351–8353. [PubMed] [Google Scholar]
- Zehring W. A., Lee J. M., Weeks J. R., Jokerst R. S., Greenleaf A. L. The C-terminal repeat domain of RNA polymerase II largest subunit is essential in vivo but is not required for accurate transcription initiation in vitro. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3698–3702. doi: 10.1073/pnas.85.11.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]