Abstract
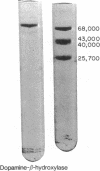
Dopamine-β-hydroxylase (EC 1.14.2.1) has been isolated as a pure protein from bovine-adrenal glands. Although the molecular weight of the native protein is well established (290,000), sodium dodecyl sulfate-gel electrophoresis under dissociating conditions gave a single band with a molecular weight of about 75,000. The enzyme contains about 4% carbohydrate, which consists of residues of mannose, glucosamine, galactose, glucose, fucose, and sialic acid. The pure enzyme also contains about 4 atoms of copper per molecule. It is concluded that dopamine-β-hydroxylase is a tetrameric glycoprotein.
Keywords: sodium dodecyl sulfate-gel electrophoresis, molecular weight, transmission of nerve impulses
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod J. Dopamine- -hydroxylase: regulation of its synthesis and release from nerve terminals. Pharmacol Rev. 1972 Jun;24(2):233–243. [PubMed] [Google Scholar]
- Belpaire F., Laduron P. Tissue fractionation and catecholamines. 3. Intracellular distribution of endogenous inhibitors of dopamine- -hydroxylase in adrenal medulla. Biochem Pharmacol. 1970 Apr;19(4):1323–1331. doi: 10.1016/0006-2952(70)90047-x. [DOI] [PubMed] [Google Scholar]
- CLARKE J. T. SIMPLIFIED "DISC" (POLYACRYLAMIDE GEL) ELECTROPHORESIS. Ann N Y Acad Sci. 1964 Dec 28;121:428–436. doi: 10.1111/j.1749-6632.1964.tb14214.x. [DOI] [PubMed] [Google Scholar]
- FELSENFELD G. The determination of cuprous ion in copper proteins. Arch Biochem Biophys. 1960 Apr;87:247–251. doi: 10.1016/0003-9861(60)90168-5. [DOI] [PubMed] [Google Scholar]
- Foldes A., Jeffrey P. L., Preston B. N., Austin L. Dopamine -hydroxylase of bovine adrenal medullae. A rapid purification procedure. Biochem J. 1972 Mar;126(5):1209–1217. doi: 10.1042/bj1261209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S., Kaufman S. 3,4-dihydroxyphenylethylamine beta-hydroxylase. Physical properties, copper content, and role of copper in the catalytic acttivity. J Biol Chem. 1965 Dec;240(12):4763–4773. [PubMed] [Google Scholar]
- Friedman S., Kaufman S. An electron paramagnetic resonance study of 3,4-dihydroxyphenylethylamine beta-hydroxylase. J Biol Chem. 1966 May 25;241(10):2256–2259. [PubMed] [Google Scholar]
- Horwitz D., Alexander R. W., Lovenberg W., Keiser H. R. Human serum dopamine- -hydroxylase. Relationship to hypertension and sympathetic activity. Circ Res. 1973 May;32(5):594–599. doi: 10.1161/01.res.32.5.594. [DOI] [PubMed] [Google Scholar]
- Hörtnagl H., Winkler H., Lochs H. Membrane proteins of chromaffin granules, dopamine -hydroxylase, a major constituent. Biochem J. 1972 Aug;129(1):187–195. doi: 10.1042/bj1290187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVIN E. Y., LEVENBERG B., KAUFMAN S. The enzymatic conversion of 3,4-dihydroxyphenylethylamine to norepinephrine. J Biol Chem. 1960 Jul;235:2080–2086. [PubMed] [Google Scholar]
- Lee C. Y., Scocca J. R. A common structural unit in asparagine-oligosaccharides of several glycoproteins from different sources. J Biol Chem. 1972 Sep 25;247(18):5753–5758. [PubMed] [Google Scholar]
- Nagatsu T., Udenfriend S. Photometric assay of dopamine- -hydroxylase activity in human blood. Clin Chem. 1972 Sep;18(9):980–983. [PubMed] [Google Scholar]
- Rush R. A., Geffen L. B. Radioimmunoassay and clearance of circulating dopamine- -hydroxylase. Circ Res. 1972 Sep;31(3):444–452. doi: 10.1161/01.res.31.3.444. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Jackson R. L., Andrews E. P., Marchesi V. T. Human erythrocyte membrane glycoprotein: a re-evaluation of the molecular weight as determined by SDS polyacrylamide gel electrophoresis. Biochem Biophys Res Commun. 1971 Jul 16;44(2):390–395. doi: 10.1016/0006-291x(71)90612-7. [DOI] [PubMed] [Google Scholar]
- Spiro R. G. Glycoproteins. Annu Rev Biochem. 1970;39:599–638. doi: 10.1146/annurev.bi.39.070170.003123. [DOI] [PubMed] [Google Scholar]
- Viveros O. H., Arqueros L., Kirshner N. Mechanism of secretion from the adrenal medulla. V. Retention of storage vesicle membranes following release of adrenaline. Mol Pharmacol. 1969 Jul;5(4):342–349. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Winkler H., Hörtnagl H., Smith A. D. Membranes of the adrenal medulla. Behaviour of insoluble proteins of chromaffin granules on gel electrophoresis. Biochem J. 1970 Jun;118(2):303–310. doi: 10.1042/bj1180303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]