Abstract
Axillary staging of patients with early-stage breast cancer is essential in the treatment planning. Currently such staging is intraoperatively performed, but there is a tendency to seek a preoperative and less invasive technique to detect lymph node metastasis. Ultrasonography is widely utilized for this purpose, many times in association with fine-needle aspiration biopsy or core needle biopsy. However, the sonographic criteria for determining malignancy in axillary lymph nodes do not present significant predictive values, producing discrepant results in studies evaluating the sensitivity and specificity of this method. The present study was aimed at reviewing the literature approaching the utilization of ultrasonography in the axillary staging as well as the main morphological features of metastatic lymph nodes.
Keywords: Breast cancer, Axillary lymph nodes, Axillary ultrasonography, Morphological features
Abstract
O estadiamento axilar nas pacientes portadoras de câncer de mama inicial é fator essencial no planejamento terapêutico. Atualmente este é realizado durante o tratamento cirúrgico, mas há uma tendência em buscar técnicas pré-operatórias e de menor morbidade para avaliação dos linfonodos axilares. A ultrassonografia é um exame amplamente usado para esta finalidade e muitas vezes associado a punção aspirativa por agulha fina ou por agulha grossa. Entretanto, os critérios ultrassonográficos de suspeição para linfonodos axilares não apresentam valores preditivos significativos, gerando resultados discrepantes em estudos sobre sensibilidade e especificidade do método. O objetivo deste trabalho é realizar uma revisão na literatura médica sobre a ultrassonografia no estadiamento axilar e as principais alterações morfológicas do linfonodo metastático.
INTRODUCTION
The presence or absence of metastatic disease in the regional lymph node chain is crucial information for the definition of staging, treatment and prognosis of breast cancer. Axillary lymphadenectomy in association with histological analysis is still the gold standard in the evaluation of such lymph nodes; but this method is associated with relevant morbidity. With the advances in breast imaging diagnosis and consequential increased incidence of cases of early stage disease, the presence of axillary lymph nodes metastasis has declined, and a less aggressive option became necessary.
The technique of sentinel lymph node biopsy (SLNB) initially introduced by Krag in 1993(1), represented a relevant advance in the treatment of patients with clinically negative axilla, currently obtaining less than 1% of axillary recurrence in patients with negative SLNB(2). In the metaanalysis by Kell et al., SLNB demonstrated to be equivalent to axillary dissection in the detection of metastasis in regional lymph nodes and with up to 75% less morbidity in patients with early stage disease(3).
According to studies in the literature, axillary dissection followed by positive results for metastasis at SLNB presented 38% to 67% of metastasis absence in the remaining lymph nodes(4). Such data raised the question of what is the actual benefit of wide lymph node dissection in cases where the sentinel lymph node is compromised. Such benefit is particularly unknown in the cases of micrometastasis and isolated tumor cells in which clinical meaning is still undetermined. The studies on the subject are still controversial, and currently most services adopt the wide axillary approach, even in those cases where the sentinel lymph node is minimally compromised(5). The identification of new prognostic markers, the better understanding of tumors behavior and the technological developments in imaging methods have a great potential of bringing changes in axillary staging in the future, by selecting patients eligible to less aggressive interventions. Recently the ACOSOG Z0011 study defined the profile of a group of patients eligible to nonaxillary chain dissection after positive SLNB(6). Such a protocol is followed by the Division of Mastology at Escola Paulista de Medicina - Universidade Federal de São Paulo since November, 2012.
Both clinical examination and mammography demonstrably do not present appropriate accuracy in the identification of axillary lymph nodes metastasis(7) and several studies approach other imaging techniques, such as: ultrasonography alone or in association with Doppler flowmetry; fine needle aspiration biopsy (FNAB) or core biopsy; computed tomography; positron emission tomography; magnetic resonance imaging; elastography.
Currently, no imaging method has enough negative predictive value to avoid a surgical approach to the axilla in cases where no lymph node involvement is identified(8), however an increasing number of studies include such methods as part of the therapeutic planning. A study is currently being undertaken at the European Oncology Institute, comparing SLNB versus observation alone when axillary ultrasonography is negative in patients with small breast cancer candidates to breast conserving surgery(9).
Axillary ultrasonography plays a relevant role in the staging and follow-up of regional lymph nodes. It is an easily accessible noninvasive method which is helpful in obtaining material for cytology and histology.
The present study was aimed at discussing the utilization of ultrasonography in axillary staging, with emphasis on the main morphological changes of metastatic lymph nodes observed at such method.
METHOD
The adopted method was the systematic bibliographical research for the production of a review article to meet the proposed objective. An active search for articles on the proposed theme was undertaken at a scientific browser (Google scholar), websites (Faculdade de Medicina da Universidade de São Paulo and Conselho Federal de Medicina), virtual reference databanks (Medline, Lilacs, Cochrane, SciELO, High Wire, Ovid), utilizing the following keywords: "linfonodos axilares" (axillary lymph nodes), "ultrassonografia" (ultrasonography), "aspectos morfológicos" (morphological features). The search was carried out in the period of January through August of 2012. In the present article, 22 references which best covered the proposed theme were utilized.
REVIEW
Ultrasonography is widely available and, as combined with FNAP or core biopsy, it is the most significant method for preoperative evaluation of axillary lymph nodes(10). In the presence of a negative cytological or histological result for axillary metastasis, the negative predictive value of SLNB is increased; on the other hand, in the presence of a positive result, the surgical time is shortened by not performing biopsy.
Another benefit from such method would be the reduction in the occurrence of inappropriate lymphatic mapping by previously identifying, by means of ultrasonography, lymph nodes with tumor cell deposits increasing the lymphatic pressure, thus reducing the radioactive colloid uptake(11).
A previously mentioned, a positive sentinel lymph node, in many cases, is the only affected lymph node, and biopsy would not be an appropriate method to evaluate the axillary involvement extent in such a case(2,6). On the other hand, the utilization of ultrasonography in association with FNAP, in the study developed by Moore et al., has demonstrated high probability of extensive axillary involvement (more than four lymph nodes) in the presence of morphological changes such as absence of hilum and/or extracapsular extension and cytological confirmation of malignancy(12).
In spite of presenting a high accuracy in many studies, the diagnostic criteria for malignancy and the indication of the method remain controversial(4).
MORPHOLOGICAL CHARACTERISTICS
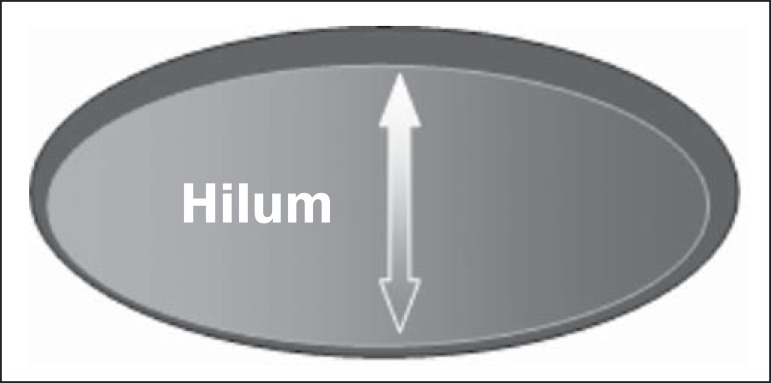
Usually, a benign lymph node is ovoid, with a hypoechogenic cortex, extremely thin or even invisible at ultrasonography with a hyperechogenic hilum due to connective tissue trabeculae, lymphatic tissue cords and medullary sinusoids. Changes such as cortical thickening, hilum decrease or absence, changes in shape or vascular pattern, are considered suspicious.
Currently many studies utilize cortical thickening and hilum absence as criteria for definition of the risk for metastasis(11,12-16). Absence of the hilum, making the lymph node completely hypoechogenic, is the most specific alteration for metastatic disease(13,16), but such finding is present only in cases of advanced disease. The great challenge in sonographic diagnosis lies in the evaluation of lymph nodes whose cortex and hilum are observed in varied forms, corresponding to early stages of metastatic disease, with such cases being responsible for the largest proportion of false-negtive cases(11-13,16).
Metastatic cells in the lymph reach the lymph nodes through afferent lymphatic vessels on the convex aspect of the organ. Then, the lymph is filtered through the cortex, paracortex and finally the hilum(17). Metastatic deposits accumulate in the lymph node peripheral area, causing enlargement of the cortex, usually focal (at early stages), or uniform. Minimum lymph node involvement, with deposits between 0.2 and 2 mm (micrometastasis), and < 0.2 mm (isolated tumor cells) is not related to significant morphological changes in the lymph node, thus limiting the usefulness of ultrasonography in such cases, so the diagnosis is made by means of histology or immunohistochemistry.
With the objective of estimating the suspicion based on image, many authors have developed classifications based on the cortical thickness. Cho et al.(11), for example, have categorized the images into five grades: grade 1, lymph nodes with cortex ≤ 1.5 mm; grade 2, > 1.5 and ≤ 2.5 mm; grade 3, > 2.5 mm and ≤ 3.5 mm; grade 4, > 3.5 mm and intact hilum; grade 5, > 3.5 mm and hilum absence. Such authors have concluded that such classification is effective in the investigation of metastasis, and that cortical thickness > 2.5 mm is an indication for cytological or histological study.
Bedi et al.(13) have created a similar classification, dividing the images into six types, as follows: type 1, without visible cortex; type 2, cortex ≤ 3 mm; type 3, cortex > 3 mm; type 4, entirely lobulated cortex; type 5, with focal lobulation; type 6, completely hypoechogenic, without hilum. Lymph nodes classified as types 5 and 6 were considered suspicious, with indication for biopsy; reactional changes were frequently observed in type 3; while type 4 was considered as probably benign, since such type comprised most falsenegative results (Figures 1 to 7).
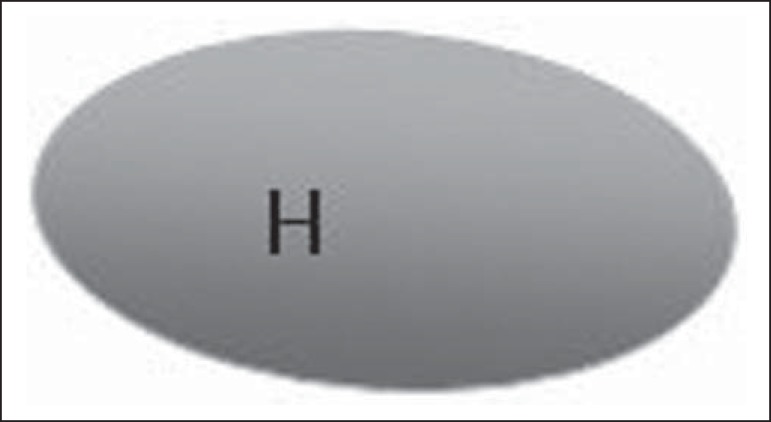
Figure 1.
Bedi type 1 lymph node. Without visible cortex. (H, hilum).
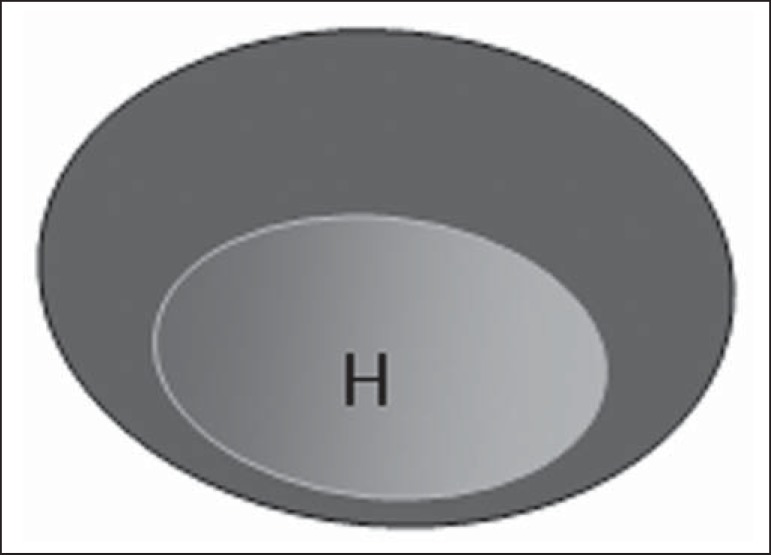
Figure 2.
Bedi type 2 lymph node. Uniform cortex ≤ 3 mm. (H, hilum).
Figure 3.
Bedi type 3 lymph node. Uniform cortex > 3 mm cortex. (H, hilum).
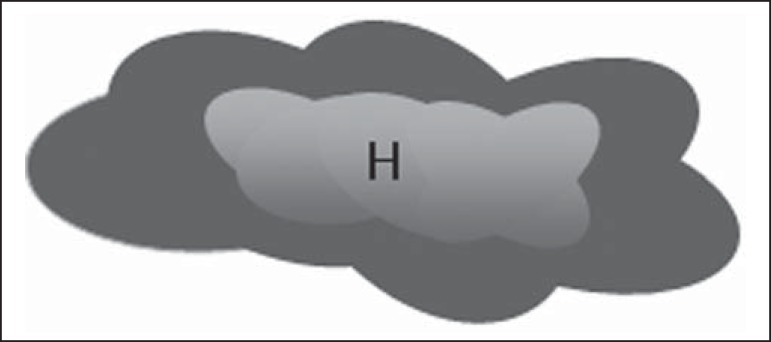
Figure 4.
Bedi type 4 lymph node. Entirely lobulated cortex. (H, Hilum).
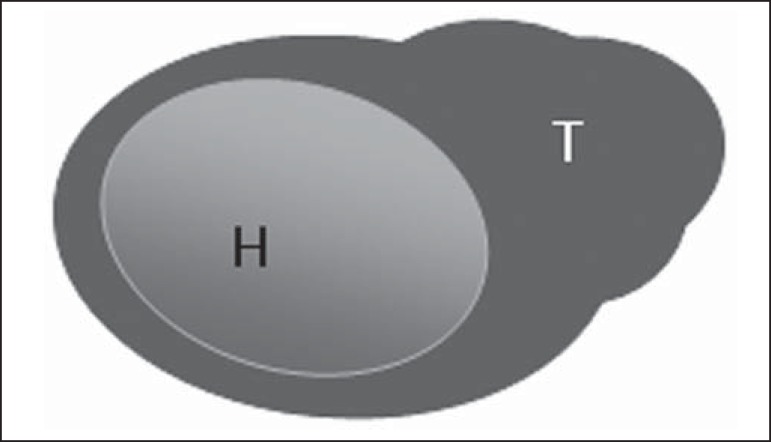
Figure 5.
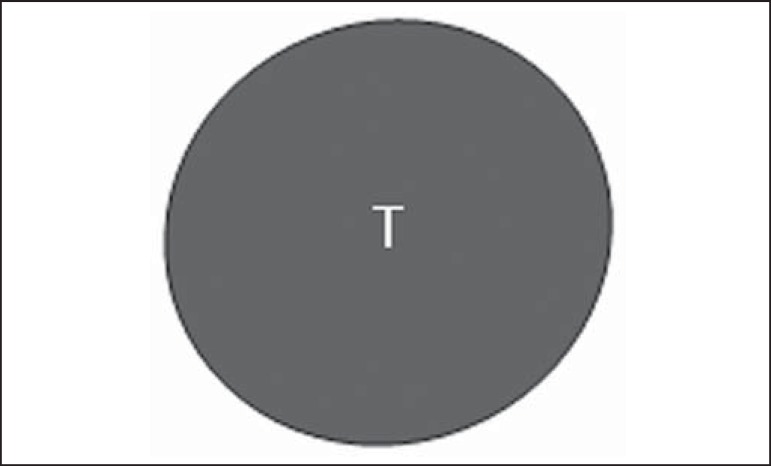
Bedi type 5 lymph node. Cortex with focal lobulation. (H, hilum; T, tumor cell deposit).
Figure 6.
Bedi type 6 lymph node. Completely hypoechogenic lymph node, absent hilum (T, tumor cell deposit).
Figure 7.
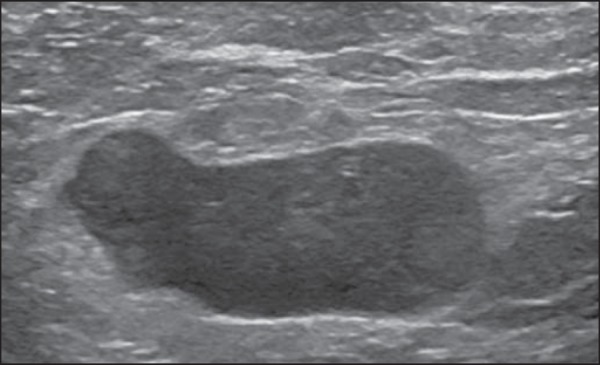
Bedi type 6 lymph node at ultrasonography. Lymph node without an apparent hilum.
Mainiero et al(14). have adopted 3 mm as the cut-off point for cortical thickness, besides the presence or not of lobulation and absence of the hilum. On the other hand, Deurloo et al.(18) have utilized 2.3 mm as a predictive factor of metastasis.
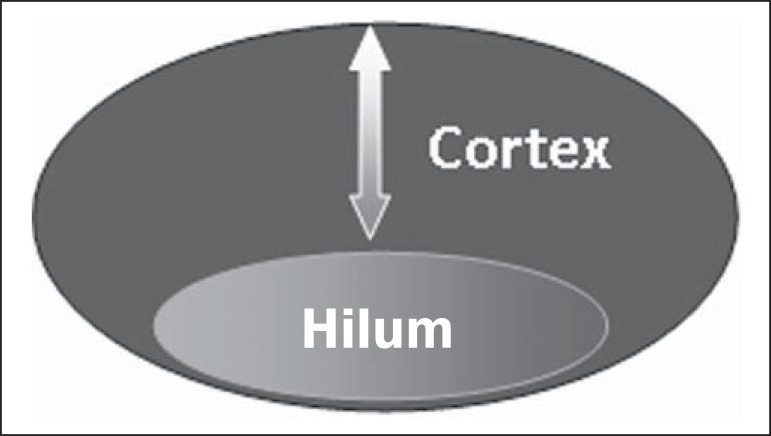
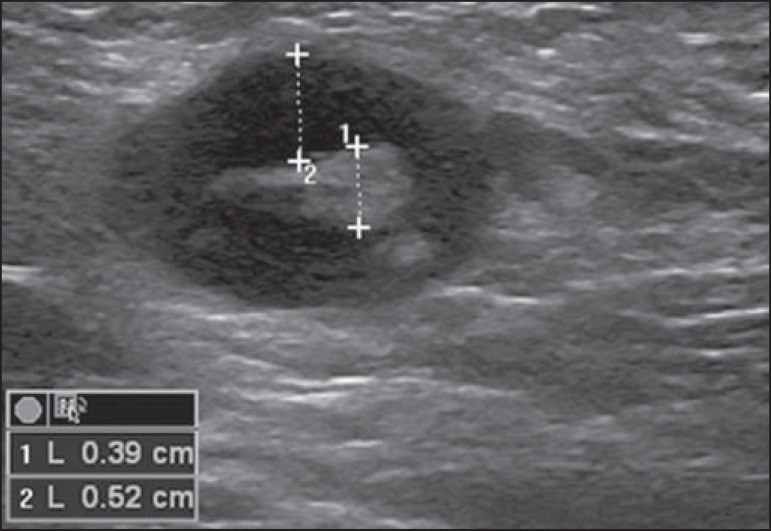
One observes that value for cortical thickness utilized as a cut-off point for metastatic disease varies a lot in the literature, as well as sensitivity and specificity of the method as a function of the selected value. Thus, some authors opted for utilizing a cortex/hilum ratio, instead of an absolute value for the cortex; in such an instance, the cortical thickening is present when the maximum cortex thickness is ≥ the thickness of the fatty hilum(16,19) (Figures 8 to 10).
Figure 8.
Representation of a cortex with a normal thickness. Measure of the fatty hilum greater than the cortex thickness.
Figure 9.
Cortical thickening representation. Cortical thickness ≤ fatty hilum thickness.
Figure 10.
Lymph node with cortical thickening at ultrasonography. Maximum cortical thickness (2) > hilum thickness (1).
As regards other morphological changes, the change in the lymph node shape generally occurs in advanced cases, in association with hilum absence. Size used to be considered a relevant criterion, but recent studies have not demonstrated a significant relationship between size and malignancy(13,20). Moore et al.(12) have observed that the combination of normal and changed lymph nodes in a single study could be an important criterion, and in their study, lymph nodes with cortical thickening not associated with normal lymph nodes corresponded to false-positive cases.
The vascularization studied at Doppler ultrasonography, basically follows two patterns, namely the central pattern, with a single hilum vascular signal or dispersed signals distributed at the center of the organ, and the peripheral pattern, where a linear signal is observed along the peripheral zone of the organ. Peripheral vascularization is more frequently found in metastatic lymph nodes, while the central pattern is more frequently found in the absence of malignancy. The indices of resistance, pulsatility and peak systolic velocity do not differentiate between malignant and benign axillary lymph nodes, but the relevance of such data has already been demonstrated in studies on cervical lymph nodes(15). The importance of the utilization of Doppler as a diagnostic criteria is observed as it is associated with other morphological characteristics and not as an isolated criteria(15,16).
INDICATION OF THE METHOD AND SELECTION OF THE PROCEDURE (FNAP × CORE BIOPSY)
The indiscriminate utilization of ultrasonography followed by FNAP or core biopsy has demonstrated to be hardly practical and expensive(12,18). The largest the primary breast tumor is, the highest is the chance of axillary metastasis, so the indication for the procedure is clear in these cases. However, the indication in those cases of primary tumors < 2 cm (T1) is not yet established. Mainiero et al.(14) have reduced sentinel lymph node biopsies by 6% by indicating ultrasonography and FNAP for patients with tumors < 1 cm. Such number increased to 17% in those cases of primary tumors between 1 and 2 cm, and to 42% in the cases of tumors measuring between 2 and 5 cm.
Once an abnormal lymph node is found, one faces the question on which procedure would be more appropriate: FNAP or core biopsy. Such a decision should be made taking into account various peculiar aspects of each method. FNAP is fast, with high sensitivity and specificity, besides being less invasive; on the other hand, it requires an experienced cytologist, a professional who is only available at a small number of institutions.
Core biopsy, albeit more expensive, provides material for histological and immunohistochemical analysis and has a higher sensitivity as compared with FNAP(21). However, a negative result by means of such a method does not exclude SLNB, as the core biopsy, as well as FNAB, presents a percentage of false-negatives as a function of small metastatic deposits(16). It is important to highlight that although core biopsy presents a higher potential for complications such as bleeding and nerve injury, it has demonstrated to be very safe with a technique described by Abe et al., where the cannula only crosses the tissue already limited by the needle(22).
CONCLUSION
Ultrasonography represents an important resource in the preoperative evaluation of axillary lymph nodes in patients with breast cancer. The utilization of this method allows for the identification of the axillary disease extent and assists in percutaneous biopsy, but it presents a limited benefit in those cases with minimum lymph node involvement, such as in those cases of micrometastasis and isolated tumor cells.
Changes such as cortical thickening and hilum absence are predictors of metastatic disease, and cytological or histological analysis is indicated in cases where such changes are present.
Axillary ultrasonography and elective puncture/biopsy should be a part of breast cancer diagnosis routine, particularly in those cases of primary tumors > 1 cm, which are the ones that benefit the most from this method.
The choice between FNAP and core biopsy should be made according to the equipment and professionals available at the institution.
Footnotes
Pinheiro DJPC, Elias S, Nazário ACP. Axillary lymph nodes in breast cancer patients: sonographic evaluation. Radiol Bras. 2014 Jul/Ago;47(4):240–244.
Study developed at Escola Paulista de Medicina - Universidade Federal de São Paulo (EPM-Unifesp), São Paulo, SP, Brazil.
REFERENCES
- 1.Krag DN, Weaver DL, Alex JC, et al. Surgical resection and radiolocalization of the sentinel lymph node in breast cancer using a gamma probe. Surg Oncol. 1993;2:335–339. doi: 10.1016/0960-7404(93)90064-6. [DOI] [PubMed] [Google Scholar]
- 2.Giuliano AE, Han SH. Local and regional control in breast cancer: role of sentinel node biopsy. Adv Surg. 2011;45:101–116. doi: 10.1016/j.yasu.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 3.Kell MR, Burke JP, Barry M, et al. Outcome of axillary staging in early breast cancer: a meta-analysis. Breast Cancer Res Treat. 2010;120:441–447. doi: 10.1007/s10549-009-0705-6. [DOI] [PubMed] [Google Scholar]
- 4.Reynolds C, Mick R, Donohue JH, et al. Sentinel lymph node biopsy with metastasis: can axillary dissection be avoided in some patients with breast cancer? J Clin Oncol. 1999;17:1720–1726. doi: 10.1200/JCO.1999.17.6.1720. [DOI] [PubMed] [Google Scholar]
- 5.Pazaiti A, Fentiman IS. Which patients need an axillary clearance after sentinel node biopsy? Int J Breast Cancer. 2011;2011: doi: 10.4061/2011/195892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252:426–432. doi: 10.1097/SLA.0b013e3181f08f32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pamilo M, Soiva M, Lavast EM. Real-time ultrasound, axillary mammography, and clinical examination in the detection of axillary lymph node metastases in breast cancer patients. J Ultrasound Med. 1989;8:115–120. doi: 10.7863/jum.1989.8.3.115. [DOI] [PubMed] [Google Scholar]
- 8.Rahbar H, Partridge SC, Javid SH, et al. Imaging axillary lymph nodes in patients with newly diagnosed breast cancer. Curr Probl Diagn Radiol. 2012;41:149–158. doi: 10.1067/j.cpradiol.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Gentilini O, Veronesi U. Abandoning sentinel lymph node in early breast cancer? A new trial in progress at the European Institute of Oncology of Milan (SOUND: Sentinel node vs Observation after axillary UltraSouND) Breast. 2012;21:678–681. doi: 10.1016/j.breast.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez S, Añorbe E, Alcorta P, et al. Role of sonography in the diagnosis of axillary lymph node metastases in breast cancer: a systematic review. AJR Am J Roentgenol. 2006;186:1342–1348. doi: 10.2214/AJR.05.0936. [DOI] [PubMed] [Google Scholar]
- 11.Cho N, Moon WK, Han W, et al. Preoperative sonographic classification of axillary lymph nodes in patients with breast cancer: nodeto-node correlation with surgical histology and sentinel node biopsy results. AJR Am J Roentgenol. 2009;193:1731–1737. doi: 10.2214/AJR.09.3122. [DOI] [PubMed] [Google Scholar]
- 12.Moore A, Hester M, Nam MW, et al. Distinct lymph nodal sonographic characteristics in breast cancer patients at high risk for axillary metastases correlate with the final axillary stage. Br J Radiol. 2008;81:630–636. doi: 10.1259/bjr/21933846. [DOI] [PubMed] [Google Scholar]
- 13.Bedi DG, Krishnamurthy R, Krishnamurthy S, et al. Cortical morphologic features of axillary lymph nodes as a predictor of metastasis in breast cancer: in vitro sonographic study. AJR Am J Roentgenol. 2008;191:646–652. doi: 10.2214/AJR.07.2460. [DOI] [PubMed] [Google Scholar]
- 14.Mainiero MB, Cinelli CM, Koelliker SL, et al. Axillary ultrasound and fine-needle aspiration in the preoperative evaluation of the breast cancer patient: an algorithm based on tumor size and lymph node appearance. AJR Am J Roentgenol. 2010;195:1261–1267. doi: 10.2214/AJR.10.4414. [DOI] [PubMed] [Google Scholar]
- 15.Yang WT, Chang J, Metreweli C. Patients with breast cancer: differences in color Doppler flow and gray-scale US features of benign and malignant axillary lymph nodes. Radiology. 2000;215:568–573. doi: 10.1148/radiology.215.2.r00ap20568. [DOI] [PubMed] [Google Scholar]
- 16.Abe H, Schmidt RA, Kulkarni K, et al. Axillary lymph nodes suspicious for breast cancer metastasis: sampling with US-guided 14gauge core-needle biopsy - clinical experience in 100 patients. Radiology. 2009;250:41–49. doi: 10.1148/radiol.2493071483. [DOI] [PubMed] [Google Scholar]
- 17.van der Valk P, Meijer CJL. Reactive lymph nodes. In: Sternberg SS, editor. Histology for pathologists. 2nd ed. PA: Lippincott Raven; 1997. pp. 651–673. [Google Scholar]
- 18.Deurloo EE, Tanis PJ, Gilhuijs KG, et al. Reduction in the number of sentinel lymph node procedures by preoperative ultrasonography of the axilla in breast cancer. Eur J Cancer. 2003;39:1068–1073. doi: 10.1016/s0959-8049(02)00748-7. [DOI] [PubMed] [Google Scholar]
- 19.Luparia A, Campanino P, Cotti R, et al. Role of axillary ultrasound in the preoperative diagnosis of lymph node metastases in patients affected by breast carcinoma. Radiol Med. 2010;115:225–237. doi: 10.1007/s11547-009-0465-8. [DOI] [PubMed] [Google Scholar]
- 20.Koelliker SL, Chung MA, Mainiero MB, et al. Axillary lymph nodes: US-guided fine-needle aspiration for initial staging of breast cancer - correlation with primary tumor size. Radiology. 2008;246:81–89. doi: 10.1148/radiol.2463061463. [DOI] [PubMed] [Google Scholar]
- 21.Rao R, Lilley L, Andrews V, et al. Axillary staging by percutaneous biopsy: sensitivity of fine-needle aspiration versus core needle biopsy. Ann Surg Oncol. 2009;16:1170–1175. doi: 10.1245/s10434-009-0421-9. [DOI] [PubMed] [Google Scholar]
- 22.Abe H, Schmidt RA, Sennett CA, et al. US-guided core needle biopsy of axillary lymph nodes in patients with breast cancer: why and how to do it. Radiographics. 2007;27(Suppl 1):S91–S99. doi: 10.1148/rg.27si075502. [DOI] [PubMed] [Google Scholar]