Abstract
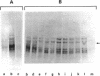
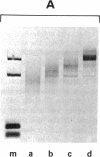
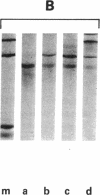
The mRNA coding for a mouse immunoglobulin L-chain was prepared from MOPC-321 myeloma polysomes specifically precipitated with antibodies directed against L-chains, followed by chemical purification on oligo(dT)-cellulose. Biological purity (capacity to program the synthesis only of L-chain) was calculated to be ≥95%. This value was based on the estimation of contamination by non-L-chain mRNA activities that were present in large abundance in RNA preparations extracted from the total polysome population. A similar degree of purity was calculated from the extent of precipitation of myeloma and nonmyeloma polysomes with anti-L-chain and non-L-chain antibodies. Chemical purity (95%) was determined from the amount of rRNA in the mRNA preparation by scanning of appropriate gels. In a cell-free system, the purified mRNA directed the synthesis of two precursors heavier than L-chain by about 1300 and 4700 daltons. Cell-free products labeled with 10 [14C]aminoacids yielded 27 out of 28 expected L-chain tryptic peptides and four additional peptides. Most probably the latter were derived from extra pieces in the precursors, and the apparent loss of one peptide was due to modifications at the N-terminus. The main fraction of L-chain mRNA was composed of two species of about 420,000 and 450,000 daltons. These molecules are much larger than that required to code for a mature L-chain (calculated about 250,000). The additional nucleotide mass can be accounted for in part for the coding of the extra piece (about 50,000) and in part for the polyadenylate moiety.
Keywords: mouse myeloma, mRNA molecular weight, L-chain precursor proteins, partial amino-acid sequence of precursor, sequence of cell-free product
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Boime I., Leder P. Protein synthesis directed by encephalomyocarditis virus RNA: properties of a transfer RNA-dependent system. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2303–2307. doi: 10.1073/pnas.68.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delovitch T. L., Baglioni C. Estimation of light-chain gene reiteration of mouse immunoglobulin by DNA-RNA hybridization. Proc Natl Acad Sci U S A. 1973 Jan;70(1):173–178. doi: 10.1073/pnas.70.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easley C. W., Zegers B. J., de Vijlder M. Application of specialized techniques for specific staining of peptide maps on various media. Biochim Biophys Acta. 1969 Feb 4;175(1):211–213. doi: 10.1016/0005-2795(69)90161-5. [DOI] [PubMed] [Google Scholar]
- Edmonds M., Caramela M. G. The isolation and characterization of adenosine monophosphate-rich polynucleotides synthesized by Ehrlich ascites cells. J Biol Chem. 1969 Mar 10;244(5):1314–1324. [PubMed] [Google Scholar]
- Lisowska-Bernstein B., Lamm M. E., Vassalli P. Synthesis of immunoglobulin heavy and light chains by the free ribosomes of a mouse plasma cell tumor. Proc Natl Acad Sci U S A. 1970 Jun;66(2):425–432. doi: 10.1073/pnas.66.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach B., Faust C., Vassalli P. Purification of 14S messenger RNA of immunoglobulin light chain that codes for a possible light-chain precursor. Proc Natl Acad Sci U S A. 1973 Feb;70(2):451–455. doi: 10.1073/pnas.70.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M. B. Tissue-specific factor required for the translation of a mammalian viral RNA. Nature. 1970 Nov 14;228(5272):661–663. doi: 10.1038/228661a0. [DOI] [PubMed] [Google Scholar]
- McKean D., Potter M., Hood L. Mouse immunoglobulin chains. Partial amino acid sequence of a kappa chain. Biochemistry. 1973 Feb;12(4):749–759. doi: 10.1021/bi00728a027. [DOI] [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Palacios R., Schimke R. T. Identification and isolation of ovalbumin-synthesizing polysomes. 3. Precipitation of ovalbumin polysomes from a heterologous cell-free protein-synthesizing system. J Biol Chem. 1973 Feb 25;248(4):1424–1430. [PubMed] [Google Scholar]
- Palacios R., Sullivan D., Summers N. M., Kiely M. L., Schimke R. T. Purification of ovalbumin messenger ribonucleic acid by specific immunoadsorption of ovalbumin-synthesizing polysomes and millipore partition of ribonucleic acid. J Biol Chem. 1973 Jan 25;248(2):540–548. [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- RAFTERY M. A., COLE R. D. Tryptic cleavage at cysteinyl peptide bonds. Biochem Biophys Res Commun. 1963 Mar 25;10:467–472. doi: 10.1016/0006-291x(63)90381-4. [DOI] [PubMed] [Google Scholar]
- Stavnezer J., Huang R. C. Synthesis of a mouse immunoglobulin light chain in a rabbit reticulocyte cell-free system. Nat New Biol. 1971 Apr 7;230(14):172–176. doi: 10.1038/newbio230172a0. [DOI] [PubMed] [Google Scholar]
- Staynov D. Z., Pinder J. C., Gratzer W. B. Molecular weight determination of nucleic acids by gel electrophoresis in non-aqueous solution. Nat New Biol. 1972 Jan 26;235(56):108–110. doi: 10.1038/newbio235108a0. [DOI] [PubMed] [Google Scholar]
- Stevens R. H., Williamson A. R. Specific IgG mRNA molecules from myeloma cells in heterogeneous nuclear and cytoplasmic RNA containing poly-A. Nature. 1972 Sep 15;239(5368):143–146. doi: 10.1038/239143a0. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Kelly R. B., Sinsheimer R. L. Denaturation of RNA with dimethyl sulfoxide. Biopolymers. 1968 Jun;6(6):793–807. doi: 10.1002/bip.1968.360060604. [DOI] [PubMed] [Google Scholar]
- Swan D., Aviv H., Leder P. Purification and properties of biologically active messenger RNA for a myeloma light chain. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1967–1971. doi: 10.1073/pnas.69.7.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uenoyama K., Ono T. Nascent catalase and its messenger RNA on rat liver polyribosomes. J Mol Biol. 1972 Mar 14;65(1):75–89. doi: 10.1016/0022-2836(72)90493-7. [DOI] [PubMed] [Google Scholar]