Abstract
Parry-Romberg syndrome is a rare disease characterized by progressive hemifacial atrophy associated with other systemic changes, including neurological symptoms. Currently, there are few studies exploring the utilization of advanced magnetic resonance sequences in the investigation of this disease. The authors report the case of a 45-year-old patient and describe the findings at structural magnetic resonance imaging and at advanced sequences, correlating them with pathophysiological data.
Keywords: Parry-Romberg syndrome, Progressive hemifacial atrophy, Hemifacial atrophy, Facial hemiatrophy of Romberg
Abstract
Síndrome de Parry-Romberg é uma doença rara caracterizada por atrofia hemifacial progressiva associada a outras alterações sistêmicas, dentre elas, neurológicas. Atualmente, são poucos os trabalhos que exploraram sequências avançadas em ressonância magnética nesta enfermidade. Neste artigo, relatamos o caso de um paciente com 45 anos e descrevemos os achados de ressonância magnética estrutural e em sequências avançadas, correlacionando com dados fisiopatológicos.
INTRODUCTION
Parry-Romberg syndrome, also known as progressive hemifacial atrophy, is a sporadic neurocutaneous disease characterized by slow and progressive hemifacial atrophy of the skin, muscles and bone structures, occasionally involving the central nervous system (CNS)(1,2). Its origin is still unknown, although some authors believe that it is a manifestation of focal scleroderma(3). As regards the physiopathology of this disease, some authors have raised the hypothesis of an inflammation meningoencephalic associated with vasculitis, and others, the hypothesis of a chronic vasomotor disorder related to hyperactivity of the sympathetic nervous system(4).
The present case report is aimed to describe the findings of structural magnetic resonance imaging (MRI) and advanced sequences to establish a physiopathological correlation.
CASE REPORT
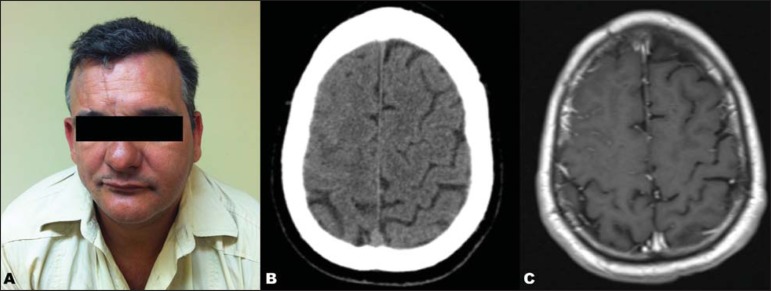
A 45-year-old male patient presenting with a 20 years history of right hemifacial alteration characterized by progressive atrophy and deformity (Figure 1A). The clinical appearance was compatible with Parry-Romberg syndrome.
Figure 1.
A: Right hemifacial alteration characterized by remarkable atrophy and deformity. B: Non-contrast-enhanced, axial, cranial CT demonstrating fading of the sulci in the right frontal lobe. C: Axial MRI, contrast-enhanced T1-weighted sequence more clearly demonstrating the fading of the sulci as well as the associated meningeal enhancement.
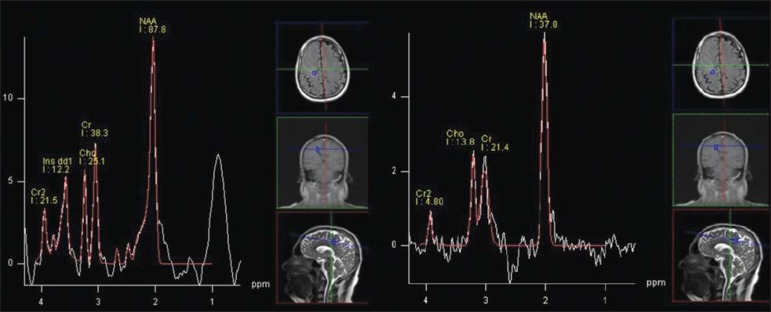
Two years ago, the patient started to present headache and paresthesia of his left upper extremity. Cranial computed tomography (CT) demonstrated fading of the sulci in the right frontal lobe (Figure 1B). Magnetic resonance imaging (MRI) identified cortical thickening and foci of hypersignal intensity on T2-weighted and FLAIR in the frontoparietal white matter, leptomeningeal enhancement (Figure 1C) and decreased perfusion (Figure 2), all ipsilateral to the affected hemiface. Proton spectroscopy demonstrated an exuberant peak at 0.8 and 0.9 ppm in the affected region characterized by a singleton with short echo-time of 30 ms and suppressed with spectroscopy performed with a long echo-time (270 ms), attributable to the presence of lipids (Figure 3).
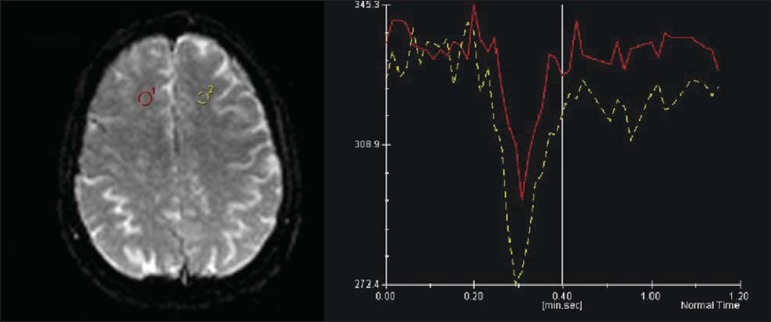
Figure 2.
Comparative analysis of the perfusion curve in the region of the lesion and in the corresponding contralateral region demonstrates a curve with smaller amplitude as compared with the healthy region, compatible with hypoperfusion.
Figure 3.
Proton spectroscopy with multivoxel technique, at left with echo time = 30 ms, and at right with echo time = 270 ms. An increase is observed in the levels of a singleton around 0.8–0.9 ppm, with echo time = 30 ms, that is suppressed with a long echo time = 270 ms (attributable to the presence of lipids).
Diffusion-weighted images did not demonstrate any significant alteration. However, the apparent diffusion coefficient (ADC) calculation demonstrated a higher value in the affected region (886.9), as compared with the corresponding contralateral region (726.3).
Furthermore, the fractional anisotropy value in the affected region (300.0) was significantly lower than in the contralateral region (555.9).
DISCUSSION
Parry-Romberg syndrome is an uncommon disorder most frequently found in women, at the first decade of life(5).
Neurological symptoms include headache, trigeminal neuralgia and focal epilepsy(4).
CT and MRI represent excellent methods for the diagnosis of CNS alterations, with the following typical findings: white matter hypersignal on T2-weighted and FLAIR sequences, leptomeningeal enhancement, intracranial calcifications and brain atrophy(4).
The differential diagnosis includes diseases coursing with cerebral hemiatrophy, such as Rasmussen encephalitis and Sturge-Weber syndrome, although the typical hemifacial alterations observed in Parry-Romberg syndrome are not found in these diseases(1,5).
Currently, the use of advanced MRI sequences in the investigation of Parry-Romberg syndrome has been poorly explored, although it could help in the understanding of the physiopathology of this disease.
In the present case, the typical alterations of Parry-Romberg syndrome were observed, including right hemifacial atrophy and the above described radiological findings, except for intracranial calcifications. At advanced MRI sequences, the presence of lipids in the affected area was observed, in addition to decreased perfusion.
Okumura et al.(6) have demonstrated a normal imaging pattern at proton spectroscopy of the white matter in the affected region, as compared with a normal curve in the contralateral region. In order to explain the physiopathology of this syndrome, some authors have raised the hypothesis of a meningoencephalic inflammation associated with vasculitis; and others have postulated a chronic vasomotor disorder related to sympathetic nervous system hyperactivity(4). The authors of the present report believe that the presence of lipids might be secondary to myelin destruction due to metabolic alterations related to a chronic vasomotor disorder.
Some authors report changes in the perfusion pattern of the affected regions observed at single photon emission computed tomography (SPECT). Okumura et al.(6) have reported a decreased flow pattern in the white matter of the affected area, with a relatively increased flow in the cortex of the affected hemisphere. DeFelipe et al.(7) have reported a case of Parry-Romberg syndrome associated with temporal lobe epilepsy and an extensive area of parieto-occipital hypoperfusion in the affected hemisphere observed at SPECT. The present case demonstrated decreased perfusion in the affected region at perfusion MRI. As a limitation, the patient did not undergo SPECT. In the literature review, the authors have not found any report about perfusion MRI findings in Parry-Romberg syndrome.
As regards of diffusion tensor imaging, some author have reported a decrease in fractional anisotropy values(6) and a reduction in the amount of white matter fibers demonstrated by tractography(8) that can be explained by a decreased myelination. In the present case, the authors observed a decreased fractional anisotropy value in relation to the contralateral region, which is in agreement with findings reported in the literature.
The ADC values were higher in the affected regions as compared with the contralateral hemisphere. The authors assume that this also has occurred as a result from decreased myelination.
CONCLUSION
Advanced MRI sequences may be useful to clarify the physiopathology of Parry-Romberg syndrome and to detect the classical findings of this disease, that are white matter hypersignal on T2-weighted and FLAIR sequences, leptomeningeal enhancement, intracranial calcifications and brain atrophy(4).
Footnotes
Alfenas R, Niemeyer B, Bahia PRV, Niemeyer R, Balbi L. Parry-Romberg syndrome: findings in advanced magnetic resonance imaging sequences – case report. Radiol Bras. 2014 Mai/Jun;47(3):186–188.
Study developed at Hospital Universitário Clementino Fraga Filho - Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil.
REFERENCES
- 1.Longo D, Paonessa A, Specchio N, et al. Parry-Romberg syndrome and Rasmussen encephalitis: possible association. Clinical and neuroimaging features. J Neuroimaging. 2011;21:188–193. doi: 10.1111/j.1552-6569.2009.00398.x. [DOI] [PubMed] [Google Scholar]
- 2.Sharma M, Bharatha A, Antonyshyn OM, et al. Case 178: Parry-Romberg syndrome. Radiology. 2012;262:721–725. doi: 10.1148/radiol.11092104. [DOI] [PubMed] [Google Scholar]
- 3.Barra FR, Gonçalves FG, Matos VL, et al. Sinais em neurorradiologia - Parte 2. Radiol Bras. 2011;44:129–133. [Google Scholar]
- 4.Cory RC, Clayman DA, Faillace WJ, et al. Clinical and radiologic findings in progressive facial hemiatrophy (Parry-Romberg syndrome) AJNR Am J Neuroradiol. 1997;18:751–757. [PMC free article] [PubMed] [Google Scholar]
- 5.El-Kehdy J, Abbas O, Rubeiz N. A review of Parry-Romberg syndrome. J Am Acad Dermatol. 2012;67:769–784. doi: 10.1016/j.jaad.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 6.Okumura A, Ikuta T, Tsuji T, et al. Parry-Romberg syndrome with a clinically silent white matter lesion. AJNR Am J Neuroradiol. 2006;27:1729–1731. [PMC free article] [PubMed] [Google Scholar]
- 7.DeFelipe J, Segura T, Arellano JI, et al. Neuropathological findings in a patient with epilepsy and the Parry-Romberg syndrome. Epilepsia. 2001;42:1198–1203. doi: 10.1046/j.1528-1157.2001.45800.x. [DOI] [PubMed] [Google Scholar]
- 8.Moon WJ, Kim HJ, Roh HG, et al. Diffusion tensor imaging and fiber tractography in Parry-Romberg syndrome. AJNR Am J Neuroradiol. 2008;29:714–715. doi: 10.3174/ajnr.A0967. [DOI] [PMC free article] [PubMed] [Google Scholar]