Abstract
Objective
To evaluate sonographic measurements of visceral and subcutaneous fat in children, and to investigate the usefulness of preperitoneal fat (PF) and the abdominal wall fat index (AFI) as parameters to determine visceral fat and presence of nonalcoholic fatty liver disease (NAFLD) in obese children.
Materials and Methods
A case-control study of a sample including 44 children (22 cases and 22 controls) matched by sex and age. The Student t test and the Fisher exact test were utilized in the descriptive and bivariate analysis.
Results
The sonographic parameters evaluated - subcutaneous cell tissue, PF and intraperitoneal fat, and NAFLD - presented high statistical association with body mass index. NAFLD was observed in eight obese patients (36.36%), and PF and AFI were the variables with highest statistical significance, with p < 0.0001.
Conclusion
Ultrasonography is useful tool in the differentiation and quantification of visceral and subcutaneous fat in children. The measures of PF and AFI are useful in the assessment of visceral fat and NAFLD in obese children.
Keywords: Ultrasonography, Child, Obesity, Fatty liver, Intra-abdominal fat
Abstract
Objetivo
Avaliar as medidas ultrassonográficas da gordura visceral e subcutânea em crianças e testar se a gordura pré-peritoneal (GPP) e o índice de gordura da parede abdominal (IGPA) são parâmetros úteis para determinar a gordura visceral e a presença de doença hepática gordurosa não alcoólica (DHGNA) em crianças obesas.
Materiais e Métodos
Estudo tipo caso-controle, com uma amostra de 44 crianças, sendo 22 casos e 22 controles, pareados conforme sexo e idade. Os resultados foram analisados de forma descritiva e bivariada, com teste t de Student e teste exato de Fischer.
Resultados
Os parâmetros ultrassonográficos avaliados - tecido celular subcutâneo, GPP e gorduras intraperitoneais, e DHGNA - obtiveram elevada associação estatística com o índice de massa corpórea. A DHGNA foi observada em oito pacientes obesos (36,36%), sendo que a GPP e o IGPA foram as variáveis com maior significância estatística, com valor de p < 0,0001.
Conclusão
A ultrassonografia permite diferenciar e quantificar a gordura visceral e subcutânea nas crianças. As medidas da GPP e do IGPA são úteis para a avaliação da gordura visceral e DHGNA em crianças obesas.
INTRODUCTION
Child obesity is a global epidemics, constituting a risk factor for cardiovascular diseases, arterial hypertension, diabetes, hyperlipidemia and nonalcoholic fatty liver disease (NAFLD)(1). Currently, the type of distribution of fat in the body, particularly the accumulation of visceral fat, is considered the most important factor for obesity, cardiovascular diseases, and metabolic syndromes(2-4).
The visceral fat may be evaluated either by measurement of the waist(5) or by means of imaging methods such as computed tomography (CT), magnetic resonance imaging (MRI) and ultrasonography (US)(6). CT is the most utilized method, but it involves exposure to ionizing radiation besides its high cost. MRI, besides its high cost and low availability in Brazil, requires a long time for images acquisition, which represents a limitation in the assessment of children. US has shown to be a reliable and convenient method to quantify the visceral fat(6,7), in addition to its low cost, wide availability and easiness, making it ideal for assessing the pediatric population(6,7).
Suzuki et al.(8) have estimated the distribution of visceral fat by means of US, measuring the thickness of the preperitoneal fat in correlation with the thickness of the subcutaneous fat, which was named abdominal wall fat index (AFI). Such authors have demonstrated a strong correlation of the AFI with the measurements of visceral fat distribution obtained by means of CT in adult individuals.
This is an observational, case-control study aimed at: 1) comparing the visceral, subcutaneous fat thicknesses and the AFI obtained by means of US, in obese and normoweight children; 2) correlating such measurements with the presence of NAFLD in obese children; 3) evaluating the role of preperitoneal fat (PF) and AFI as parameter to evaluate the presence of visceral fat and NAFLD in obese children.
MATERIALS AND METHODS
Study population
The present study population included 44 children with ages between 7 and 14, assisted at Hospital Infantil Joana de Gusmão (HIJG), Florianópolis, SC, Brazil. The case group included 22 obese children with body mass index (BMI) > the 95th percentile on the National Center for Health Statistics BMI chart, and the control group included 22 normoweight children paired by age and sex.
The study was approved by the HIJG Committee for Ethics in Research under the number 029/2009.
Sonographic study
The sonographic evaluations were performed with a Philips EnVisor apparatus with a convex, broadband 3.5- 7.0 MHz transducer and a linear, broadband 5.0-12.0 MHz transducer. The time gain compensation curve was adjusted in the neutral position and the general gain was calibrated in a way that fluid structures such as the gallbladder contents, inferior vena cava and aorta were presented anechoic. All the sonographic measurements were performed with no pressure on the transducer.
The following parameters were evaluated with basis on the arithmetic means of three measurements:
a) Subcutaneous fat (S) - distance from the skin to the linea alba, measured on the hemi-sternal line, 1 cm above the umbilical scar, utilizing the linear transducer in a longitudinal section (Figure 1);
Figure 1.
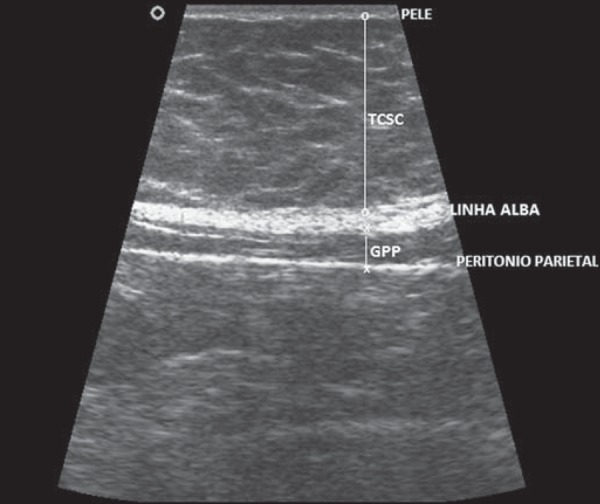
Ultrasonography demonstrating measurement of the thickness of subcutaneous fat (S) and of preperitoneal fat (P).
b) Preperitoneal fat (P) - distance from the linea alba to the anterior parietal peritoneum, measured on the hemisternal line, 1 cm above the umbilical scar, utilizing the linear transducer in a longitudinal section (Figure 1);
c) Intraperitoneal fat (IPF) - was measured with the convex transducer in three ways, as follows: i) IPF 1 - distance between the anterior peritoneum and the anterior wall of the aorta measured on the hemi-sternal line, 1 cm above the umbilical scar, in an axial section (Figure 2); ii) IPF 2- distance between the anterior parietal peritoneum and the posterior wall of the aorta measured on the hemi-sternal line, 1 cm above the umbilical scar, in an axial section (Figure 2); iii) IPF 3 - thickness of the small omentum: distance between the posterior surface of the left liver lobe and the anterior wall of the aorta measured at the level of the emergence of the celiac trunk at the midline of the epigastric region on a longitudinal section (Figure 3);
Figure 2.

Ultrasonography demonstrating measurement of IPF 1 – distance between the anterior parietal peritoneum and the anterior wall of the aorta, and IPF 2 – distance between the anterior parietal peritoneum and the posterior wall of the aorta. AO, aorta.
Figure 3.
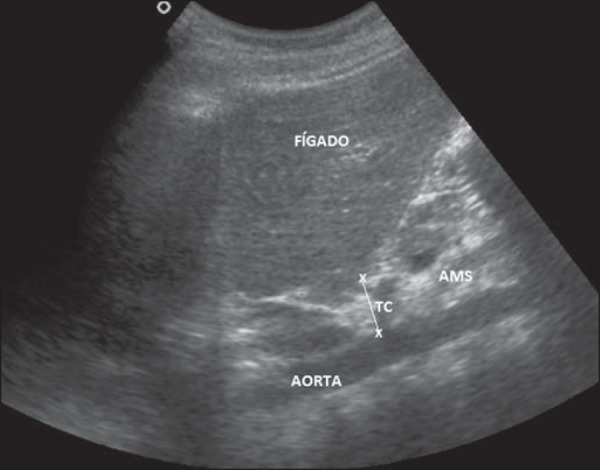
Ultrasonography demonstrating measurement of the distance between the posterior surface of the left liver lobe and the anterior wall of the aorta (IPF 3). TC, celiac trunk; AMS, superior mesenteric artery.
d) Abdominal wall fat index (AFI) - was calculated by division of the largest measurement of the P (maximum P) by the smallest measurement of the S (minimum S) (Figure 4).
Figure 4.
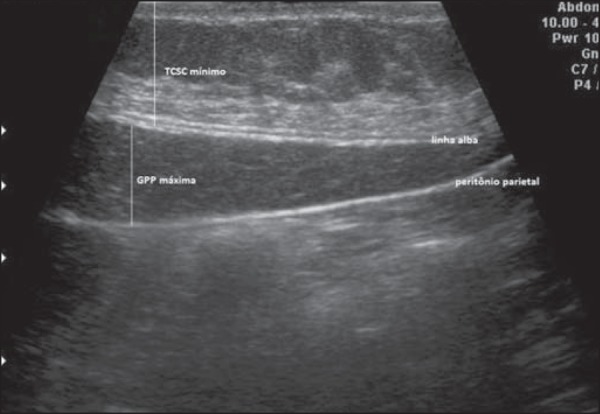
Ultrasonography demonstrating measurement of preperitoneal fat (maximum P) and of subcutaneous fat (minimum S).
At US, the following finding corresponded to presence of NAFLD: atypical increased echogenicity of the liver parenchyma as compared with the right renal parenchyma, with posterior ultrasound beam attenuation.
In order to rule out other causes of liver disease, the patients who presented with sonographic signs of liver steatosis had material collected for the following laboratory tests: serology for hepatitis A, B and C; protein electrophoresis; antinuclear factor; anti-smooth muscle antibody; serum ceruloplasmin and cooper; serum alpha 1 antitrypsin.
The Student's t test and the exact Fisher test were utilized in the statistical analysis, with a significance level corresponding to p < 0.05.
RESULTS
All the sonographic parameters presented great significance, highlighting the measurements of subcutaneous and visceral fat as the obese and non-obese groups were compared, as shown on Table 1.
Table 1.
Sonographic parameters for patients, cases group and control group, in the evaluation of visceral and subcutaneous fat in obese children.
| Cases (n = 22) | Controls (n = 22) | |||
|---|---|---|---|---|
| Parameters | Mean (SD) | Mean (SD) | p value | |
| Steatosis | 0.63 | (± 0.95) | 0 | 0.003 |
| S | 2.98 | (± 0.81) | 1.23 (± 0.89) | < 0.0001 |
| Minimum S | 2.69 | (± 0.77) | 1.11 (± 0.78) | < 0.0001 |
| Maximum S | 3.18 | (± 0.85) | 1.33 (± 0.9) | < 0.0001 |
| P | 0.52 | (± 0.29) | 0.22 (± 0.13) | < 0.0001 |
| Minimum P | 0.45 | (± 0.28) | 0.19 (± 0.12) | 0.0002 |
| Maximum P | 0.58 | (± 0.32) | 0.25 (± 0.14) | < 0.0001 |
| IPF 1 | 4.20 | (± 1.39) | 2.77 (± 0.63) | < 0.0001 |
| IPF 2 | 5.12 | (± 1.27) | 3.86 (± 0.65) | 0.0002 |
| IPF 3 | 1.70 | (± 0.48) | 1.01 (± 0.23) | < 0.0001 |
SD, standard deviation; S, subcutaneous fat; P, preperitoneal fat; IPF, intraperitoneal fat.
NAFLD was found in eight (36.36%) children among obese patients, and in none of the control group. No alteration was observed at screening tests for other livers diseases in NAFLD patients.
Considering only the cases group (obese patients), a new outcome (either presence or absence of steatosis) was correlated with the sonographic measurements of visceral fat, SCT, and with the AFI, as shown on Table 2.
Table 2.
Sonographic parameters for obese patients (cases), comparing patients with and with NAFLD, in the evaluation of visceral and subcutaneous fat in obese children.
| With NAFLD (n = 8) | Without NAFLD (n = 14) | ||
|---|---|---|---|
| Parameters | Mean (SD) | Mean (SD) | p value |
| S | 3.37 (± 0.79) | 2.76 (± 0.76) | 0.09 |
| Minimum S | 3.16 (± 0.81) | 2.42 (± 0.63) | 0.02 |
| Maximum S | 3.64 (± 0.81) | 2.91 (± 0.78) | 0.05 |
| P | 0.86 (± 0.22) | 0.32 (± 0.06) | < 0.0001 |
| Minimum P | 0.78 (± 0.21) | 0.27 (± 0.06) | < 0.0001 |
| Maximum P | 0.93 (± 0.23) | 0.37 (± 0.07) | < 0.0001 |
| IPF 1 | 4.97 (± 1.21) | 3.72 (± 1.30) | 0.04 |
| IPF 2 | 5.75 (± 0.92) | 4.78 (± 1.33) | 0.10 |
| IPF 3 | 1.87 (± 0.48) | 1.61 (± 0.46) | 0.22 |
| AFI | 0.30 (± 0.07) | 0.15 (±0.02) | < 0.0001 |
NAFLD, non-alcoholic fatty liver disease; SD, standard deviation; S, subcutaneous fat; P, preperitoneal fat; IPF, intra-peritoneal fat; AFI, abdominal wall fat index.
The variables with greater statistical significance were PF and AFI, with a value corresponding to p < 0.0001.
Considering the statistical significance of P, the sample strength was calculated taking into consideration that the standard deviation for the group of obese patients was 0.29 and 0.13 for the group of normoweight children, the difference between the means was 0.29, with a type 1 (alpha) error of 0.05, the sample strength was 99%.
DISCUSSION
Indirect indicators of body composition are commonly utilized as substitute measurements to evaluate adiposity in adults and in children. The most common of such indicators include skinfolds, BMI, waist and hip circumference. The utilization of such indicators is based on the assumption that they are correlated with more direct adiposity measurements, such as visceral fat and SCT, but they cannot differentiate these two measurements(9,10). According to Camhi et al.(9), the waist circumference and BMI may not be faithful parameters to measure intra-abdominal fat. Additionally, alterations of skinfold measurements are variable during the peak of growth and specifically in relation to the peak of growth velocity(10). Therefore, the utilization of some imaging method is required for a more specific in relation to intra-abdominal fat.
Up until now, there are not much studies approaching methods for a rapid and precise evaluation of visceral fat in children. In a study developed by Mook-Kanamori et al.(11), it was observed that CT may be replaced by US to determine measurements of intra-abdominal fat, and it is suggested that US is a valid method to measure PF and visceral fat in children for the purposes of epidemiological and clinical trials. In the present study, the authors observed statistical significance of intra-abdominal fat and SCT in relation to obesity, a result similar to those reported by Radominski et al.(12), who have found correlations significantly positive with the BMI both in relation to intra-abdominal thickness and subcutaneous tissue thickness measured by US.
In the study developed by Hirooka et al.(13), the fat volume was defined with basis on three measurements, as follows: a) distance between the internal surface of the abdominal muscle and the splenic vein; b) distance between the internal surface of the abdominal muscle and the posterior wall of the aorta, at the level of the umbilicus; c) thickness of the fat layer posterior to the right renal wall. In the present study, there was a similarity in relation to the statistical significance of the distance between the internal surface of the abdominal muscle and the posterior wall of the aorta (IPF 2), but neither the measurement of the distance between the posterior layer of fat and the right renal wall, nor the distance between the internal surface of the abdominal muscle and the splenic vein were measured, because the latter includes pancreatic measurements and the other involves retroperitoneal fat, regions where the accumulation of fat is not frequent in children. In order to replace such measurements, the authors measured the distance between the anterior peritoneum and the anterior wall of the aorta (IPF 1) and the distance between the posterior surface of the left liver lobe and the anterior wall of the aorta (IPF 3), considering that the latter evaluates the small omentum. In the study developed by Hirooka et al.(13), like in the present study, it was observed a correlation between fat volume and BMI, and the patients who presented body weight loss also presented a reduction of the fat volume, both at CT and US. Thus, US also would be useful to evaluate the effectiveness of the therapy with diet and physical exercises.
In the present study, the visceral adiposity was evaluated in the measurements of IPF (IPF1, IPF2 and IPF3) and PF. In spite of being in the abdominal wall, the preperitoneal fat is considered as visceral, since it is covered by the peritoneum. Amongst all the measurements, the PF is most easily reproducible at US. On the other hand, the measurement of IPF is more difficult to be performed because it involves measurements related to the aorta and is more susceptible to the variation in the pressure of the transducer during the evaluation.
Liver biopsy, in spite of being the gold standard for the diagnosis of NAFLD, is an invasive and expensive method(14). According to El-Koofy et al.(15), ultrasonography has 100% sensitivity and specificity in the detection of NAFLD, thus it is a relevant and noninvasive method to evaluate liver steatosis. Other studies have investigated the use of CT and MRI in the evaluation of liver steatosis, but both methods are expensive and many times require sedation of the child, besides the exposure to radiation in the case of CT, which makes its utilization in the routine investigation of NAFLD in children unfeasible(14,16).
In the present study, US demonstrated the presence of steatosis only in the group of obese children and adolescents (36.36%), corroborating the significant association between obesity and NAFLD(17-20).
Considering the high prevalence of NAFLD in obese children, the authors have compared the sonographic parameters among obese patients with and without NAFLD, observing a high statistical significance of the measurements of PF and AFI. It is important to note that the sample strength was calculated for PF (99%), demonstrating that, despite the small size of the sample, the association was highly significant.
The high statistical significance observed between NAFLD and visceral fat thickness is in agreement with other studies(21,22) which have obtained similar results, observing a high association between liver steatosis severity and accumulation of visceral fat at CT, independently of the BMI.
Based on the study developed by Suzuki et al.(8) validating the AFI measured by US, other authors(13,23,24) have demonstrated a high reproducibility of such method in the evaluation of visceral fat. Sogabe et al.(25) have found similar results regarding the AFI at US, observing a greater propensity to liver dysfunction and liver steatosis in the population whose the maximum PF/minimum SCT ratio was ≥ 1. All the mentioned studies validating the AFI were developed with adult individuals.
Considering that all the sonographic data, PF inclusive, presented great significance, the authors believe that studies approaching PF and AFI are quite relevant, since the accumulation of fat is responsible for decrease in the glucose metabolism, lipid disorders and systemic arterial hypertension; thus it is considered a key factor in the development of metabolic syndrome(26).
The knowledge acquired by means of the present study may be useful to guide the utilization of a new method of visceral fat evaluation and also to investigate sonographic parameters which change with the increase in BMI.
CONCLUSION
Finally, both the visceral fat and subcutaneous fat thickness as well as the AFI measured by US have shown significantly positive correlation with the BMI, and the measurements of PF and AFI, with the presence of NAFLD in obese children. Because of its easiness, noninvasiveness and reproducibility, the measurements of PF and AFI are useful to evaluate the visceral fat and NAFLD in obese children.
Footnotes
Sakuno T, Tomita LM, Tomita CM, Giuliano ICB, Ibagy A, Perin NMM, Poeta LS. Sonographic evaluation of visceral and subcutaneous fat in obese children. Radiol Bras. 2014 Mai/Jun;47(3):149–153.
Study developed at Hospital Infantil Joana de Gusmão (HIJG), Florianópolis, SC, Brazil.
REFERENCES
- 1.Abrams P, Levitt Katz LE. Metabolic effects of obesity causing disease in childhood. Curr Opin Endocrinol Diabetes Obes. 2011;18:23–27. doi: 10.1097/MED.0b013e3283424b37. [DOI] [PubMed] [Google Scholar]
- 2.Edwards LA, Bugaresti JM, Buchholz AC. Visceral adipose tissue and the ratio of visceral to subcutaneous adipose tissue are greater in adults with than in those without spinal cord injury, despite matching waist circumferences. Am J Clin Nutr. 2008;87:600–607. doi: 10.1093/ajcn/87.3.600. [DOI] [PubMed] [Google Scholar]
- 3.Matsuzawa Y. Pathophysiology and molecular mechanisms of visceral fat syndrome: the Japanese experience. Diabetes Metab Rev. 1997;13:3–13. doi: 10.1002/(sici)1099-0895(199703)13:1<3::aid-dmr178>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 4.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 5.Li X, Katashima M, Yasumasu T, et al. Visceral fat area, waist circumference and metabolic risk factors in abdominally obese Chinese adults. Biomed Environ Sci. 2012;25:141–148. doi: 10.3967/0895-3988.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Schwenzer NF, Springer F, Schraml C, et al. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol. 2009;51:433–445. doi: 10.1016/j.jhep.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 7.Dâmaso AR, do Prado WL, de Piano A, et al. Relationship between nonalcoholic fatty liver disease prevalence and visceral fat in obese adolescents. Dig Liver Dis. 2008;40:132–139. doi: 10.1016/j.dld.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki R, Watanabe S, Hirai Y, et al. Abdominal wall fat index, estimated by ultrasonography, for assessment of the ratio of visceral fat to subcutaneous fat in the abdomen. Am J Med. 1993;95:309–314. doi: 10.1016/0002-9343(93)90284-v. [DOI] [PubMed] [Google Scholar]
- 9.Camhi SM, Bray GA, Bouchard C, et al. The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: sex and race differences. Obesity (Silver Spring) 2011;19:402–408. doi: 10.1038/oby.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katzmarzyk PT, Shen W, Baxter-Jones A, et al. Adiposity in children and adolescents: correlates and clinical consequences of fat stored in specific body depots. Pediatr Obes. 2012;7:e42–e61. doi: 10.1111/j.2047-6310.2012.00073.x. [DOI] [PubMed] [Google Scholar]
- 11.Mook-Kanamori DO, Holzhauer S, Hollestein LM, et al. Abdominal fat in children measured by ultrasound and computed tomography. Ultrasound Med Biol. 2009;35:1938–1946. doi: 10.1016/j.ultrasmedbio.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Radominski RB, Vezozzo DP, Cerri GG, et al. O uso da ultra-sonografia na avaliação da distribuição de gordura abdominal. Arq Bras Endocrinol Metab. 2000;44:5–12. [Google Scholar]
- 13.Hirooka M, Kumagi T, Kurose K, et al. A technique for the measurement of visceral fat by ultrasonography: comparison of measurements by ultrasonography and computed tomography. Intern Med. 2005;44:794–799. doi: 10.2169/internalmedicine.44.794. [DOI] [PubMed] [Google Scholar]
- 14.Nobili V, Alisi A, Raponi M. Pediatric non-alcoholic fatty liver disease: preventive and therapeutic value of lifestyle intervention. World J Gastroenterol. 2009;15:6017–6022. doi: 10.3748/wjg.15.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Koofy N, El-Karaksy H, El-Akel W, et al. Ultrasonography as a non-invasive tool for detection of nonalcoholic fatty liver disease in overweight/obese Egyptian children. Eur J Radiol. 2012;81:3120–3123. doi: 10.1016/j.ejrad.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 16.Fishbein MH, Mogren C, Gleason T, et al. Relationship of hepatic steatosis to adipose tissue distribution in pediatric nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2006;42:83–88. [PubMed] [Google Scholar]
- 17.Dixon JB, Bhathal PS, O'Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- 18.Souza MR, Diniz MF, Medeiros-Filho JE, et al. Metabolic syndrome and risk factors for non-alcoholic fatty liver disease. Arq Gastroenterol. 2012;49:89–96. doi: 10.1590/s0004-28032012000100015. [DOI] [PubMed] [Google Scholar]
- 19.Duarte MA, Silva GA. Hepatic steatosis in obese children and adolescents. J Pediatr (Rio J) 2011;87:150–156. doi: 10.2223/JPED.2065. [DOI] [PubMed] [Google Scholar]
- 20.Janczyk W, Socha P. Non-alcoholic fatty liver disease in children. Clin Res Hepatol Gastroenterol. 2012;36:297–300. doi: 10.1016/j.clinre.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 21.Eguchi Y, Mizuta T, Sumida Y, et al. The pathological role of visceral fat accumulation in steatosis, inflammation, and progression of nonalcoholic fatty liver disease. J Gastroenterol. 2011;46(1):70–78. doi: 10.1007/s00535-010-0340-3. [DOI] [PubMed] [Google Scholar]
- 22.Eguchi Y, Eguchi T, Mizuta T, et al. Visceral fat accumulation and insulin resistance are important factors in nonalcoholic fatty liver disease. J Gastroenterol. 2006;41:462–469. doi: 10.1007/s00535-006-1790-5. [DOI] [PubMed] [Google Scholar]
- 23.Nakanishi S, Matsuura B, Hirooka M, et al. Clinical usefulness of quantitative evaluation of visceral fat by ultrasonography. J Med Ultrasonics. 2007;34:151–157. doi: 10.1007/s10396-007-0149-8. [DOI] [PubMed] [Google Scholar]
- 24.Kawamoto R, Ohtsuka N, Nakamura S, et al. Preperitoneal fat thickness by ultrasonography and obesity-related disorders. J Med Ultrasonics. 2007;34:93–99. doi: 10.1007/s10396-007-0137-z. [DOI] [PubMed] [Google Scholar]
- 25.Sogabe M, Okahisa T, Hibino S, et al. Usefulness of differentiating metabolic syndrome into visceral fat type and subcutaneous fat type using ultrasonography in Japanese males. J Gastroenterol. 2012;47:293–299. doi: 10.1007/s00535-011-0489-4. [DOI] [PubMed] [Google Scholar]
- 26.Matsuzawa Y. The role of fat topology in the risk of disease. Int J Obes (Lond) 2008;32(Suppl 7):S83–S92. doi: 10.1038/ijo.2008.243. [DOI] [PubMed] [Google Scholar]


