Abstract
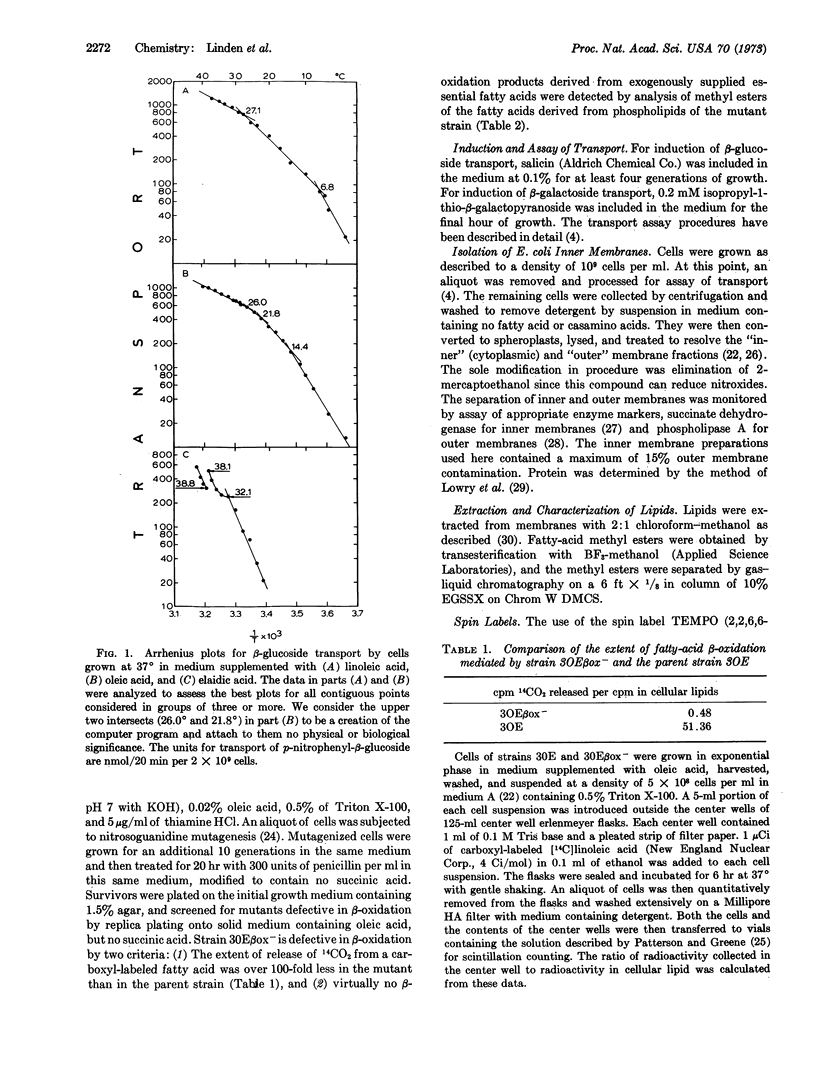
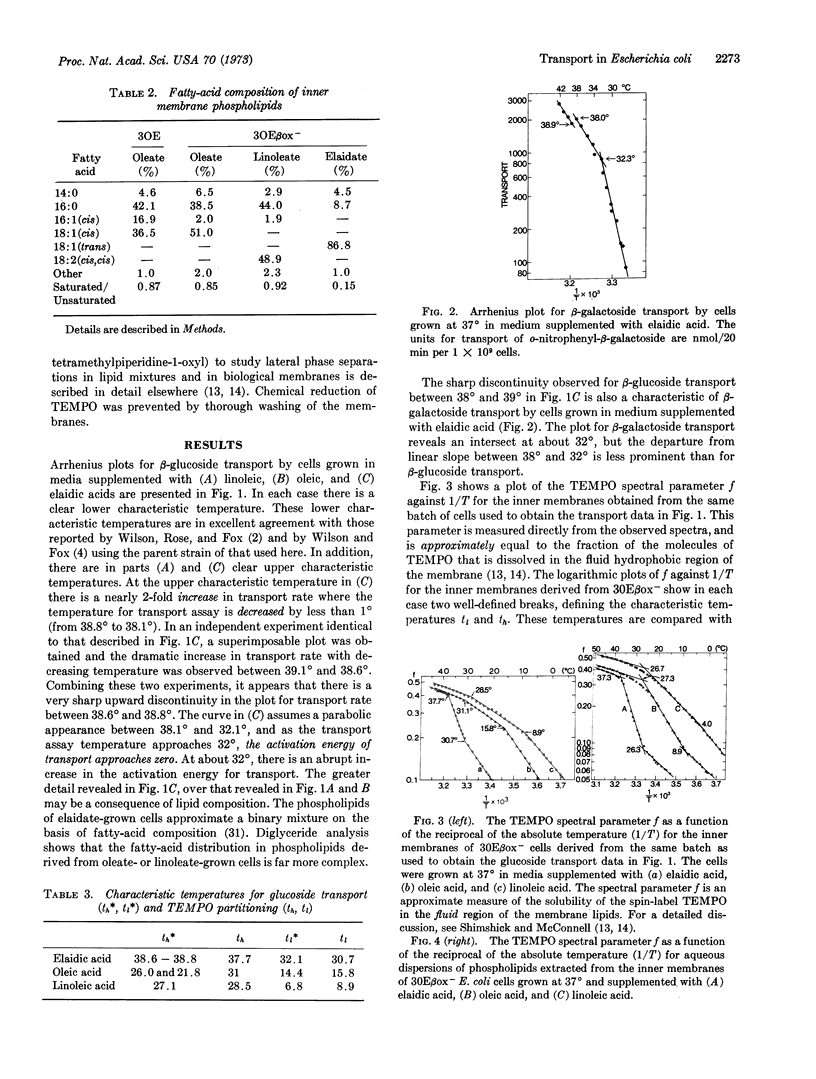
Changes in slope of Arrhenius plots for transport can, in some instances, be detected at two different temperatures for cells that have a relatively simple fatty-acid composition in the membrane lipids. These characteristic temperatures correlate with the characteristic temperatures that define changes of state in membrane phospholipids as revealed by the paramagnetic resonance of the spin label TEMPO (2,2,6,6-tetramethylpiperidine-1-oxyl). The higher of these characteristic temperatures is that at which the formation of solid patches of membrane lipids is first detected. The lower is the end point of the course of lateral phase separations, at which all the membrane lipids are in a solid phase. For cells enriched for elaidic acid, the rate of transport increase by as much as 2-fold as the temperature is decreased by less than 1°, at the higher characteristic temperature. At this characteristic temperature, lateral phase separations begin in the membrane phospholipids. This is also the temperature where one predicts a striking increase in the lateral compressibility of the membrane lipids. These data are thus interpreted to indicate that a component of the transport system vertically penetrates one or both monolayer faces of the membrane during transport, or that some other event involving the lateral compression of the phospholipids is important for transport.
Keywords: β-oxidationless fatty-acid auxotrophs, spin labels
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux P., McConnell H. M. Lateral diffusion in spin-labeled phosphatidylcholine multilayers. J Am Chem Soc. 1972 Jun 28;94(13):4475–4481. doi: 10.1021/ja00768a600. [DOI] [PubMed] [Google Scholar]
- Engelman D. M. X-ray diffraction studies of phase transitions in the membrane of Mycoplasma laidlawii. J Mol Biol. 1970 Jan 14;47(1):115–117. doi: 10.1016/0022-2836(70)90407-9. [DOI] [PubMed] [Google Scholar]
- Epstein W., Fox C. F. Mapping of a locus for unsaturated fatty acid biosynthesis in Escherichia coli. J Bacteriol. 1970 Jul;103(1):273–274. doi: 10.1128/jb.103.1.273-274.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esfahani M., Limbrick A. R., Knutton S., Oka T., Wakil S. J. The molecular organization of lipids in the membrane of Escherichia coli: phase transitions. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3180–3184. doi: 10.1073/pnas.68.12.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. F., Law J. H., Tsukagoshi N., Wilson G. A density label for membranes. Proc Natl Acad Sci U S A. 1970 Oct;67(2):598–605. doi: 10.1073/pnas.67.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C. C., Fox C. F. Induction of the lactose transport system in a lipid-synthesis-defective mutant of Escherichia coli. J Bacteriol. 1970 Aug;103(2):410–416. doi: 10.1128/jb.103.2.410-416.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James R., Branton D., Wisnieski B., Keith A. Composition, structure and phase transition in yeast fatty acid auxotroph membranes: spin labels and freeze-fracture. J Supramol Struct. 1972;1(1):38–49. doi: 10.1002/jss.400010106. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mavis R. D., Vagelos P. R. The effect of phospholipid fatty acid composition in membranous enzymes in Escherichia coli. J Biol Chem. 1972 Feb 10;247(3):652–659. [PubMed] [Google Scholar]
- Overath P., Hill F. F., Lamnek-Hirsch I. Biogenesis of E. coli membrane: evidence for randomization of lipid phase. Nat New Biol. 1971 Dec 29;234(52):264–267. doi: 10.1038/newbio234264a0. [DOI] [PubMed] [Google Scholar]
- Overath P., Schairer H. U., Stoffel W. Correlation of in vivo and in vitro phase transitions of membrane lipids in Escherichia coli. Proc Natl Acad Sci U S A. 1970 Oct;67(2):606–612. doi: 10.1073/pnas.67.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Phillips M. C., Ladbrooke B. D., Chapman D. Molecular interactions in mixed lecithin systems. Biochim Biophys Acta. 1970 Jan 6;196(1):35–44. doi: 10.1016/0005-2736(70)90163-x. [DOI] [PubMed] [Google Scholar]
- SLATER E. C., BORNER W. D., Jr The effect of fluoride on the succinic oxidase system. Biochem J. 1952 Oct;52(2):185–196. doi: 10.1042/bj0520185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackmann E., Träuble H. Studies of the crystalline-liquid crystalline phase transition of lipid model membranes. I. Use of spin labels and optical probes as indicators of the phase transition. J Am Chem Soc. 1972 Jun 28;94(13):4482–4491. doi: 10.1021/ja00768a013. [DOI] [PubMed] [Google Scholar]
- Scandella C. J., Devaux P., McConnell H. M. Rapid lateral diffusion of phospholipids in rabbit sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2056–2060. doi: 10.1073/pnas.69.8.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandella C. J., Kornberg A. A membrane-bound phospholipase A1 purified from Escherichia coli. Biochemistry. 1971 Nov 23;10(24):4447–4456. doi: 10.1021/bi00800a015. [DOI] [PubMed] [Google Scholar]
- Schairer H. U., Overath P. Lipids containing trans-unsaturated fatty acids change the temperature characteristic of thiomethylgalactoside accumulation in Escherichia coli. J Mol Biol. 1969 Aug 28;44(1):209–214. doi: 10.1016/0022-2836(69)90416-1. [DOI] [PubMed] [Google Scholar]
- Shimshick E. J., McConnell H. M. Lateral phase separation in phospholipid membranes. Biochemistry. 1973 Jun 5;12(12):2351–2360. doi: 10.1021/bi00736a026. [DOI] [PubMed] [Google Scholar]
- Tsukagoshi N., Fox C. F. Hybridization of membranes by sonic irradiation. Biochemistry. 1971 Aug 17;10(17):3309–3313. doi: 10.1021/bi00793a023. [DOI] [PubMed] [Google Scholar]
- Verkleij A. J., Ververgaert P. H., van Deenen L. L., Elbers P. F. Phase transitions of phospholipid bilayers and membranes of Acholeplasma laidlawii B visualized by freeze fracturing electron microscopy. Biochim Biophys Acta. 1972 Nov 2;288(2):326–332. doi: 10.1016/0005-2736(72)90253-2. [DOI] [PubMed] [Google Scholar]
- Wilson G., Fox C. F. Biogenesis of microbial transport systems: evidnce for coupled incorporation of newly synthesized lipids and proteins into membrane. J Mol Biol. 1971 Jan 14;55(1):49–60. doi: 10.1016/0022-2836(71)90280-4. [DOI] [PubMed] [Google Scholar]
- Wilson G., Rose S. P., Fox C. F. The effect of membrane lipid unsaturation on glycoside transport. Biochem Biophys Res Commun. 1970 Feb 20;38(4):617–623. doi: 10.1016/0006-291x(70)90625-x. [DOI] [PubMed] [Google Scholar]



