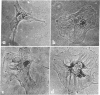
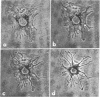
Abstract
Adenosine, dibutyryl cyclic AMP, monobutyryl cyclic AMP, cyclic AMP, and 5′-AMP have a remarkable morphogenetic effect on cultured iris epithelial cells obtained from adult newt. They alter the broad undulating membrane of the cell into branching strands of cytoplasm, a configuration that has been named “stellate.” Theophylline, a phosphodiesterase inhibitor also induces the stellate configuration. This transient morphological alteration is detectable by 30 min and becomes maximal 80 min after treatment. In the continued presence of the effective compounds the altered cells return to their normal shape, although the recovery period is variable. The morphological alteration of iris epithelial cells in vitro observed in the present experiment is reminiscent of that which occurs during the dedifferentiation phase in lens regeneration in vivo. These observations suggest that induction of the stellate configuration is relevant to the mechanism of dedifferentiation of newt iris epithelial cells during Wolffian lens regeneration.
Keywords: cell dedifferentiation, cytoplasmic projections, morphological alterations
Full text
PDF

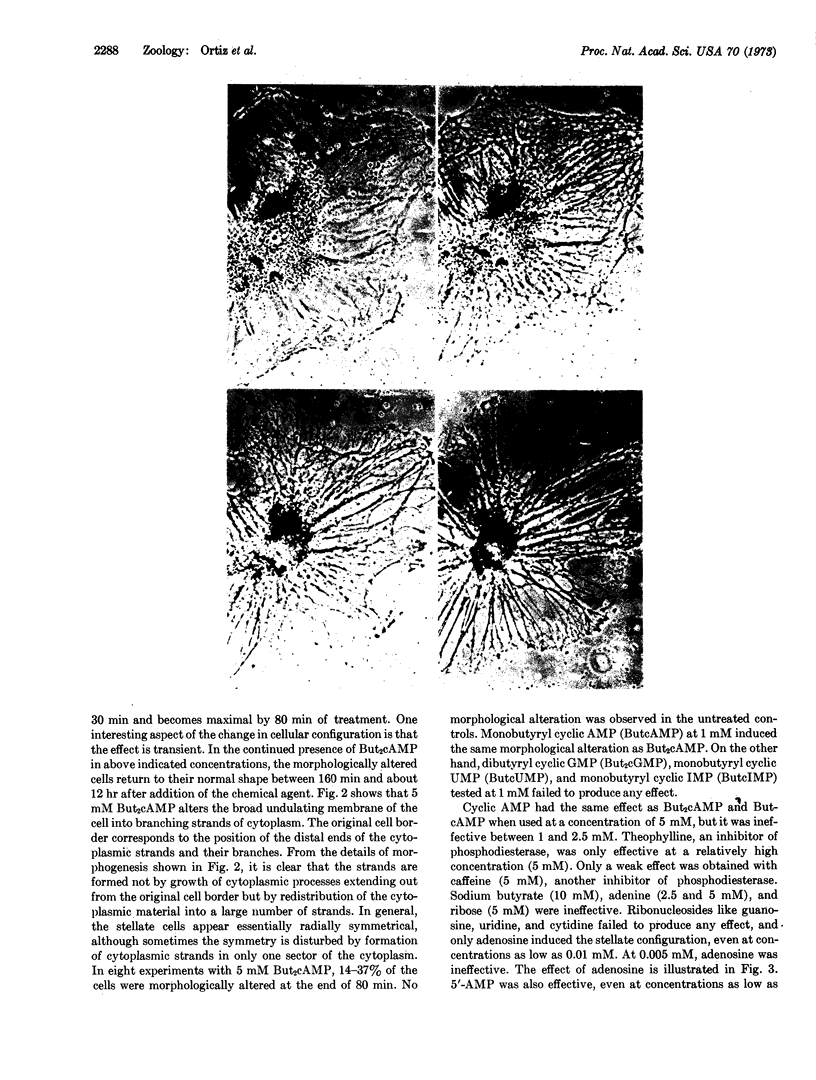


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., Butcher R. W., Nicholson W. E., Baird C. E., Liddle R. A., Liddle G. W. Adenosine 3',5'-monophosphate (cyclic AMP) as the mediator of the actions of melanocyte stimulating hormone (MSH) and norepinephrine on the frog skin. Endocrinology. 1969 Feb;84(2):362–368. doi: 10.1210/endo-84-2-362. [DOI] [PubMed] [Google Scholar]
- Balls M., Ruben L. N. Cultivation in vitro of normal and neoplastic cells of Xenopus laevis. Exp Cell Res. 1966 Oct;43(3):694–695. doi: 10.1016/0014-4827(66)90048-6. [DOI] [PubMed] [Google Scholar]
- Bitensky M. W., Burstein S. R. Effects of cyclic adenosine monophosphate and melanocyte-stimulating hormone on frog skin in vitro. Nature. 1965 Dec 25;208(5017):1282–1284. doi: 10.1038/2081282a0. [DOI] [PubMed] [Google Scholar]
- Dumont J. N., Yamada T. Dedifferentiation of iris epithelial cells. Dev Biol. 1972 Dec;29(4):385–401. doi: 10.1016/0012-1606(72)90079-6. [DOI] [PubMed] [Google Scholar]
- Hsie A. W., Jones C., Puck T. T. Further changes in differentiation state accompanying the conversion of Chinese hamster cells of fibroblastic form by dibutyryl adenosine cyclic 3':5'-monophosphate and hormones. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1648–1652. doi: 10.1073/pnas.68.7.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire J., Moellmann G., McKeon F. Cytochalasin B and pigment granule translocation. Cytochalasin B reverses and prevents pigment granule dispersion caused by dibutyryl cyclic AMP and theophylline in Rana pipiens Melanocytes. J Cell Biol. 1972 Mar;52(3):754–758. doi: 10.1083/jcb.52.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K. N., Hsie A. W. Morphologic differentiation of mouse neuroblastoma cells induced in vitro by dibutyryl adenosine 3':5'-cyclic monophosphate. Nat New Biol. 1971 Sep 29;233(39):141–142. doi: 10.1038/newbio233141a0. [DOI] [PubMed] [Google Scholar]
- Puck T. T., Waldren C. A., Hsie A. W. Membrane dynamics in the action of dibutyryl adenosine 3':5'-cyclic monophosphate and testosterone on mammalian cells. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1943–1947. doi: 10.1073/pnas.69.7.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYER R. W. Regeneration of the lens in the amphibian eye. Q Rev Biol. 1954 Mar;29(1):1–46. doi: 10.1086/399936. [DOI] [PubMed] [Google Scholar]
- Sattin A., Rall T. W. The effect of adenosine and adenine nucleotides on the cyclic adenosine 3', 5'-phosphate content of guinea pig cerebral cortex slices. Mol Pharmacol. 1970 Jan;6(1):13–23. [PubMed] [Google Scholar]
- Shapiro D. L. Morphological and biochemical alterations in foetal rat brain cells cultured in the presence of monobutyryl cyclic AMP. Nature. 1973 Jan 19;241(5386):203–204. doi: 10.1038/241203a0. [DOI] [PubMed] [Google Scholar]
- Torpier G., Montagnier L. Modifications ultrastructurales de la surface des cellules BHK 21-13 transformées, dépendant de nucléotides de l'adénine. Int J Cancer. 1970 Nov 15;6(3):529–535. doi: 10.1002/ijc.2910060324. [DOI] [PubMed] [Google Scholar]
- Yamada T. Cellular and subcellular events in Wolffian lens regeneration. Curr Top Dev Biol. 1967;2:247–283. doi: 10.1016/s0070-2153(08)60290-2. [DOI] [PubMed] [Google Scholar]
- Yokota T., Gots J. S. Requirement of adenosine 3', 5'-cyclic phosphate for flagella formation in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1970 Aug;103(2):513–516. doi: 10.1128/jb.103.2.513-516.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]