Abstract
A critical task for psychotherapy research is to create treatments that can be used by community clinicians. Streamlining of psychotherapies is a necessary first step for this purpose. We suggest that neurobiological knowledge has reached the point of providing biologically meaningful behavioral targets thus guiding the development of effective, simplified psychotherapies. This view is supported by the Research Domain Criteria (RDoC) Project, which reflects the field’s consensus and recognizes the readiness of neurobiology to guide research in treatment development.
“Engage” is an example of such a streamlined therapy. It targets behavioral domains of late-life depression grounded on RDoC constructs using efficacious behavioral strategies selected for their simplicity. “Reward exposure” targeting the behavioral expression of positive valence systems’ dysfunction is the principal therapeutic vehicle of “Engage.” Its first three sessions consist of direct “reward exposure,” but the therapists search for barriers in three behavioral domains, i.e., “negativity bias” (negative valence), “apathy” (arousal), and “emotional dysregulation” (cognitive control), and add strategies targeting these domains when needed. The end result is a structured, stepped approach using neurobiological constructs as targets and as a guide to personalization.
We argue that the “reduction” process needed in order to arrive to simplified effective therapies can be achieved in three steps: 1) Identify RDoC constructs driving the syndrome’s psychopathology; 2) Create a structured intervention utilizing behavioral and ecosystem modification techniques targeting behaviors related to these constructs; 3) Examine whether the efficacy of the new intervention is mediated by change in behaviors related to the targeted RDoC constructs.
Evidence-based psychotherapies are rarely employed and sustained in the community (1-4). A major barrier to their implementation is their complexity, which makes them inaccessible to a large number of mental health professionals (5-8). Streamlining and simplification of psychotherapies is a necessary first step for their large-scale use.
A Model for Streamlining Psychotherapy
Ideally, to develop a simplified, efficacious psychotherapy, one should rely on accepted concepts and findings on the neurobiology of the targeted domains of function. In theory, using neurobiological constructs to guide the selection of targets would assure that the streamlined psychotherapy addresses biologically driven, core aspects of psychopathology that perpetuate maladaptive behavior and suffering. Identifying distinct, biologically driven behavioral targets could sharpen the selection of behavioral interventions from a larger list of interventions that earlier psychotherapy research has found effective in changing behavior. Simplifying the language and delivery of such interventions can make them accessible to community clinicians. Further, biological constructs of function can be used to structure the personalization of psychotherapy. Personalization using a stepped approach can start with interventions targeting psychopathology driven by the core biological disturbance of the targeted disorder. Depending on the response to early interventions and on the nature of remaining psychopathology and its presumed biological determinants, new targeted interventions can be added. Of course, a central question is: “Is our neurobiology strong enough to support this process?”
We argue that advances in neurobiology have reached a point that can meaningfully guide psychotherapy development. This view is supported by the Research Domain Criteria (RDoC) Project, which reflects the field’s current consensus and recognizes the readiness of neurobiology to guide research on mechanisms and treatment of mental disorders.
The Role of the RDoC Project
The RDoC Project’s intent has been to develop a research program to promote knowledge on pathophysiology, identify targets for treatment development, detect subgroups for treatment selection, and facilitate the use of neurobiology findings in clinical decision making (9). Its principal assumptions have been that mental illnesses are disorders of brain circuits and that dysfunction of relevant brain circuits can be identified by tools of clinical neuroscience. Accordingly, the RDoC research program focuses on circuitry, with levels of analysis progressing upwards from measures of circuitry function to clinical manifestations or downwards to the genetic and molecular/cellular factors influencing such functions.
To organize research on brain circuits, the RDoC Project identified five broad domains of function (negative valence, positive valence, cognition, social processes, and arousal/modulation). These domains of function may contribute to psychopathology in varying degrees across a range of clinically defined psychiatric disorders. Accordingly, they serve as areas in which circuit functions and their determinants may be investigated. Measures in such investigations range from genetic, molecular, cellular, circuitry, and physiological functions to observed behavior and self-reports (10). The ultimate goal of this process is to integrate behavioral and neurobiological findings.
Beyond proposing an organized research approach, the RDoC Project initiated workshops and a consensus development process. This process led to the identification of constructs encompassed within each domain of function. Each construct is a behavioral function with evidence that it has behavioral validity and that it maps onto a specific biological system such as a circuit (10). The RDoC recognizes that understanding the role of brain circuits is a work in progress. However, the consensus development process led to an agreement of what constitutes valid knowledge at this point and where to start further research. It also signified an agreement that there is adequate readiness for neurobiology to play a role in treatment development.
Bottom-up, Top-down
Optimally, an intervention for a mental disorder and its neurobiological contributors should be built from the bottom up. In theory, drugs, direct electrical or magnetic stimulation, and gene therapies can target specific brain circuits implicated in abnormal behaviors. Such “surgical precision” has not been accomplished so far. Most of treatments have broad targets and their putative mechanisms of action are based on post hoc interpretations once there is a signal of efficacy. Psychotherapies face even greater challenges in targeting distinct brain circuits. Even the simplest techniques consist of complex interventions processed by interacting brain circuits. For this reason, the available psychotherapies cannot be viewed as treatments targeting specific brain circuits promoting psychopathology.
We argue that a “reduction” process is feasible and may result in a psychotherapy targeting neuroscience-driven constructs of function (RDoC domains) that have a biological basis. Such a reduction can be achieved in three steps. Step 1: Identification of RDoC constructs driving the psychopathology of the targeted syndrome; Step 2: Creation of a structured psychotherapy utilizing behavioral and ecosystem modification approaches targeting these constructs. Step 3: A study examining whether the efficacy of the new psychotherapy is mediated by change in behaviors related to the targeted RDoC constructs. Identification of such behavioral mediators of efficacy would open the field to biological investigations using measures (units of analysis) at the physiology, circuit, cell, molecule and gene levels. The results of such studies can clarify the new psychotherapy’s mechanism of action.
The Example of “Engage”
“Engage” was conceived as a streamlined psychotherapy using neurobiological constructs to identify behavioral targets; select behavioral interventions; and structure a stepped, personalized approach to the treatment of late-life depression. “Engage” relied on the RDoC workshop proceedings and related findings to select behavioral targets. Accordingly, “Engage” theorized that dysfunction of Positive Valence Systems (especially in Reward Valuation, Effort Valuation, Action Selection, Preference-based Decision Making, and Reward Learning) is a central process perpetuating late-life depression. For this reason, “Engage” used “reward exposure” (reactivation and retraining of positive valence systems) as its principal intervention. Barriers to direct “reward exposure” may be posed by the behavioral expression of neurobiological system dysfunctions frequently occurring in late-life depression. “Negativity bias” (a behavioral expression of Negative Valence Systems dysfunction; construct: Loss), “apathy” (an expression of the Arousal and Regulatory System dysfunction; construct: Arousal) and “emotional dysregulation” (an expression of Cognitive Systems Dysfunction; construct: Cognitive Control) have both a neurobiological basis and occur in large numbers of depressed older adults. To address behavioral abnormalities related to the above neurobiologically-based domains of function, “Engage” selected cognitive-behavioral and ecosystem modification strategies that literature has found efficacious. The end result is a streamlined psychotherapy consisting of simple to learn strategies targeting domains of function grounded on accepted neurobiological constructs in late-life depression.
The Focus on Positivity Valence Systems
“Engage” conceptualizes depression as a dysfunction of the positivity valence systems leading to inadequate experience of pleasure or meaning from rewards. In animals, precipitants of depression, including chronic stress, alter gene expression and the morphology, functional activity, and connectivity of networks mediating reward-related depression-like behaviors (11). The molecular and cellular basis of these behaviors includes changes in glutamatergic and GABAergic synaptic plasticity, dopamine neuron excitability, epigenetic and transcriptional mechanisms, and neurotrophic factors (12-18). Tools, such as mutations in mice, gene transfer and optogenetics, have made it possible to identify specific proteins acting within specific cells within reward circuits in mediating depression-like behavior.
Depressed young adults have abnormalities in reward processing. Delay discounting studies showed high impulsivity and time inconsistency in inter-temporal action for gain and loss in depression suggesting a dysfunction in the RDoC construct of reward valuation (19). Depressive symptoms were associated with deficits in reward-based decisions and high variability in selection of action (20) consistent with impairment in the RDoC constructs of action selection and preference based decision-making. Further, small caudate volume has been observed in depressed patients as well as low activation of the nucleus accumbens and the caudate during rewarding outcomes in a monetary gain paradigm (21), impaired reward reversal accuracy, and attenuated response of the anteroventral striatum to unexpected reward during reward reversal learning (22, 23).
Aging influences reward processing (24). Older adults present impaired learning under reward uncertainty because of inability to update value (outcome) representations using prediction error signals. Aging influences the flexibility of adaptation to changes in stimulus-reward contingencies during reversal learning. Furthermore, older adults have high sensitivity to loss (punishment) in learning tasks when the acquisition of behavior depends on outcome processing. In depressed older adults, history of suicide attempts was associated with lower putamen grey matter volume and high delay discounting of rewards (25). These observations are consistent with impairment in the RDoC constructs of action selection and preference based decision-making.
“Reward Exposure”: The Principal Treatment Vehicle
Impairment in the positivity valence systems leads to the abandonment of rewarding activities, strengthening the conviction that little meaning or value is left in life and promoting depressive symptoms. Accordingly, “reward exposure” is the principal treatment vehicle of “Engage.” It is devoted to reinforcing engagement of patients in once rewarding social and physical activities that have been abandoned during depression.
“Reigniting and retraining” the positivity valence systems has been the goal of drug treatment and psychotherapy for depression. On a behavioral level, there are least three reasons to focus on “reward exposure”: 1) Increasing rewarding activities has been a central component of most psychotherapies for depression (26-29). 2) Pleasant Activity Scheduling is an effective component of integrated care models for depression; the IMPACT treatment model is one such example (30, 31). 3) Depressed elders understand and often respond to direct attempts to encourage their social and physical activities (29, 31).
Based on this view, the first three sessions of “Engage” are devoted to direct facilitation of rewarding activities. In the context of a supportive relationship, therapists guide patients to develop a list of rewarding social and physical activity goals and select 2-3 activities in which to engage between sessions. Facilitation of rewarding activities utilizes a simplified problem solving approach. During sessions, they: 1) select a goal (i.e., a rewarding activity); 2) develop a list of ideas on what to do in order to meet this goal; 3) choose one of these ideas; and 4) make an “action plan” with concrete steps to address obstacles to implementation.
Reward exposure serves both as the principal therapeutic tool and as a behavioral probe to identify depressed patients who cannot be engaged or who fail to experience the value of reward engagement. Throughout the first three sessions, therapists conduct a structured assessment to determine whether “action plans” progress well or are inhibited by barriers in three behavioral domains, i.e., “negativity bias,” “apathy” leading to behavioral inertia, or “emotional dysregulation.” Each of these constructs is related to impairment in domains of function identified by the RDoC workshops, and each has been the target of learning-based therapies of depression. If a barrier is identified, therapists choose strategies to mitigate it so that “reward exposure” can progress unhindered.
Mitigating Negativity Bias
Negativity bias refers to selective attention to negative rather than positive information and experiences, and it is a behavioral expression of the construct of loss of the RDoC negative valence domain. According to classical cognitive theorists, negativity bias constitutes a “cognitive vulnerability” factor for depression (32). Several biological correlates of negativity bias have been identified. Negativity bias is associated with greater psychophysiological reactivity and early neural excitation to signals of potential danger, heightened startle amplitude and higher heart rate responses to conscious and non-conscious fear conditions (33-35). Correlates of negativity bias include high activation of the brainstem, amygdala, anterior cingulate cortex (ACC), and ventral and dorsal medial prefrontal cortex to conscious and non-conscious fear probes and differences in electrical brain measures of working memory and EEG theta power (33, 35). Hyper-reactivity of the amygdala in carriers of the short allele of the 5-HTTLPR serotonin transporter has been associated with sensitivity to negative stimuli (36) and negative bias in the processing or interpretation of emotional stimuli (35, 37, 38).
Strategies for coping with negativity bias teach patients to recognize when they are excessively focusing on negative cues and to redirect their attention to neutral or positive aspects of the situation. “Engage” uses simplified cognitive behavioral strategies to overcome negativity bias when patients encounter difficulties with “reward exposure.” These include Playing Devil’s Advocate, Weighing the Evidence, Practicing Positivity Bias, keeping a “Negative Thought Log,” writing Alternative Positive Explanations to negative thoughts, and noticing and learning how positive people respond to negative situations.
Remedying Apathy
The geriatric literature is replete with descriptions of apathy syndromes of depression (depression without sadness, masked depression, etc.). Apathy is defined as “lack of motivation not attributable to diminished level of consciousness, cognitive impairment, or emotional distress” (39-41). Apathy affects more than one third of non-demented individuals with major depression, is most prevalent in older adults (42-44), and is associated with poor treatment response of depression (45-47).
Apathy is a clinical expression of dysfunction in arousal/modulation, a construct of the RDoC part of the Arousal and Regulatory System. Apathy occurs in various conditions impairing the ventral striatum, dorsal ACC, ventral globus pallidus, and medial thalamus (48). In Parkinson’s disease, apathy was inversely correlated with dopamine and norepinephrine transporter binding (11C-RTI-32) in the ventral striatum (49). In frontotemporal dementia, apathy was associated with abnormal metabolism in the ventral striatum, ACC, orbitofrontal cortex, and caudate (50). More than 60% of patients with focal frontal lobe lesions had apathy, while apathy was identified in 40% of patients with basal ganglia lesions (50). Apathy of late-life depression is accompanied by low resting functional connectivity (FC) of the NAcc with the amygdala, caudate, putamen, globus pallidus, and thalamus and increased FC with the dmPFC, superior frontal cortex, and the insula (51). Persistence of apathy of late-life depression after SSRI treatment was correlated with smaller posterior subgenual cingulate gray matter volume and lower uncinate fasciculus white matter integrity (by DTI) (Yuen et al ACNP 2012).
Apathetic patients have difficulty initiating activities and, when they do, they cannot maintain interest and stay on task. Creating an action plan may help patients with mild apathy. However, patients with prominent apathy need prompts to trigger their action plan (52, 53). Examples of prompts include checklists for action plans, placing signs and equipment for daily activities in full view around the patient’s home, using labels and electronic devices (e.g., tape recorders and menu-driven electronic instructions) to get the plan started, calling the patient during the week to prompt the action plan, engaging family and friends to prompt patients to start an action plan, and starting the action plan in session. Easily distracted patients may respond to the removal of distracting items from their environment and to the designation of a place where they can review their action plans and other assignments.
Improving Emotional Dysregulation
Emotional dysregulation is a behavioral correlate of the cognitive control construct of the RDoC Cognitive System domain of function. Classical theories suggest that emotional regulation depends on an interaction between the medial prefrontal cortex (mPFC), implicated in the governance of emotional functions (54), and the subcortical circuits associated with emotion processing (55, 56). Recent findings suggest that rostral areas of the mPFC/ACC participate in conscious threat appraisal and mid regions of the dmPFC/dACC are part of a "core" fear network that is activated irrespective of how fear was learned (57-59). Ventral-rostral portions of the ACC and mPFC have a regulatory role of limbic regions generating emotional responses (57). Anxiety is associated with increased dmPFC-amygdala connectivity during the processing of fearful faces and with faster behavioral responses (60). Normal elders react less than younger adults to negative situations, ignore irrelevant negative stimuli better, and remember relatively more positive than negative information (61). A potential explanation is that older adults are less influenced by interoceptive cues, have lower amygdala and higher prefrontal activation in response to emotional stimuli (62), and have greater medial prefrontal control over negative input as demonstrated in multimodal studies (63). However, when vascular disease leads to additional prefrontal white matter damage, older adults have fewer cognitive control mechanisms available to regulate their emotions (62).
Strategies for emotional dysregulation interfering with “reward exposure” aim to modulate emotions, e.g., overwhelming disappointment, anxiety, etc. Effective emotion management strategies are distraction, meditation, relaxation exercises, deep breathing, and imagery (64-67). Therapist and patient select the most appropriate strategy based on the patient’s previous experiences. Patients practice their selected strategy first in session and then on their own, so that they master it and can use it when they sense an emotional overflow while pursuing “reward exposure.”
Personalization
“Engage” uses a stepped approach to personalization and relies on two principles of stepped care. First, the initial step should be the easiest for clinicians to deliver and for patients to adhere to. Second, the intervention should be self-correcting (68); this approach is analogous to the “measurement-based care” of STAR*D (69-73).
Accordingly, the first three sessions of “Engage” are devoted to “reward exposure” (Figure 1). The therapist guides patients to select rewarding, meaningful activities (e.g. social engagement, exercise, intellectual exchange) and form “action plans” for their pursuit. Simultaneously, therapists assess whether patients: 1) learned how to form “action plans”; 2) engaged in planned activities; and 3) showed improvement of depression. If all conditions are met, patients continue with “reward exposure” (Step 1). If not, therapists identify the most prominent barrier to “reward exposure” by session 3 and proceed with Step 2 by adding strategies to mitigate the identified barrier. A similar assessment is made between session 3 and the end of session 6. Patients doing well in Step 2 (e.g., “reward exposure” plus strategies for negativity bias) continue with the Step 2. For those still experiencing difficulties, therapists assess whether another barrier exists and add a strategy to counteract it (Step 3). Starting with direct facilitation of rewarding activities is parsimonious and instructive. Obstacles to rewarding activities that are not immediately apparent may emerge after attempts to pursue these activities (Manual in Appendix).
Figure 1.
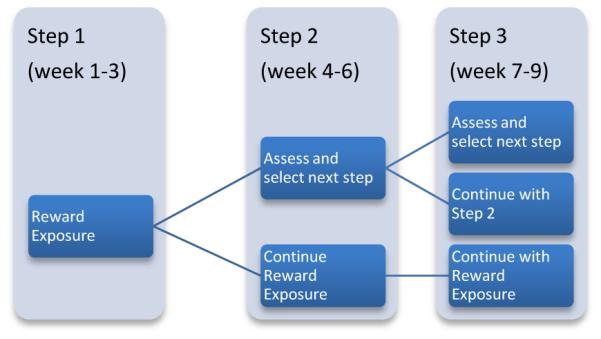
Steps of “Engage”
Executive dysfunction has been associated with poor and/or slow response of late-life depression to pharmacotherapy (74-76). However, patients with depression and executive dysfunction respond well to problem solving therapy (PST) (77, 78). PST does not use direct “reward exposure,” but trains patients to problem solve and indirectly offers the skills to reengage in rewarding activities. “Engage” offers direct “reward exposure,” and uses additional strategies in patients with emotional dysregulation or apathy with assumed Cognitive Control or Arousal and Regulatory Systems abnormalities. Thus, “Engage” offers a personalized approach to barriers imposed by behaviors related to executive dysfunction.
Neurobiology Studies
The Manual of “Engage” offers guidance on how and when to evaluate patients for the four behavioral expressions of RDoC domain dysfunctions in late-life depression. Instruments for “reward exposure” ( e.g., Behavioral Activation for Depression Scale; BADS) (79), “negativity bias” (e.g., Brain Resource Inventory for Social Cognition (BRISC) (80), “apathy” (e.g., Apathy Evaluation Scale) (40) and “emotional dysregulation” (e.g., BRISC) exist. Such instruments can offer further guidance for focusing clinical evaluation. These instruments are also easy to use and are available on mobile platforms, further simplifying the process of targeting and tailoring treatment.
“Engage” conceptualizes reactivation and retraining of the Positive Valence Systems as its principal mechanism of action achieved through “reward exposure,” a treatment component that all depressed patients receive. Constructs of the Positive Valence System such as Reward Valuation, Effort Valuation, Action Selection, Preference-based Decision Making, and Reward Learning can be targets for mediation analysis aimed to identify “How Engage works.” Measures at different levels of analysis may be used for this purpose.
While some depressed patients require only reactivation of the Positive Valence Systems, others may need interventions targeting barriers to reward exposure, i.e., negativity bias, apathy, and/or emotional dysregulation related to Negative Valence, Arousal and Regulatory, and Cognitive Control Systems respectively. Mediation analysis of measures of these systems in depressed patients with specific barriers to “reward exposure” may offer information on the question “For whom does Engage work?”
Conclusion
The “holy grail” of psychotherapy research is the creation of therapies that can be used by community clinicians and are acceptable to patients. The need for such therapies is urgent in late-life depression. The baby boomers are expected to overwhelm the health care system and have been afflicted by depression more than past cohorts (NCS data). Late-life depression has a modest response to pharmacotherapy, promotes disability, worsens the outcomes of most medical illnesses, increases expense, and undermines treatment adherence (81). Two IOM reports (82, 83) predict that the workforce in geriatrics will be overwhelmed by the aging population, a problem that will reach cataclysmic proportions when ACA programs come into being. Older adults seek services in community settings where the providers of initial care for depression are social workers and nurses without in-depth training in psychotherapies. “Engage” is designed for such clinicians and settings and has potential for broad scalability (use by many professionals) and reach by offering many depressed seniors access to effective psychotherapy. Its derivation from neurobiology responds to the NIMH Council report “From Discovery to Cure” (84) and its potential for scalability and reach to the “Bridging Science and Service” report (85). The focus of “Engage” on behaviors related to the positivity bias systems may have neuroeconomic implications for suicide prevention, an area of particular concern in depressed elders who have a high suicide risk (86).
“Engage” can serve as a template for streamlining therapies for patients with a variety of psychiatric syndromes. Key features in this process are:
Identification of one or more domains of function central to the psychopathology of the targeted syndrome. Reliance on the RDoC Project or similar consensus processes would assure that the targets of the streamlined psychotherapy are consistent with currently accepted neurobiological constructs.
Development of a hierarchy of these domains of functions giving higher status to domains commonly occurring in patients with the targeted syndrome and thought to be driving its psychopathology.
Creation of a structured, personalized, stepped therapy in which the first step consists of techniques targeting the domain of function highest in the hierarchy. Additional domains of function should be targeted with appropriate behavioral techniques but only in patients unresponsive to the first step.
Selection of behavioral and ecosystem modification strategies to target abnormal domains of function based on evidence of efficacy and simplicity so that training of community clinicians is feasible.
Use of each step of treatment both as a therapeutic intervention and as an assessment tool to identify whether other domains of function need to be targeted. The end result of this process is a stepped therapy that structures both the selection of behavioral strategies and the personalization process on the basis of accepted neurobiology rather than psychological theories.
Uptake of an intervention requires organization infrastructure changes, but an effective intervention is easier to implement and sustain it if it is feasible and acceptable to clinicians. For this reason, streamlined therapies based on the above principles may have potential for broad scalability (used by many professionals) and reach by offering many patients access to effective care.
Supplementary Material
Acknowledgements
This paper was supported by P30 MH085943 (Alexopoulos), MH074717 (Arean), MH075900 (Arean), and MH077192 (Arean).
This paper was supported by P30 MH085943 (Alexopoulos), R01 MH075897 (Alexopoulos), K24 MH074717 (Arean), R01 MH075900 (Arean).
Footnotes
Conflict of Interest: Dr. Alexopoulos had a research grant from Forest; consulted to Pfizer and Otsuka; and serves on the speaker’s bureaus of Astra Zeneca, Avanir, Novartis, and Sunovion. Dr. Arean reports no conflicts.
References
- 1.Thornicroft G. Evidence-based mental health care and implementation science in low- and middle-income countries. Epidemiol Psychiatr Sci. 2012;21(3):241–4. doi: 10.1017/S2045796012000261. [DOI] [PubMed] [Google Scholar]
- 2.Chambers DA. Advancing the science of implementation: a workshop summary. Adm Policy Ment Health. 2008;35(1-2):3–10. doi: 10.1007/s10488-007-0146-7. [DOI] [PubMed] [Google Scholar]
- 3.Stahmer AC, Aarons G. Attitudes Toward Adoption of Evidence-Based Practices: A comparison of Autism Early Intervention Providers and Children's Mental Health Providers. Psychol Serv. 2009;6(3):223–34. doi: 10.1037/a0010738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldman HH, Azrin ST. Public policy and evidence-based practice. Psychiatr Clin North Am. 2003;26(4):899–917. doi: 10.1016/s0193-953x(03)00068-6. [DOI] [PubMed] [Google Scholar]
- 5.Gehart D. The core competencies and MFT education: practical aspects of transitioning to a learning-centered, outcome-based pedagogy. J Marital Fam Ther. 2011;37(3):344–54. doi: 10.1111/j.1752-0606.2010.00205.x. [DOI] [PubMed] [Google Scholar]
- 6.Sburlati ES, Schniering CA, Lyneham HJ, Rapee RM. A model of therapist competencies for the empirically supported cognitive behavioral treatment of child and adolescent anxiety and depressive disorders. Clin Child Fam Psychol Rev. 2011;14(1):89–109. doi: 10.1007/s10567-011-0083-6. [DOI] [PubMed] [Google Scholar]
- 7.Spruill J, Rozensky RH, Stigall TT, Vasquez M, Bingham RP, De Vaney Olvey C. Becoming a competent clinician: basic competencies in intervention. J Clin Psychol. 2004;60(7):741–54. doi: 10.1002/jclp.20011. [DOI] [PubMed] [Google Scholar]
- 8.Mellman LA, Beresin E. Psychotherapy competencies: development and implementation. Acad Psychiatry. 2003;27(3):149–53. doi: 10.1176/appi.ap.27.3.149. [DOI] [PubMed] [Google Scholar]
- 9.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. The American journal of psychiatry. 2010;167(7):748–51. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 10.Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC medicine. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nature reviews Neuroscience. 2013;14(9):609–25. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo M, Lu Y, Garza JC, Li Y, Chua SC, Zhang W, et al. Forebrain glutamatergic neurons mediate leptin action on depression-like behaviors and synaptic depression. Translational psychiatry. 2012;2:e83. doi: 10.1038/tp.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493(7433):537–41. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warner-Schmidt JL, Schmidt EF, Marshall JJ, Rubin AJ, Arango-Lievano M, Kaplitt MG, et al. Cholinergic interneurons in the nucleus accumbens regulate depression-like behavior. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(28):11360–5. doi: 10.1073/pnas.1209293109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun H, Kennedy PJ, Nestler EJ. Epigenetics of the depressed brain: role of histone acetylation and methylation. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38(1):124–37. doi: 10.1038/npp.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vialou V, Robison AJ, Laplant QC, Covington HE, 3rd, Dietz DM, Ohnishi YN, et al. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nature neuroscience. 2010;13(6):745–52. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blendy JA. The role of CREB in depression and antidepressant treatment. Biological psychiatry. 2006;59(12):1144–50. doi: 10.1016/j.biopsych.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(6):2669–74. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi T, Oono H, Inoue T, Boku S, Kako Y, Kitaichi Y, et al. Depressive patients are more impulsive and inconsistent in intertemporal choice behavior for monetary gain and loss than healthy subjects--an analysis based on Tsallis' statistics. Neuro endocrinology letters. 2008;29(3):351–8. [PubMed] [Google Scholar]
- 20.Kunisato Y, Okamoto Y, Ueda K, Onoda K, Okada G, Yoshimura S, et al. Effects of depression on reward-based decision making and variability of action in probabilistic learning. Journal of behavior therapy and experimental psychiatry. 2012;43(4):1088–94. doi: 10.1016/j.jbtep.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. The American journal of psychiatry. 2009;166(6):702–10. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson OJ, Cools R, Carlisi CO, Sahakian BJ, Drevets WC. Ventral striatum response during reward and punishment reversal learning in unmedicated major depressive disorder. The American journal of psychiatry. 2012;169(2):152–9. doi: 10.1176/appi.ajp.2011.11010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wacker J, Dillon DG, Pizzagalli DA. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG, fMRI, and volumetric techniques. NeuroImage. 2009;46(1):327–37. doi: 10.1016/j.neuroimage.2009.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eppinger B, Hammerer D, Li SC. Neuromodulation of reward-based learning and decision making in human aging. Annals of the New York Academy of Sciences. 2011;1235:1–17. doi: 10.1111/j.1749-6632.2011.06230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dombrovski AY, Siegle GJ, Szanto K, Clark L, Reynolds CF, Aizenstein H. The temptation of suicide: striatal gray matter, discounting of delayed rewards, and suicide attempts in late-life depression. Psychological medicine. 2012;42(6):1203–15. doi: 10.1017/S0033291711002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gawrysiak MJ, Carvalho JP, Rogers BP, Nicholas CR, Dougherty JH, Hopko DR. Neural Changes following Behavioral Activation for a Depressed Breast Cancer Patient: A Functional MRI Case Study. Case Rep Psychiatry. 2012;2012:152916. doi: 10.1155/2012/152916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dirmaier J, Steinmann M, Krattenmacher T, Watzke B, Barghaan D, Koch U, et al. Non-pharmacological treatment of depressive disorders: a review of evidence-based treatment options. Rev Recent Clin Trials. 2012;7(2):141–9. doi: 10.2174/157488712800100233. [DOI] [PubMed] [Google Scholar]
- 28.Hunnicutt-Ferguson K, Hoxha D, Gollan J. Exploring sudden gains in behavioral activation therapy for Major Depressive Disorder. Behav Res Ther. 2012;50(3):223–30. doi: 10.1016/j.brat.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moss K, Scogin F, Di Napoli E, Presnell A. A self-help behavioral activation treatment for geriatric depressive symptoms. Aging Ment Health. 2012;16(5):625–35. doi: 10.1080/13607863.2011.651435. [DOI] [PubMed] [Google Scholar]
- 30.Unutzer J, Katon W, Williams JW, Jr., Callahan CM, Harpole L, Hunkeler EM, et al. Improving primary care for depression in late life: the design of a multicenter randomized trial. Med Care. 2001;39(8):785–99. doi: 10.1097/00005650-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Riebe G, Fan MY, Unutzer J, Vannoy S. Activity scheduling as a core component of effective care management for late-life depression. International journal of geriatric psychiatry. 2012;27(12):1298–304. doi: 10.1002/gps.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. The American journal of psychiatry. 2008;165(8):969–77. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- 33.Gordon E, Barnett KJ, Cooper NJ, Tran N, Williams LM. An "integrative neuroscience" platform: application to profiles of negativity and positivity bias. Journal of integrative neuroscience. 2008;7(3):345–66. [PubMed] [Google Scholar]
- 34.Williams LM, Gatt JM, Grieve SM, Dobson-Stone C, Paul RH, Gordon E, et al. COMT Val(108/158)Met polymorphism effects on emotional brain function and negativity bias. NeuroImage. 2010;53(3):918–25. doi: 10.1016/j.neuroimage.2010.01.084. [DOI] [PubMed] [Google Scholar]
- 35.Williams LM, Gatt JM, Schofield PR, Olivieri G, Peduto A, Gordon E. 'Negativity bias' in risk for depression and anxiety: brain-body fear circuitry correlates, 5-HTT-LPR and early life stress. NeuroImage. 2009;47(3):804–14. doi: 10.1016/j.neuroimage.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Munafo MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biological psychiatry. 2008;63(9):852–7. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dannlowski U, Ohrmann P, Bauer J, Kugel H, Arolt V, Heindel W, et al. Amygdala reactivity to masked negative faces is associated with automatic judgmental bias in major depression: a 3 T fMRI study. Journal of psychiatry & neuroscience : JPN. 2007;32(6):423–9. [PMC free article] [PubMed] [Google Scholar]
- 38.Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton JL, 3rd, et al. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. The American journal of psychiatry. 2008;165(1):90–8. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- 39.Marin RS. Differential diagnosis and classification of apathy. The American journal of psychiatry. 1990;147(1):22–30. doi: 10.1176/ajp.147.1.22. [DOI] [PubMed] [Google Scholar]
- 40.Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res. 1991;38(2):143–62. doi: 10.1016/0165-1781(91)90040-v. [DOI] [PubMed] [Google Scholar]
- 41.Marin RS, Firinciogullari S, Biedrzycki RC. The sources of convergence between measures of apathy and depression. J Affect Disord. 1993;28(1):7–14. doi: 10.1016/0165-0327(93)90072-r. [DOI] [PubMed] [Google Scholar]
- 42.Mehta M, Whyte E, Lenze E, Hardy S, Roumani Y, Subashan P, et al. Depressive symptoms in late life: associations with apathy, resilience and disability vary between young-old and old-old. International journal of geriatric psychiatry. 2008;23(3):238–43. doi: 10.1002/gps.1868. [DOI] [PubMed] [Google Scholar]
- 43.Forsell Y, Jorm AF, Fratiglioni L, Grut M, Winblad B. Application of DSM-III-R criteria for major depressive episode to elderly subjects with and without dementia. The American journal of psychiatry. 1993;150(8):1199–202. doi: 10.1176/ajp.150.8.1199. [DOI] [PubMed] [Google Scholar]
- 44.Lampe IK, Heeren TJ. Is apathy in late-life depressive illness related to age-at-onset, cognitive function or vascular risk? Int Psychogeriatr. 2004;16(4):481–6. doi: 10.1017/s1041610204000766. [DOI] [PubMed] [Google Scholar]
- 45.Chaturvedi SK, Sarmukaddam SB. Prediction of outcome in depression by negative symptoms. Acta psychiatrica Scandinavica. 1986;74(2):183–6. doi: 10.1111/j.1600-0447.1986.tb10603.x. [DOI] [PubMed] [Google Scholar]
- 46.Lavretsky H, Lesser IM, Wohl M, Miller BL, Mehringer CM. Clinical and neuroradiologic features associated with chronicity in late-life depression. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 1999;7(4):309–16. [PubMed] [Google Scholar]
- 47.Levkovitz Y, Sheer A, Harel EV, Katz LN, Most D, Zangen A, et al. Differential effects of deep TMS of the prefrontal cortex on apathy and depression. Brain stimulation. 2011;4(4):266–74. doi: 10.1016/j.brs.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 48.Bonelli RM, Cummings JL. Frontal-subcortical circuitry and behavior. Dialogues in clinical neuroscience. 2007;9(2):141–51. doi: 10.31887/DCNS.2007.9.2/rbonelli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Remy P, Doder M, Lees A, Turjanski N, Brooks D. Depression in Parkinson's disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain : a journal of neurology. 2005;128:1314–22. doi: 10.1093/brain/awh445. Pt 6. [DOI] [PubMed] [Google Scholar]
- 50.Chase TN. Apathy in neuropsychiatric disease: diagnosis, pathophysiology, and treatment. Neurotoxicity research. 2011;19(2):266–78. doi: 10.1007/s12640-010-9196-9. [DOI] [PubMed] [Google Scholar]
- 51.Alexopoulos GS, Hoptman MJ, Yuen G, Kanellopoulos D, Seirup JK, Lim KO, et al. Functional connectivity in apathy of late-life depression: A preliminary study. J Affect Disord. 2013;149(1-3):398–405. doi: 10.1016/j.jad.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Livingston G, Johnston K, Katona C, Paton J, Lyketsos CG. Old Age Task Force of the World Federation of Biological P. Systematic review of psychological approaches to the management of neuropsychiatric symptoms of dementia. The American journal of psychiatry. 2005;162(11):1996–2021. doi: 10.1176/appi.ajp.162.11.1996. [DOI] [PubMed] [Google Scholar]
- 53.Verkaik R, van Weert JC, Francke AL. The effects of psychosocial methods on depressed, aggressive and apathetic behaviors of people with dementia: a systematic review. International journal of geriatric psychiatry. 2005;20(4):301–14. doi: 10.1002/gps.1279. [DOI] [PubMed] [Google Scholar]
- 54.Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 1996;351(1346):1413–20. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- 55.Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383(6603):812–5. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- 56.Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16(2):331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 57.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in cognitive sciences. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maier S, Szalkowski A, Kamphausen S, Perlov E, Feige B, Blechert J, et al. Clarifying the Role of the Rostral dmPFC/dACC in Fear/Anxiety: Learning, Appraisal or Expression? PloS one. 2012;7(11):e50120. doi: 10.1371/journal.pone.0050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mechias ML, Etkin A, Kalisch R. A meta-analysis of instructed fear studies: implications for conscious appraisal of threat. NeuroImage. 2010;49(2):1760–8. doi: 10.1016/j.neuroimage.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 60.Robinson OJ, Charney DR, Overstreet C, Vytal K, Grillon C. The adaptive threat bias in anxiety: amygdala-dorsomedial prefrontal cortex coupling and aversive amplification. NeuroImage. 2012;60(1):523–9. doi: 10.1016/j.neuroimage.2011.11.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mather M. The emotion paradox in the aging brain. Annals of the New York Academy of Sciences. 2012;1251:33–49. doi: 10.1111/j.1749-6632.2012.06471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roalf DR, Pruis TA, Stevens AA, Janowsky JS. More is less: emotion induced prefrontal cortex activity habituates in aging. Neurobiology of aging. 2011;32(9):1634–50. doi: 10.1016/j.neurobiolaging.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Williams LM, Brown KJ, Palmer D, Liddell BJ, Kemp AH, Olivieri G, et al. The mellow years?: neural basis of improving emotional stability over age. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26(24):6422–30. doi: 10.1523/JNEUROSCI.0022-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Szanton SL, Wenzel J, Connolly AB, Piferi RL. Examining mindfulness-based stress reduction: perceptions from minority older adults residing in a low-income housing facility. BMC Complement Altern Med. 2011;11:44. doi: 10.1186/1472-6882-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Howell CA, Turnbull DA, Beilby JJ, Marshall CA, Briggs N, Newbury WL. Preventing relapse of depression in primary care: a pilot study of the "Keeping the blues away" program. Med J Aust. 2008;188(12 Suppl):S138–41. doi: 10.5694/j.1326-5377.2008.tb01878.x. [DOI] [PubMed] [Google Scholar]
- 66.Price RB, Paul B, Schneider W, Siegle GJ. Neural Correlates of Three Neurocognitive Intervention Strategies: A Preliminary Step Towards Personalized Treatment for Psychological Disorders. Cognitive therapy and research. 2013;37(4):657–72. doi: 10.1007/s10608-012-9508-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang W, Li X, Liu X, Duan X, Wang D, Shen J. Distraction reduces theta synchronization in emotion regulation during adolescence. Neuroscience letters. 2013;550:81–6. doi: 10.1016/j.neulet.2013.05.070. [DOI] [PubMed] [Google Scholar]
- 68.Kendall T, McGoey L, Jackson E. If NICE was in the USA. Lancet. 2009;374(9686):272–3. doi: 10.1016/S0140-6736(09)60781-9. [DOI] [PubMed] [Google Scholar]
- 69.Morris DW, Trivedi MH. Measurement-based care for unipolar depression. Current psychiatry reports. 2011;13(6):446–58. doi: 10.1007/s11920-011-0237-8. [DOI] [PubMed] [Google Scholar]
- 70.Shelton RC, Trivedi MH. Challenges and algorithm-guided treatment in major depressive disorder. J Clin Psychiatry. 2011;72(4):e14. doi: 10.4088/JCP.10027tx2cc. [DOI] [PubMed] [Google Scholar]
- 71.Trivedi MH. Tools and strategies for ongoing assessment of depression: a measurement-based approach to remission. J Clin Psychiatry. 2009;70(Suppl 6):26–31. doi: 10.4088/JCP.8133su1c.04. [DOI] [PubMed] [Google Scholar]
- 72.Trivedi MH, Rush AJ, Gaynes BN, Stewart JW, Wisniewski SR, Warden D, et al. Maximizing the adequacy of medication treatment in controlled trials and clinical practice: STAR(*)D measurement-based care. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2007;32(12):2479–89. doi: 10.1038/sj.npp.1301390. [DOI] [PubMed] [Google Scholar]
- 73.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. The American journal of psychiatry. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 74.Alexopoulos GS, Kiosses DN, Heo M, Murphy CF, Shanmugham B, Gunning-Dixon F. Executive dysfunction and the course of geriatric depression. Biological psychiatry. 2005;58(3):204–10. doi: 10.1016/j.biopsych.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 75.Alexopoulos GS, Kiosses DN, Murphy C, Heo M. Executive dysfunction, heart disease burden, and remission of geriatric depression. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004;29(12):2278–84. doi: 10.1038/sj.npp.1300557. [DOI] [PubMed] [Google Scholar]
- 76.Sheline YI, Pieper CF, Barch DM, Welsh-Bohmer K, McKinstry RC, MacFall JR, et al. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Archives of general psychiatry. 2010;67(3):277–85. doi: 10.1001/archgenpsychiatry.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arean PA, Raue P, Mackin RS, Kanellopoulos D, McCulloch C, Alexopoulos GS. Problem-solving therapy and supportive therapy in older adults with major depression and executive dysfunction. The American journal of psychiatry. 2010;167(11):1391–8. doi: 10.1176/appi.ajp.2010.09091327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alexopoulos GS, Raue PJ, Kiosses DN, Mackin RS, Kanellopoulos D, McCulloch C, et al. Problem-solving therapy and supportive therapy in older adults with major depression and executive dysfunction: effect on disability. Archives of general psychiatry. 2011;68(1):33–41. doi: 10.1001/archgenpsychiatry.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kanter J, Mulick P, Busch A, Berlin K, Martell C. The Behavioral Activation for Depression Scale (BADS): Psychometric properties and factor structure. Psychopathological Behavior Assessment. 2007;29:191–202. [Google Scholar]
- 80.Williams LM, Cooper NJ, Wisniewski SR, Gatt JM, Koslow SH, Kulkarni J, et al. Sensitivity, specificity, and predictive power of the "Brief Risk-resilience Index for SCreening," a brief pan-diagnostic web screen for emotional health. Brain and behavior. 2012;2(5):576–89. doi: 10.1002/brb3.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alexopoulos GS. Depression in the elderly. Lancet. 2005;365(9475):1961–70. doi: 10.1016/S0140-6736(05)66665-2. [DOI] [PubMed] [Google Scholar]
- 82.IOM report highlights inadequacies of the geriatric behavioral health workforce. Psychiatr Serv. 2012;63(8):841–2. doi: 10.1176/appi.ps.2012p841a. [DOI] [PubMed] [Google Scholar]
- 83.Institute of Medicine . Retooling for an aging America: Building the health care workforce. National Academies Press; Washington, DC: 2008. [PubMed] [Google Scholar]
- 84.NIMH Council Report: From Discovery to Cure: Accelerating the Development of New and Personalized Interventions for Mental Illnesses. 2010 http://www.nimh.nih.gov/about/advisory-boards-and-groups/namhc/reports/fromdiscoverytocure.pdf.
- 85.NIMH Council Report: Bridging Science and Service. 1998 http://wwwapps.nimh.nih.gov/ecb/archives/nimhbridge.pdf.
- 86.Centers for Disease Control and Prevention, National Center for Injury Prevention and Control Web-based Injury Statistics Query and Reporting System (WISQARS) 2010 www.cdc.gov/injury/wisqars/index.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


